Investigation of Carboxymethyl Cellulose (CMC) on Mechanical Properties of Cold Water Fish Gelatin Biodegradable Edible Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Preparation
2.2. Mechanical Properties
- - Tensile Stress (also called tensile strength), σ, expressed in MPa. This corresponds to the measured force (N) required rupturing the section of the specimen [13]:where F is the force in Newton (N) and A is the area of the section of the test piece (thickness width in mm2).
- - Elongation (also called strain), ε (unit less). This is the ratio of displacement to length of reference sample [13]:where l is displacement (mm) and l0 is reference length (mm). Elongation at break is reported as a percentage relative to comparison flexibility of films.
- - Young’s modulus E (MPa). This parameter corresponds to the slope in the linear stress-strain curve for low elongations [13]:
2.3. Sorption Isotherm
3. Results and Discussion
3.1. Mechanical Properties
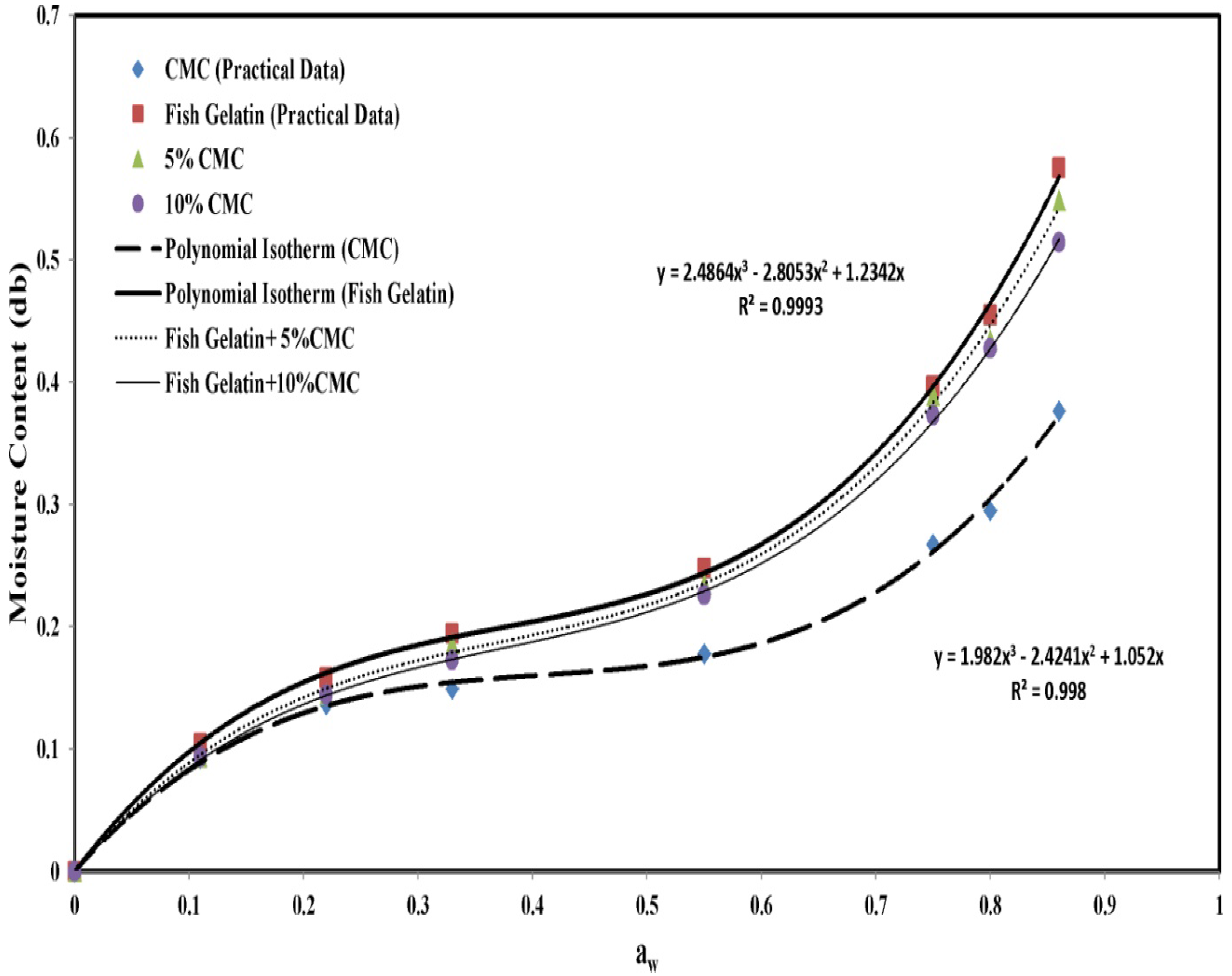
3.2. Absorption Isotherm
3.3. Equilibrium Adsorption Diagrams
4. Conclusions
- 1-
- An increase in the concentration of carboxymethyl cellulose reduced the equilibrium moisture and improved the mechanical properties of composite films compared with the control film. It also increased the tensile stress and film hardness and reduced the film elongation.
- 2-
- Since these films are made of biopolymer materials and one of the properties of biopolymers is their biodegradability, these films are biodegradable in the environment
- 3-
- One of the advantages of fish gelatin over other gelatins is its extraction from fish wastes. Composite films are also cost-effective.
- 4-
- Considering the obtained results, composite films showed better physical and mechanical properties than the control film and they can be used in the packaging industry.
- 5-
- In addition, cold water fish gelatin blended with carboxymethyl cellulose can be an alternative package material of some natural and synthetic products.
Acknowledgments
Author Contributions
Conflicts of Interest
Correction Statement
References
- Hu, Z.; Hong, P.; Liao, M.; Kong, S.; Huang, N.; Ou, C.; Li, S. Preparation and characterization of chitosan—agarose composite films. Materials 2016, 9, 816. [Google Scholar] [CrossRef]
- Larotonda, F.D.S.; Matsui, K.N.; Sobral, P.J.A.; Laurindo, J.B. Hygroscopicity and water vapor permeability of kraft paper impregnated with starch acetate. J. Food Eng. 2005, 71, 394–402. [Google Scholar] [CrossRef]
- Choo, K.; Ching, Y.C.; Chuah, C.H.; Julai, S.; Liou, N.S. Preparation and characterization of polyvinyl alcohol-chitosan composite films reinforced with cellulose nanofiber. Materials 2016, 9, 644. [Google Scholar] [CrossRef]
- Mariniello, L.; Giosafatto, C.V.L.; Moschetti, G.; Aponte, M.; Masi, P.; Sorrentino, A.; Porta, R. Fennel waste-based films suitable for protecting cultivations. Biomacromolecules 2007, 8, 3008–3014. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-M.; Liu, S.-Y.; Chu, F.-B.; Pang, S.; Liang, Y.-R.; Guan, Y.; Peng, F.; Sun, R.-C. Preparation and characterization of blended films from quaternized hemicelluloses and carboxymethyl cellulose. Materials 2016, 9, 4. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Rudhziah, S.; Ahmad, A.; Mohamed, N.S. Biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Polymers 2014, 6, 2371–2385. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Physicochemical and bioactivity of cross-linked chitosan-PVA film for food packaging applications. Int. J. Biol. Macromol. 2009, 45, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Irwandi, J.; Faridayanti, S.; Mohamed, E.S.M.; Hamzah, M.S.; Torla, H.H.; Che Man, Y.B. Extraction and characterization of gelatin from different marine fish species in Malaysia. Int. Food Res. J. 2009, 16, 381–389. [Google Scholar]
- Hamaguchp, P.Y.; Shiku, Y.; Tanaka, M. Property improvement of fish water soluble protein films by dialdehyde starch (DAS) and/or sodium dodecyl sulfate (SDS) treatments. Pack. Sci. Tech. 2003, 12, 270–282. [Google Scholar]
- Gomez-Guillen, M.C.; Perez-Mateos, M.; Gomez-Estaca, J.; Lopez-Caballero, E.; Gimenez, B.; Montero, P. Fish gelatin: A renewable material for developing active biodegradable films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, B.; Musavi, M.; Oromiehie, A.R.; Razavi, K.; Razmi Rad, E.; Milani, J. Effect of plasticizing sugars on water vapor permeability, surface energy and microstructure properties of zein films. LWT Food Sci. Technol. 2007, 40, 1191–1197. [Google Scholar] [CrossRef]
- Masclaux, C.; Gouanvé, F.; Espuche, E. Experimental and modelling studies of transport in starch nanocomposite films as affected by relative humidity. J. Membr. Sci. 2010, 363, 221–231. [Google Scholar] [CrossRef]
- Annual Book of ASTM Standards (ASTM). Standard Test Method for Tensile Properties of Thin Plastic Sheeting D882-10; ASTM: Philadelphia, PA, USA, 2010. [Google Scholar]
- Van den Berg, C. Water activity. In Concentration and Drying of Foods; MacCarthy, D., Ed.; Elsevier Applied Science Publishers: London, UK, 1986; pp. 11–36. [Google Scholar]
- Bertuzzi, M.A.; Castro Vidaurre, E.F.; Armada, M.; Gottifredi, J.C. Water vapor permeability of edible starch based films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Chambi, H.; Grosso, C. Edible films produced with gelatin and casein cross-linked with transglutaminase. Food Res. Int. 2006, 39, 458–466. [Google Scholar] [CrossRef]
- Lee, K.Y.; Shim, J.; Lee, H.G. Mechanical properties of gellan and gelatin composite films. Carbohydr. Polym. 2004, 56, 251–254. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuk, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Su, J.F.; Huang, Z.; Yuan, X.Y.; Wang, X.Y.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Mu, C.; Guo, J.; Li, X.; Lin, W.; Li, D. Preparation and properties of dialdehyde carboxymethyl cellulose crosslinked gelatin edible films. Food Hydrocolloids 2012, 27, 22–29. [Google Scholar] [CrossRef]
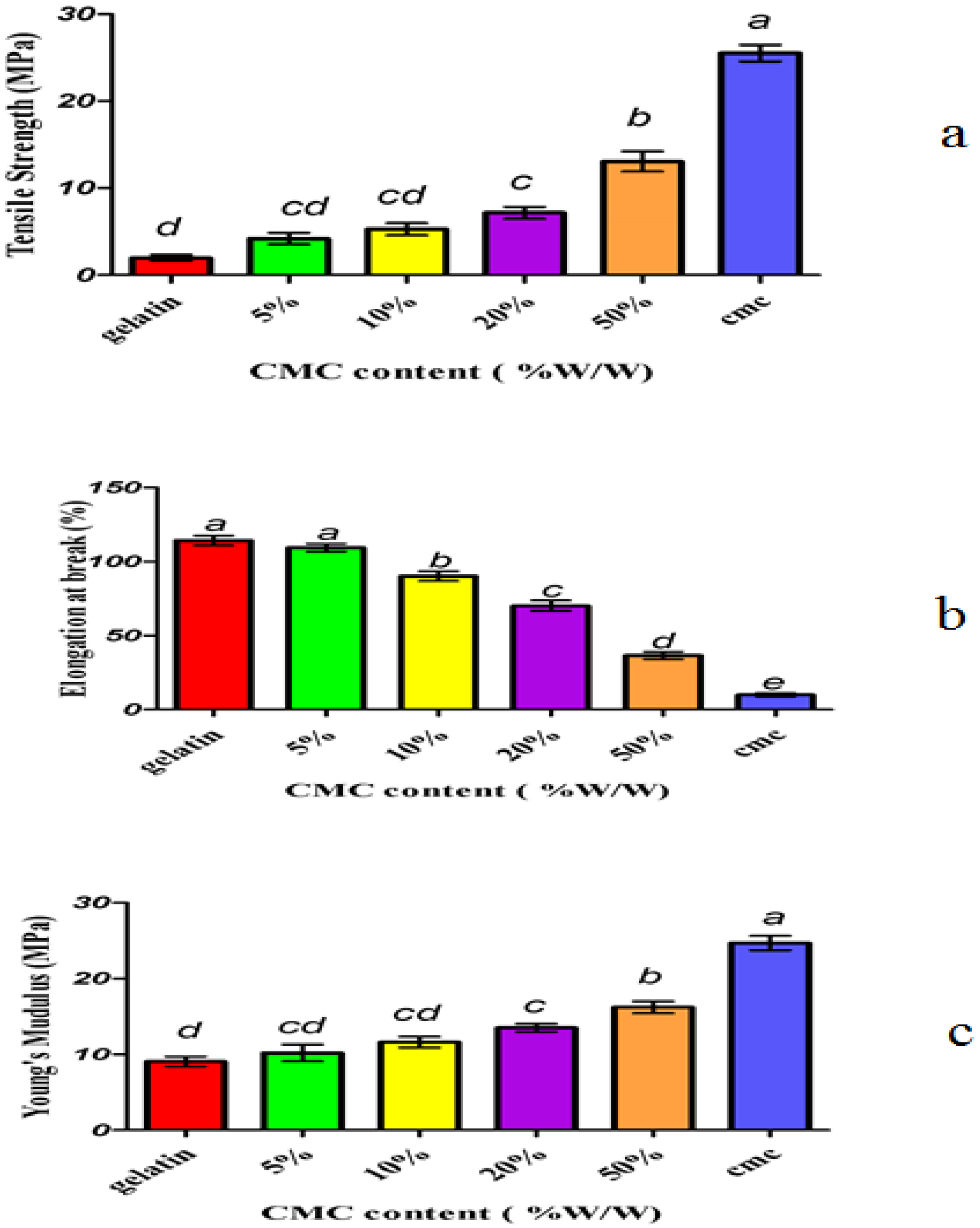
| Concentration | Tensile Stress | Elongation | Young’s Modulus |
|---|---|---|---|
| Control Fish Gelatin | 1.9643 ± 0.024 | 114.335 ± 0.322 | 9.0625 ± 0.386 |
| Control CMC (without Fish Gelatin) | 25.5132 ± 0.020 | 9.9887 ± 0.054 | 24.7013 ± 0.386 |
| Fish Gelatin with 5% CMC | 4.1970 ± 0.015 | 109.505 ± 0.073 | 10.1975 ± 0.206 |
| Fish Gelatin with 10% CMC | 5.2875 ± 0.012 | 90.2236 ± 0.106 | 11.6250 ± 0.247 |
| Fish Gelatin with 20% CMC | 7.1542 ± 0.027 | 70.2231 ± 0.052 | 13.5125 ± 0.201 |
| Fish Gelatin with 50% CMC | 13.0677 ± 0.013 | 36.5075 ± 0.030 | 16.2275 ± 0.248 |
| Concentration | M0 | C | K | E (%) |
|---|---|---|---|---|
| CMC Control | 0.101 | 147.492 | 0.845 | 7.91 |
| Gelatin Control | 0.140 | 29.545 | 0.889 | 7.13 |
| 5% CMC | 0.139 | 29.588 | 0.882 | 7.99 |
| 10% CMC | 0.137 | 29.796 | 0.873 | 6.49 |
| 20% CMC | 0.129 | 67.460 | 0.869 | 6.54 |
| 50% CMC | 0.114 | 73.291 | 0.853 | 6.78 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabari, M. Investigation of Carboxymethyl Cellulose (CMC) on Mechanical Properties of Cold Water Fish Gelatin Biodegradable Edible Films. Foods 2017, 6, 41. https://doi.org/10.3390/foods6060041
Tabari M. Investigation of Carboxymethyl Cellulose (CMC) on Mechanical Properties of Cold Water Fish Gelatin Biodegradable Edible Films. Foods. 2017; 6(6):41. https://doi.org/10.3390/foods6060041
Chicago/Turabian StyleTabari, Mahsa. 2017. "Investigation of Carboxymethyl Cellulose (CMC) on Mechanical Properties of Cold Water Fish Gelatin Biodegradable Edible Films" Foods 6, no. 6: 41. https://doi.org/10.3390/foods6060041
APA StyleTabari, M. (2017). Investigation of Carboxymethyl Cellulose (CMC) on Mechanical Properties of Cold Water Fish Gelatin Biodegradable Edible Films. Foods, 6(6), 41. https://doi.org/10.3390/foods6060041





