Contribution of Chemical Modifications and Conformational Epitopes to IgE Binding by Ara h 3
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Sera
2.2. Extract Preparation and Protein Purification
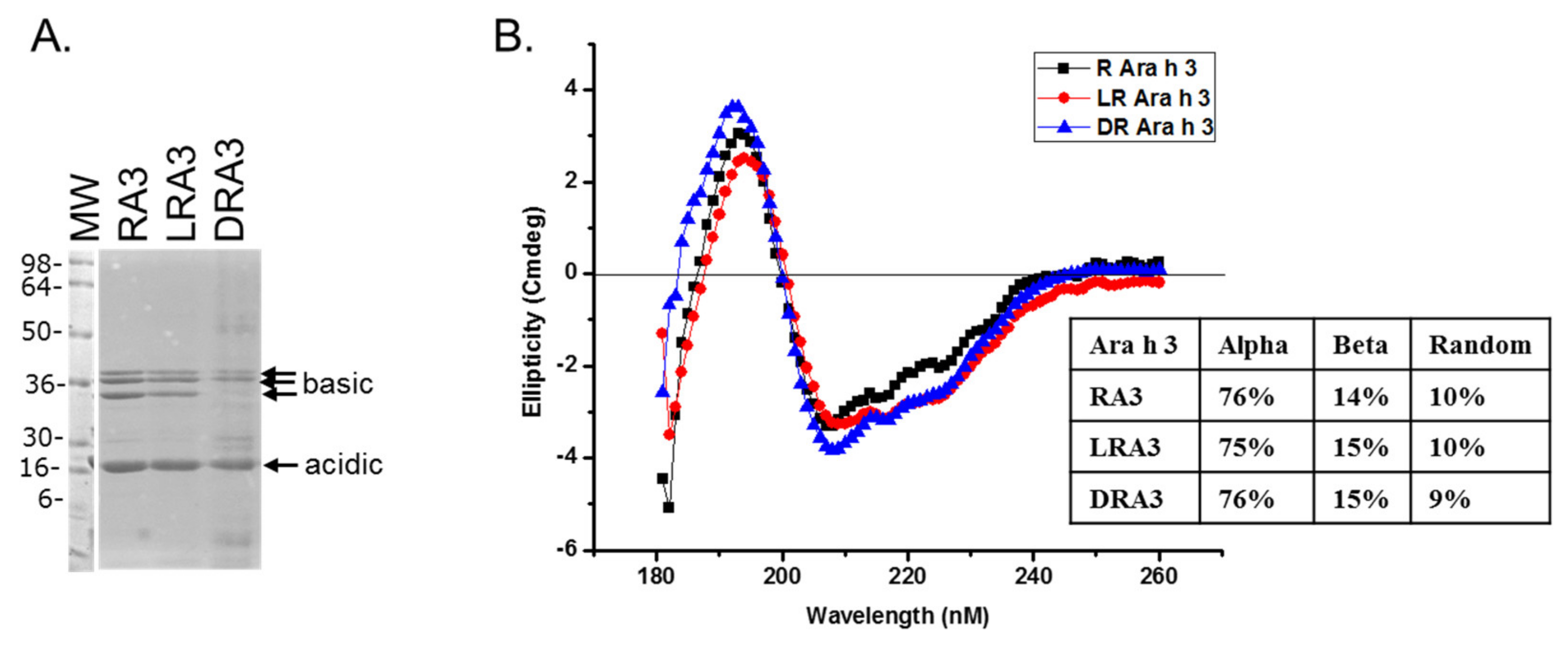
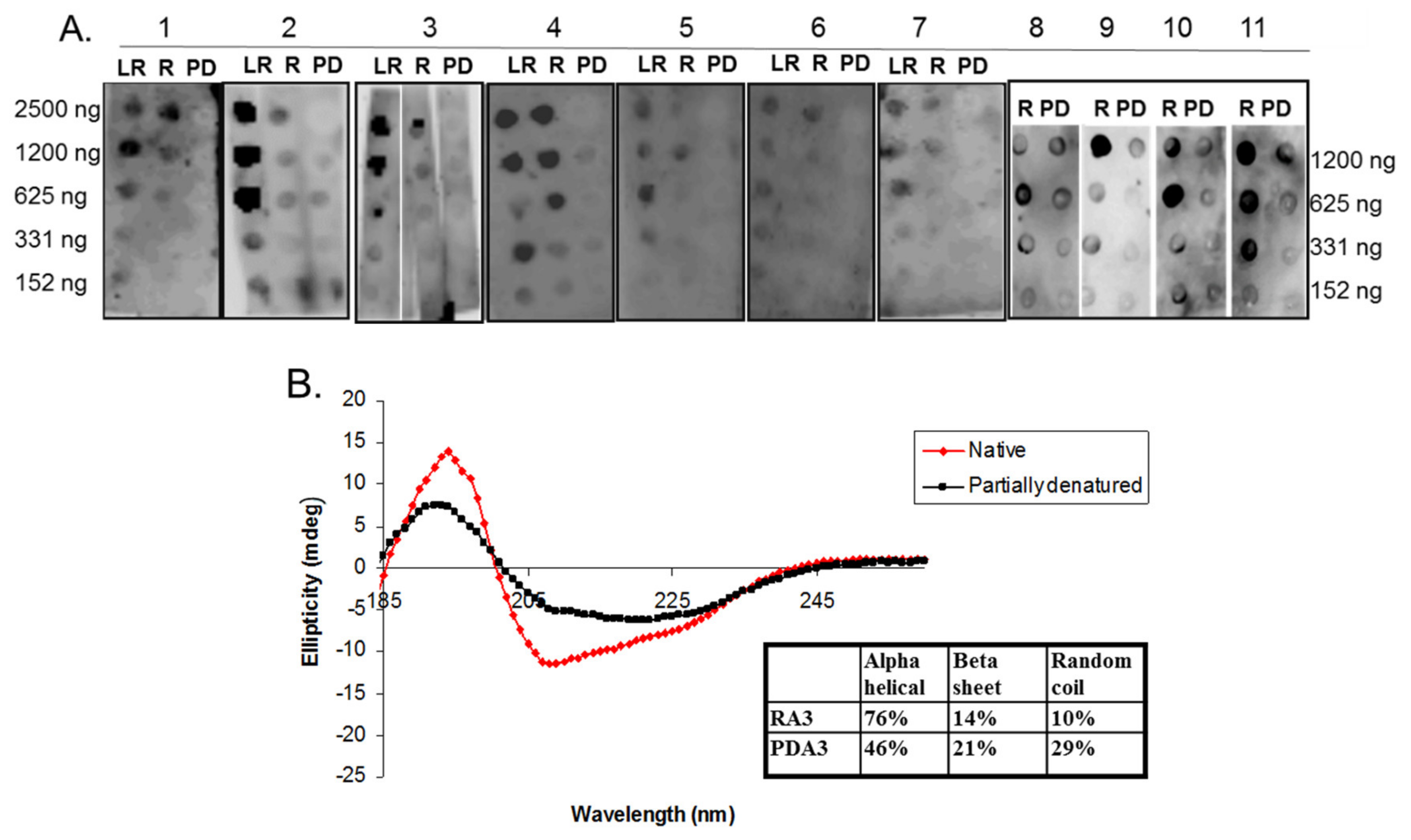
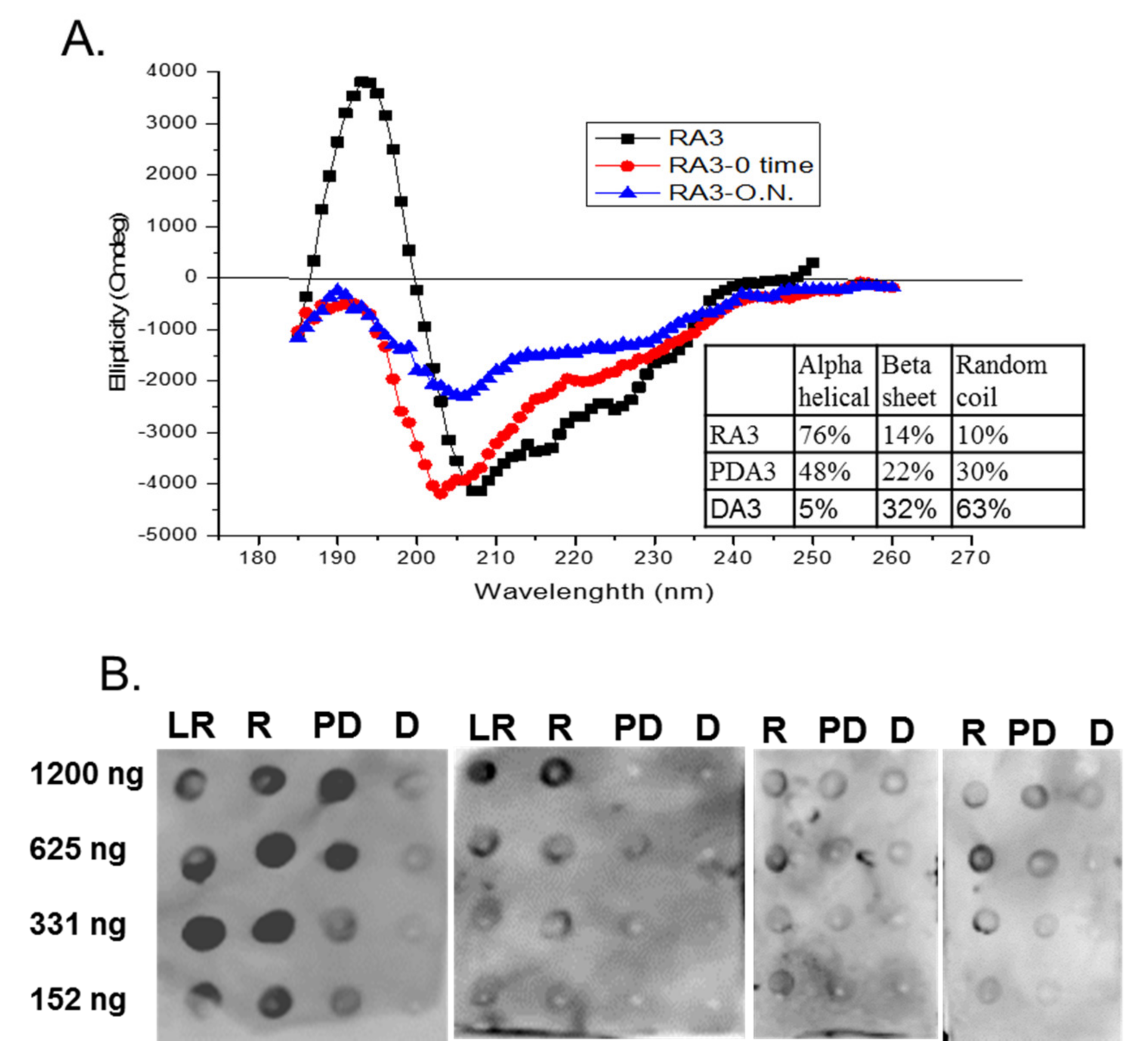
2.3. Circular Dichroism of Purified Ara h 3
2.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.5. Spot Blot Analysis of Raw (R), Light Roast (LR) and Partially Denatured (PD) Ara h 3
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, R.S.; Springston, E.E.; Warrier, M.R.; Smith, B.; Kumar, R.; Pongracic, J.; Holl, J.L. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011, 128, e9–e17. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.H.; Jaramillo, R.; Sicherer, S.H.; Wood, R.A.; Bock, S.A.; Burks, A.W.; Massing, M.; Cohn, R.D.; Zeldin, D.C. National prevalence and risk factors for food allergy and relationship to asthma: Results from the National Health and Nutrition Examination Survey 2005–2006. J. Allergy Clin. Immunol. 2010, 126, 798–806.e14. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.A.; Atkins, F.M. The natural history of peanut allergy. J. Allergy Clin. Immunol. 1989, 83, 900–904. [Google Scholar] [CrossRef]
- Netting, M.; Makrides, M.; Gold, M.; Quinn, P.; Penttila, I. Heated allergens and induction of tolerance in food allergic children. Nutrients 2013, 5, 2028–2046. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.W.; Campbell, D.E.; Turner, P.J.; Kakakios, A.; Wong, M.; Mehr, S.; Joshi, P. Baked egg food challenges—Clinical utility of skin test to baked egg and ovomucoid in children with egg allergy. Clin. Exp. Allergy 2013, 43, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.A.; Sampson, H.A.; Sicherer, S.H.; Noone, S.; Moshier, E.L.; Godbold, J.; Nowak-Wegrzyn, A. Dietary baked egg accelerates resolution of egg allergy in children. J. Allergy Clin. Immunol. 2012, 130, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Huang, F.R.; Sampson, H.A.; Nowak-Wegrzyn, A. Outcomes of 100 consecutive open, baked-egg oral food challenges in the allergy office. J. Allergy Clin. Immunol. 2012, 129, 1682–1684. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Mehr, S.; Joshi, P.; Tan, J.; Wong, M.; Kakakios, A.; Campbell, D.E. Safety of food challenges to extensively heated egg in egg-allergic children: A prospective cohort study. Pediatr. Allergy Immunol. 2013, 24, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Bartnikas, L.M.; Phipatanakul, W. Turning up the heat on skin testing for baked egg allergy. Clin. Exp. Allergy 2013, 43, 1095–1096. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Chung, S.Y.; Champagne, E.T.; Raufman, J.P. The effects of roasting on the allergenic properties of peanut proteins. J. Allergy Clin. Immunol. 2000, 106, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J. Food processing: Effects on allergenicity. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Maillard, L.C.; Gautier, M.A. The reaction of amino acids with sugars: Mechanisms of melanoid formation. CR Seances Acad. Sci. 1912, III, 66–68. [Google Scholar]
- Chung, S.Y.; Maleki, S.J.; Champagne, E.T. Allergenic properties of roasted peanut allergens may be reduced by peroxidase. J. Agric. Food Chem. 2004, 52, 4541–4545. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Chen, X.M.; Jing, H. Demonstration of antioxidant and anti-inflammatory bioactivities from sugar-amino acid maillard reaction products. J. Agric. Food Chem. 2012, 60, 6718–6727. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ho, C.T. Process-induced chemical changes in foods. An overview. Adv. Exp. Med. Biol. 1998, 434, 1–3. [Google Scholar] [PubMed]
- Maleki, S.J.; Schmitt, D.A.; Galeano, M.; Hurlburt, B.K. Comparison of the digestibility of the major peanut allergens in thermally processed peanuts and in pure form. Foods 2014, 3, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Nesbit, J.B.; Hurlburt, B.K.; Schein, C.H.; Cheng, H.; Wei, H.; Maleki, S.J. Ara h 1 structure is retained after roasting and is important for enhanced binding to IgE. Mol. Nutr. Food Res. 2012, 56, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Pedrosa, M.M.; Rodriguez, J.; Muzquiz, M.; Maleki, S.J.; Cuadrado, C.; Burbano, C.; Crespo, J.F. Influence of enzymatic hydrolysis on the allergenicity of roasted peanut protein extract. Int. Arch. Allergy Immunol. 2011, 157, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.A.; Nesbit, J.B.; Hurlburt, B.K.; Cheng, H.; Maleki, S.J. Processing can alter the properties of peanut extract preparations. J. Agric. Food Chem. 2010, 58, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, R.; Mortazavi, S.A.; Sankian, M.; Shahidi, F.; Maleki, S.J.; Nasiraii, L.R.; Falak, R.; Sima, H.R.; Varasteh, A. Influence of processing on the allergenic properties of pistachio nut assessed in vitro. J. Agric. Food Chem. 2010, 58, 10231–10235. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.M.; Cheng, H.; Nesbit, J.B.; Su, W.J.; Cao, M.J.; Maleki, S.J. Effects of boiling on the ige-binding properties of tropomyosin of shrimp (Litopenaeus vannamei). J. Food Sci. 2010, 75, T1–T5. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Hurlburt, B.K. Structural and functional alterations in major peanut allergens caused by thermal processing. J. AOAC Int. 2004, 87, 1475–1479. [Google Scholar] [PubMed]
- Maleki, S.J.; Viquez, O.; Jacks, T.; Dodo, H.; Champagne, E.T.; Chung, S.Y.; Landry, S.J. The major peanut allergen, Ara h 2, functions as a trypsin inhibitor, and roasting enhances this function. J. Allergy Clin. Immunol. 2003, 112, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.A.; Maleki, S.J.; Johnson, K.; Hurlburt, B.K.; Cheng, H.; Ruan, S.; Nesbit, J.B.; Pomes, A.; Edwards, L.L.; Schorzman, A.; et al. Identification of Maillard reaction products on peanut allergens that influence binding to the receptor for advanced glycation end products. Allergy 2013, 68, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Chung, S.; Champagne, E.; Khalifah, R. Allergic and biophysical properties of peanut proteins before and after roasting. Food Allergy Tolerance 2001, 2, 211–221. [Google Scholar]
- Koppelman, S.J.; Vlooswijk, R.A.; Knippels, L.M.; Hessing, M.; Knol, E.F.; van Reijsen, F.C.; Bruijnzeel-Koomen, C.A. Quantification of major peanut allergens Ara h 1 and Ara h 2 in the peanut varieties Runner, Spanish, Virginia, and Valencia, bred in different parts of the world. Allergy 2001, 56, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Rabjohn, P.; Helm, E.M.; Stanley, J.S.; West, C.M.; Sampson, H.A.; Burks, A.W.; Bannon, G.A. Molecular cloning and epitope analysis of the peanut allergen Ara h 3. J. Clin. Investig. 1999, 103, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Rouge, P.; Culerrier, R.; Sabatier, V.; Granier, C.; Rance, F.; Barre, A. Mapping and conformational analysis of IgE-binding epitopic regions on the molecular surface of the major Ara h 3 legumin allergen of peanut (Arachis hypogaea). Mol. Immunol. 2009, 46, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Guo, F.; Chen, Y.W.; Howard, A.; Zhang, Y.Z. Crystal structure of Ara h 3, a major allergen in peanut. Mol. Immunol. 2009, 46, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Maleki, S.J.; Cheng, H.; Novak, N. Differences in the uptake of Ara h 3 from raw and roasted peanut by monocyte-derived dendritic cells. Int. Arch. Allergy Immunol. 2018, 177, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Hurlburt, B.K.; Schmitt, D.; Isleib, T.G.; Cheng, H.; Garvey, C.; Koenig, R.L.; Maleki, S.J. Production of pure protein and antibodies and development of immunoassays to detect Ara h 3 levels in peanut varieties. Int. J. Food Sci. Technol. 2011, 46, 1477–1484. [Google Scholar] [CrossRef]
- Vissers, Y.M.; Iwan, M.; Adel-Patient, K.; Stahl Skov, P.; Rigby, N.M.; Johnson, P.E.; Mandrup Muller, P.; Przybylski-Nicaise, L.; Schaap, M.; Ruinemans-Koerts, J.; et al. Effect of roasting on the allergenicity of major peanut allergens Ara h 1 and Ara h 2/6: The necessity of degranulation assays. Clin. Exp. Allergy 2011, 41, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
| ISAC Values for Ara h 3 (Fluorescence Intensity) | |||||
|---|---|---|---|---|---|
| Patient number | 1 | 2 | 3 | 4 | 5 |
| Ara h 3 sIgE | 1.57 | 10.6 | 1.03 | 2.61 | 0.78 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyer, S.; Nesbit, J.B.; Cabanillas, B.; Cheng, H.; Hurlburt, B.K.; Maleki, S.J. Contribution of Chemical Modifications and Conformational Epitopes to IgE Binding by Ara h 3. Foods 2018, 7, 189. https://doi.org/10.3390/foods7110189
Dyer S, Nesbit JB, Cabanillas B, Cheng H, Hurlburt BK, Maleki SJ. Contribution of Chemical Modifications and Conformational Epitopes to IgE Binding by Ara h 3. Foods. 2018; 7(11):189. https://doi.org/10.3390/foods7110189
Chicago/Turabian StyleDyer, Scott, Jacqueline B. Nesbit, Beatriz Cabanillas, Hsiaopo Cheng, Barry K. Hurlburt, and Soheila J. Maleki. 2018. "Contribution of Chemical Modifications and Conformational Epitopes to IgE Binding by Ara h 3" Foods 7, no. 11: 189. https://doi.org/10.3390/foods7110189
APA StyleDyer, S., Nesbit, J. B., Cabanillas, B., Cheng, H., Hurlburt, B. K., & Maleki, S. J. (2018). Contribution of Chemical Modifications and Conformational Epitopes to IgE Binding by Ara h 3. Foods, 7(11), 189. https://doi.org/10.3390/foods7110189





