Extraction, Identification and Photo-Physical Characterization of Persimmon (Diospyros kaki L.) Carotenoids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fruit Materials
2.3. Sample Preparation and Characterization
2.4. Accelerated Solvent Extraction of Carotenoids
2.5. Preparation of ASE Carotenoids Extracts for HPLC Semi-Preparative Purification
2.6. Semi Preparative HPLC Reversed Phase Carotenoid Purifications
2.7. Identification of Unknown Carotenoids
2.8. Photophysical Properties
3. Results and Discussion
3.1. Particle Size Distribution of Persimmon Freeze-Dried Powder
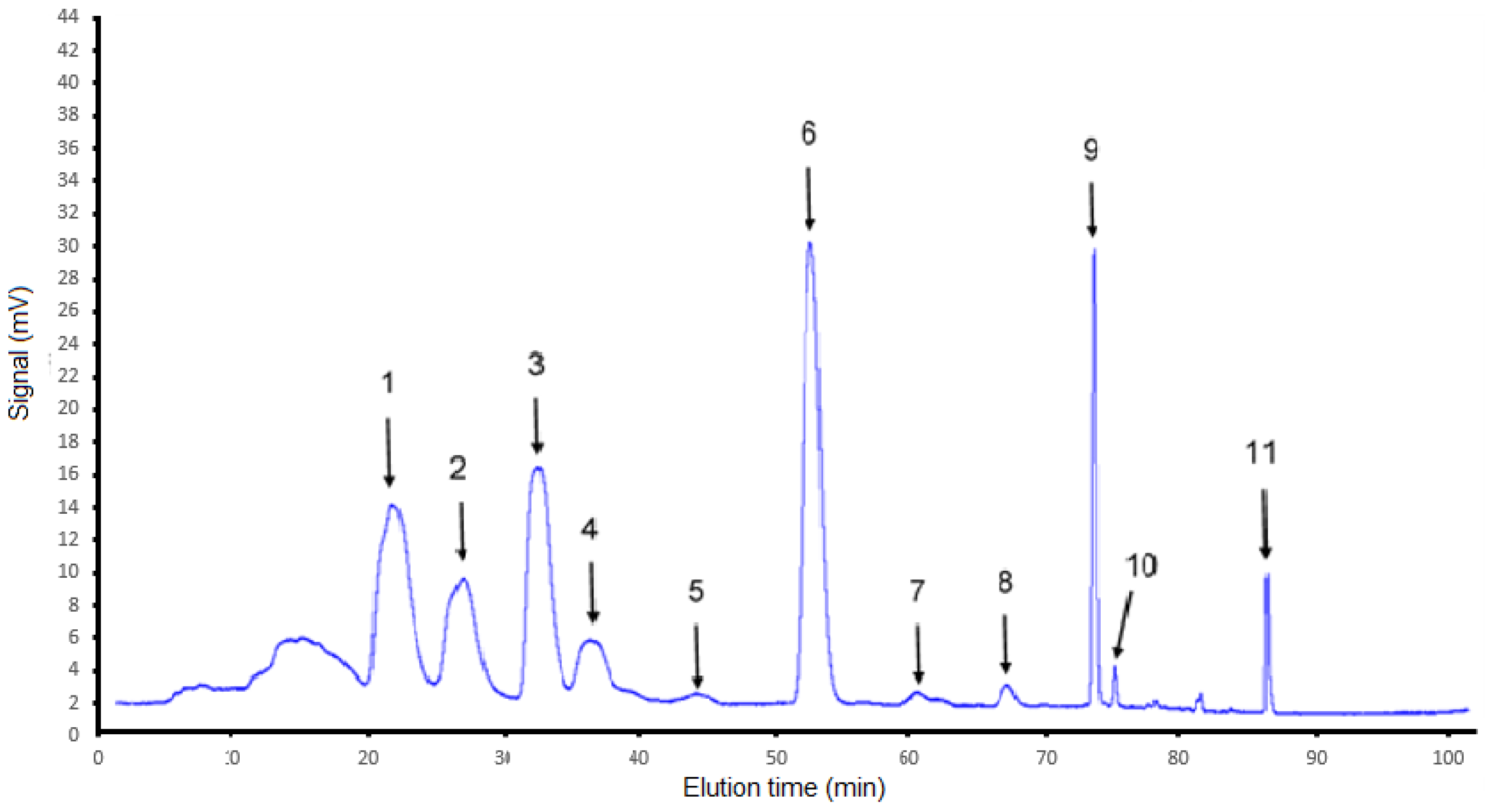
3.2. Semi-Preparative HPLC Purification and Identification of Carotenoids in the ASE Extract
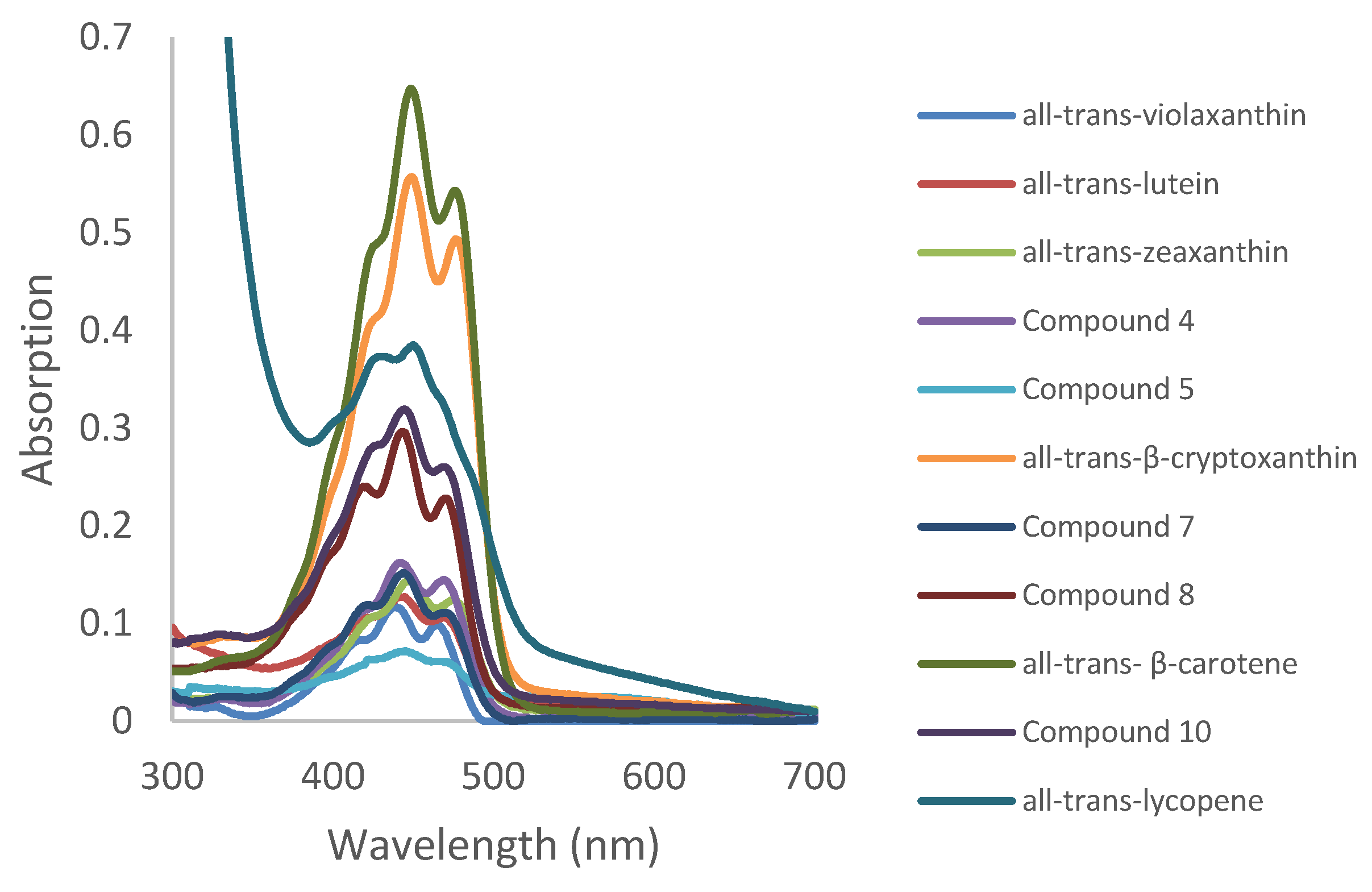
- All trans-violaxanthin (peak 1): [M + H]+ m/z, λmax, absence of cis-peak and a short retention time are in perfect agreement with the identification of all-trans-violaxanthin, which contains two hydroxyl and two epoxy groups. All-trans-violaxanthin had already been identified in persimmon [27,30]. However the % (III/II) observed here was 50 instead of 98, which may be due to the origin of the petroleum ether that we used. It should be noted that petroleum ether is not a well-defined molecule but a mixture of C5 and C6 hydrocarbons.
- All trans-lutein (peak 2): [M + H]+ m/z, λmax, absence of cis-peak and a short retention time similar to that of the standard are in perfect agreement with the identification of all-trans-lutein, which contains two OH groups and should have a slightly longer retention time than violaxanthin. All-trans-lutein had already been identified in persimmon [30] However, the % (III/II) observed here was 25 instead of 60, which may be due to the origin of the petroleum ether that we used.
- All trans-zeaxanthin (peak 3): the characteristics found fit the data perfectly from the literature [28]. The retention time is also in agreement with that of the standard.
- Not identified (peak 4): The [M + H]+ m/z value of 601 indicates a xanthophyll with four oxygens. The fact that, despite these fours oxygens, the fact that this xanthophyll has a longer retention time than lutein, and even zeaxanthin, indicates that this is a cis-form of xanthophyll and indeed we observed a slight cis-peak at 330 nm and an associated % AB:AII of 14%.
- Suspected 5,6-epoxy-α-carotene (peak 5): [M + H]+ m/z, λmax, the absence of a cis-peak, as well as the % III:II, all suggest the presence of 5,6-epoxy-α-carotene.
- All-trans-β-cryptoxanthin (peak 6): the characteristics found fit the data from the literature [29]. The retention time is also in agreement with that of the standard. This xanthophyll is known to be the most prevalent carotenoid in persimmon.
- Unidentified (peak 7): [M + H]+ m/z values of 553 and 537 indicate a mixture of a single-oxygenated xanthophyll and of one carotene, with retention times that do not allow separation by the preparative chromatography fraction collector. A very slight increase in absorbance is observed at 330 nm, which could indicate a cis-form. It is not possible to be more precise.
- Cis-isomer of β-carotene, supposed position 13 (peak 8): the mass indicates that this is a carotene and the presence of a slight peak at 330 nm indicates a cis-form of carotene. λmax as well as the % (III:II) indicates that it is most likely a cis-isomer of β-carotene. However, the % AB:AII is lower than that found by De Rosso [29], but it should be noted that they used a different solvent. Therefore, this position 13 of cis-isomerization can only be presumed.
- All-trans-β-carotene (peak 9): the characteristics found fit the data from the literature perfectly [28]. The retention time is also in agreement with that of the standard.
- Cis-isomer of β-carotene, supposed position 9 (peak 10): the mass indicates that this is a carotene and the presence of a slight peak at 330 nm indicates a cis-form of carotene. λmax as well as the % (III:II) indicates that it is very likely a cis-isomer of β-carotene. However, the % AB:AII is higher than that found by De Rosso [29], but it should be noted that they used a different solvent. This % should also be lower than that of 13-cis-β-carotene, which is not the case. Thus, position 9 of the cis-isomerization can only be presumed.
- All-trans-lycopene (peak 11): all-trans lycopene was precisely identified, especially due to its characteristic set of λmax values [28].
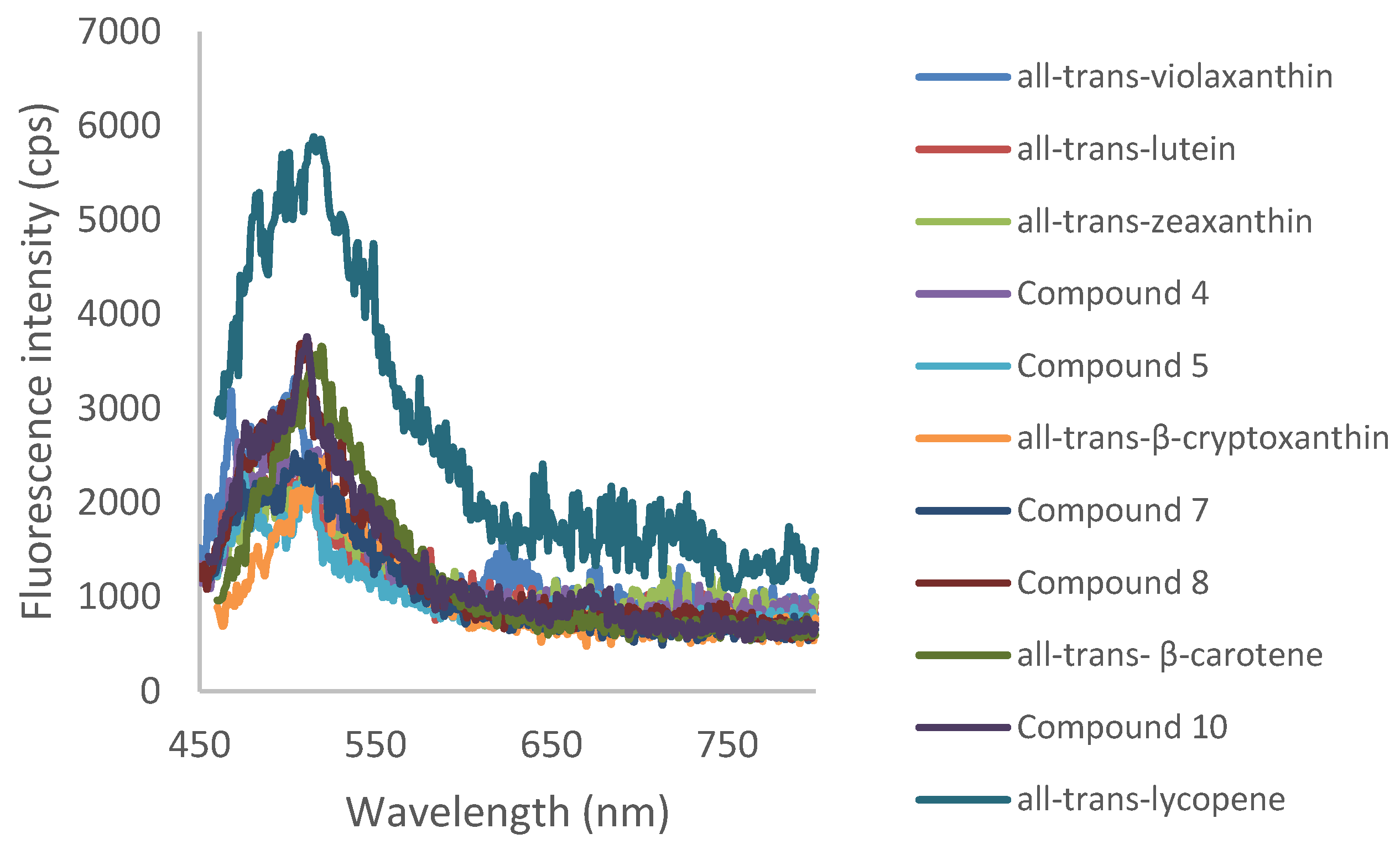
3.3. Details on UV-Visible and Fluorescence Spectra of Purified Carotenoids
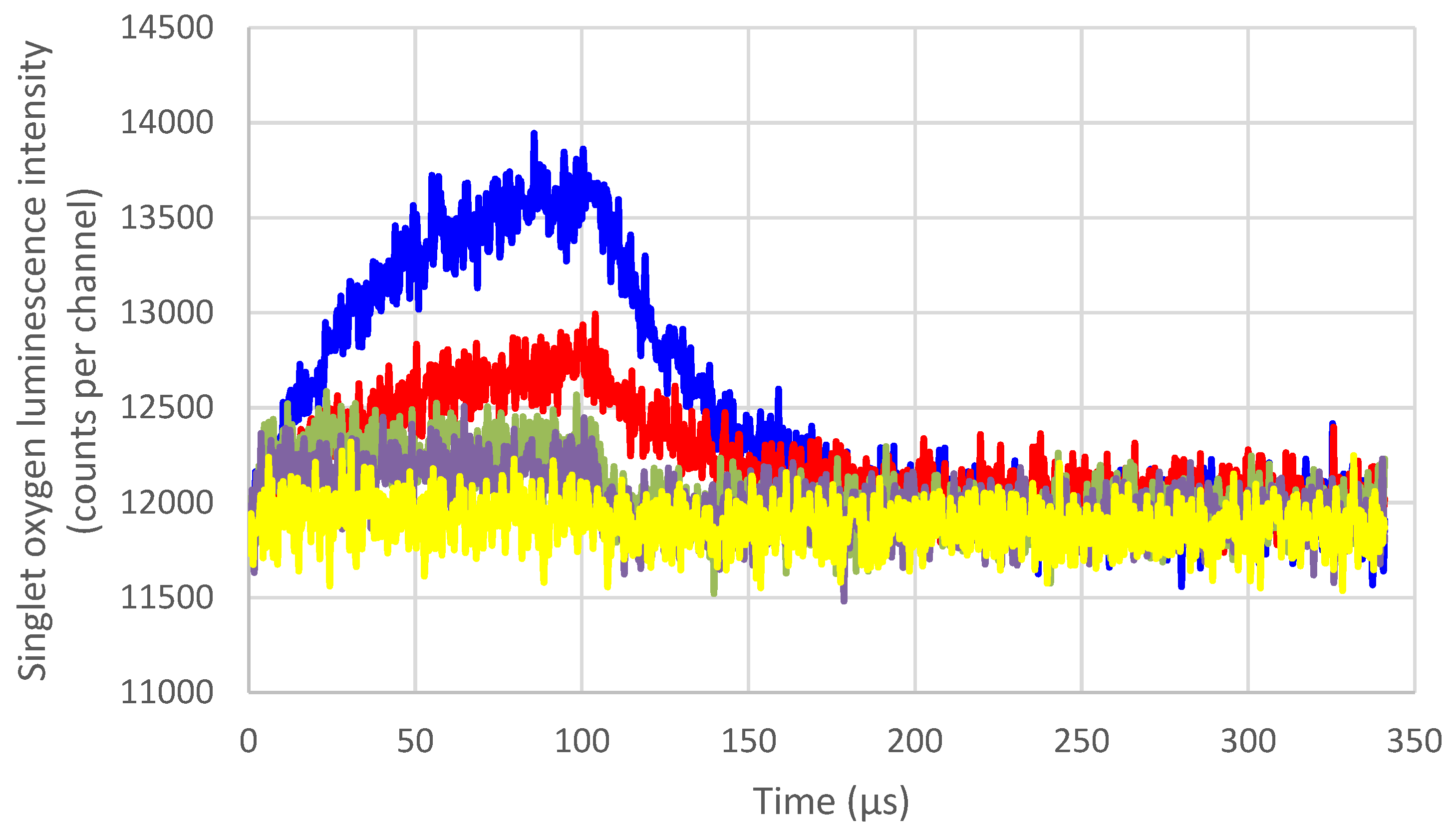
3.4. Singlet Oxygen Quenching by Carotenoids
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| APCI | atmospheric pressure chemical ionization |
| ASE | accelerated solvent extraction |
| BHT | butylhydroxytoluene |
| Car | carotenoid |
| DHIR | 3,3-dihydroxyisorenieratene |
| DMA | 9,10-dimethylanthrancene |
| DMNO2 | 1,4-dimethyl-1,4-naphthalene endoperoxide |
| DPBF | 1,3-Diphenylisobenzofuran |
| EP-1 | 3-(1,4-epidioxy-4-methyl-1,4-dihydro-1-naphtyl propionic acid) |
| EtOH | ethanol |
| Hex | hexane |
| 1-HP | 1H-Phenalen-1-one |
| LC-MS | liquid chromatography- mass spectrometry |
| LDL | low-density lipoprotein |
| MB | methylene blue |
| MTBE | methyl-tert-butyl-ether |
| NDPO2 | 3,3′-(1,4-naphthalylene dipropionate) |
| 1-NN | (1-nitronaphtalene) |
| PBA | 4-(1-pyrene)butyric acid |
| P1COOH | 5-(4-carboxyphenyl)-10,15,20-triphenylporphyrin |
| PDT | photodynamic therapy |
| PMB | photomolecular beacons |
| RB | Rose Bengal |
| RM | system of sodium (bis-2-ethylhexanyl)sulfosuccinate in (hexane/H2O) |
| TEA | triethylamine |
| TPP | tetraphenylporphyrin |
References
- Rodriguez-Amaya, D.B.; Rodriguez, E.B.; Amaya-Farfan, J. Advances in food carotenoids research: Chemical and technological aspects, implications in human health. Malays. J. Nutr. 2006, 12, 101–121. [Google Scholar]
- Rodriguez-Concepcion, M. Supply of precursors for carotenoid biosynthesis in plants. Arch. Biochem. Biophys. 2010, 504, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids. Nutrition and Health; Birkhäuser Verlag: Basel, Switzerland, 2009; Volume 5, p. 431. [Google Scholar]
- LaFountain, A.M.; Pacheco, C.; Prum, R.O.; Frank, H.A. Nuclear magnetic resonance analysis of carotenoids from the burgundy plumage of the Pompadour Cotinga (Xipholena punicea). Arch. Biochem. Biophys. 2013, 539, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Ibañez, E.; Herrero, M.; Mendiola, J.; Castro-Puyana, M. Extraction and Characterization of Bioactive Compounds with Health Benefits from Marine Resources: Macro and Micro Algae, Cyanobacteria, and Invertebrates. In Marine Bioactive Compounds; Springer: New York, NY, USA, 2011; pp. 55–98. [Google Scholar]
- Rivera, S.; Canela, R. Influence of sample processing on the analysis of carotenoids in maize. Molecules 2012, 17, 11255–11268. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Namitha, K.K.; Negi, P.S. Chemistry and biotechnology of carotenoids. Crit. Rev. Food Sci. Nutr. 2010, 50, 728–760. [Google Scholar] [CrossRef] [PubMed]
- Perera, C.O.; Yen, G.M. Functional properties of carotenoids in human health. Int. J. Food Prop. 2007, 10, 201–230. [Google Scholar] [CrossRef]
- Koh, W.P.; Yuan, J.M.; Wang, R.; Lee, Y.P.; Lee, B.L.; Yu, M.C.; Ong, C.N. Plasma carotenoids and risk of acute myocardial infarction in the singapore chinese health study. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Elis, A.; Aviram, M. Hypocholesterolemic effect of lycopene and beta-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochem. Biophys. Res. Commun. 1997, 233, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef] [PubMed]
- Wertz, K.; Siler, U.; Goralczyk, R. Lycopene: Modes of action to promote prostate health. Arch. Biochem. Biophys. 2004, 430, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, C.J.; Eto, I.; Juliana, M.M.; Whitaker, L.M. Effect of canthaxanthin on chemically-induced mammary carcinogenesis. Oncology 1991, 48, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Onogi, N.; Okuno, M.; Matsushima-Nishiwaki, R.; Fukutomi, Y.; Moriwaki, H.; Muto, Y.; Kojima, S. Antiproliferative effect of carotenoids on human colon cancer cells without conversion to retinoic acid. Nutr. Cancer 1998, 32, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Hirose, Y.; Yamada, Y.; Hata, K.; Qiang, S.H.; Asano, N.; Oyama, T.; Zhi, H.L.; Iwasaki, T.; Kobayashi, H.; et al. Chemoprevention of mouse urinary bladder carcinogenesis by fermented brown rice and rice bran. Oncol. Rep. 2006, 15, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kawamori, T.; Ohnishi, M.; Makita, H.; Mori, H.; Satoh, K.; Hara, A. Suppression of azoxymethane-induced rat colon carcinogenesis by dietary administration of naturally occurring xanthophylls astaxanthin and canthaxanthin during the postinitiation phase. Carcinogenesis 1995, 16, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids Natural Function; Birkhäuser Verlag: Basel, Switzerland, 2008; Volume 4, p. 370. [Google Scholar]
- Foote, C.S.; Denny, R.W.; Weaver, L.; Chang, Y.; Peters, J. Quenching of singlet oxygen. Ann. N. Y. Acad. Sci. 1970, 171, 139–148. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, J.; Stefflova, K.; Jarvi, M.; Li, H.; Wilson, B.C. Photodynamic molecular beacon as an activatable photosensitizer based on protease-controlled singlet oxygen quenching and activation. Proc. Natl. Acad. Sci. USA 2007, 104, 8989–8994. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Stefflova, K.; Niedre, M.J.; Wilson, B.C.; Chance, B.; Glickson, J.D.; Zheng, G. Protease-triggered photosensitizing beacon based on singlet oxygen quenching and activation. J. Am. Chem. Soc. 2004, 126, 11450–11451. [Google Scholar] [CrossRef] [PubMed]
- Verhille, M.; Benachour, H.; Ibrahim, A.; Achard, M.; Arnoux, P.; Barberi-Heyob, M.; Andre, J.C.; Allonas, X.; Baros, F.; Vanderesse, R.; et al. Photodynamic molecular beacons triggered by MMP-2 and MMP-9: Influence of the distance between photosensitizer and quencher onto photophysical properties and enzymatic activation. Curr. Med. Chem. 2012, 19, 5580–5594. [Google Scholar] [CrossRef] [PubMed]
- Zaghdoudi, K.; Pontvianne, S.; Framboisier, X.; Achard, M.; Kudaibergenova, R.; Ayadi-Trabelsi, M.; Kalthoum-Cherif, J.; Vanderesse, R.; Frochot, C.; Guiavarc’h, Y. Accelerated solvent extraction of carotenoids from: Tunisian kaki (Diospyros kaki L.), peach (Prunus persica L.) and apricot (Prunus armeniaca L.). Food Chem. 2015, 184, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Chouikrat, R.; Champion, A.; Vanderesse, R.; Frochot, C.; Moussaron, A. Microwave-assisted synthesis of zinc 5-(4-carboxyphenyl)-10,15,20-triphenylporphyrin and zinc 5-(4-carboxyphenyl)-10,15,20-triphenylchlorin. J. Porphyr. Phthalocyanines 2015, 19, 595–600. [Google Scholar] [CrossRef]
- Thermo Scientific, Technical note 208. 2013. Available online: http://www.dionex.com/en-us/webdocs/4736-TN208_FINAL.pdf (accessed on 9 July 2016).
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. International Life Sciences. In A Guide to Carotenoid Analysis in Foods; Omni, I., Ed.; ILSI Press: Washington, DC, USA, 2001. [Google Scholar]
- De Rosso, V.V.; Mercadante, A.Z. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.H.; Zhao, D.Q.; Sheng, Y.L.; Tao, J.; Yang, Y. Carotenoids in fruits of different persimmon cultivars. Molecules 2011, 16, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Dreuw, A.; Fleming, G.R.; Head-Gordon, M. Chlorophyll fluorescence quenching by xanthophylls. PCCP 2003, 5, 3247–3256. [Google Scholar] [CrossRef]
- Naqvi, K.R.; Melo, T.B.; Raju, B.B.; Javorfi, T.; Simidjiev, I.; Garab, G. Quenching of chlorophyll a singlets and triplets by carotenoids in light-harvesting complex of photosystem II: Comparison of aggregates with trimers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1997, 53, 2659–2667. [Google Scholar] [CrossRef]
- Cantrell, A.; McGarvey, D.J.; Truscott, T.G.; Rancan, F.; Böhm, F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys. 2003, 412, 47–54. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Cuine, S.; Triantaphylides, C.; Ravanat, J.-L.; Havaux, M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 2012, 158, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Fiedor, L.; Haessner, R.; Scheer, H. Cyclic endoperoxides of beta-carotene, potential pro-oxidants, as products of chemical quenching of singlet oxygen. Biochim. Biophys. Acta 2005, 1709, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Dedic, R.; Svoboda, A.; Hala, J. TPP and singlet oxygen quenching by carotene in solution. J. Mol. Struct. 2011, 993, 474–476. [Google Scholar] [CrossRef]
- Chantrell, S.J.; McAuliffe, C.A.; Munn, R.W.; Pratt, A.C.; Land, E.J. Excited-states of protoporphyrin-IX dimethyl ester—Reaction of triplet with carotenoids. J. Chem. Soc. Faraday Trans. 1 1977, 73, 858–865. [Google Scholar] [CrossRef]
- Conn, P.F.; Schalch, W.; Truscott, T.G. The singlet oxygen and carotenoid interaction. J. Photochem. Photobiol. B Biol. 1991, 11, 41–47. [Google Scholar] [CrossRef]
- Devasagayam, T.P.A.; Werner, T.; Ippendorf, H.; Martin, H.D.; Sies, H. Synthetic carotenoids, novel polyene polyketones and new capsorubin isomers as efficient quenchers of singlet molecular oxygen. Photochem. Photobiol. 1992, 55, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, A.; Aizawa, K.; Iwasaki, Y.; Inakuma, T.; Terao, J.; Nagaoka, S.-I.; Mukai, K. Kinetic study of the quenching reaction of singlet oxygen by carotenoids and food extracts in solution. Development of a singlet oxygen absorption capacity (SOAC) assay method. J. Agric. Food Chem. 2010, 58, 9967–9978. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Di Mascio, P.; Sundquist, A.R.; Devasagayam, T.P.A.; Sies, H. Assay of Lycopene and Other Carotenoids as Singlet Oxygen Quenchers. In Methods in Enzymology; Carotenoids, Part A: Chemistry, Separation, Quantitation, and Antioxidation; Academic Press: New York, NY, USA, 1992; Volume 213, pp. 429–438. [Google Scholar]
- Beutner, S.; Bloedorn, B.; Frixel, S.; Blanco, I.H.; Hoffmann, T.; Martin, H.D.; Mayer, B.; Noack, P.; Ruck, C.; Schmidt, M.; et al. Quantitative assessment of antioxidant properties of natural colorants and phytochemicals: Carotenoids, flavonoids, phenols and indigoids. The role of ss-carotene in antioxidant functions. J. Sci. Food Agric. 2001, 81, 559–568. [Google Scholar] [CrossRef]
- Böhm, F.; Edge, R.; Truscott, T.G. Interactions of dietary carotenoids with singlet oxygen (1O2) and free radicals: Potential effects for human health. Acta Biochim. Pol. 2011, 59, 27–30. [Google Scholar]
- Montenegro, M.A.; Nazareno, M.A.; Durantini, E.N.; Borsarelli, C.D. Singlet molecular oxygen quenching ability of carotenoids in a reverse-micelle membrane mimetic system. Photochem. Photobiol. 2002, 75, 353–361. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Physical quenching of singlet oxygen and cis-trans isomerization of carotenoids. Ann. N. Y. Acad. Sci. 1993, 691, 10–19. [Google Scholar] [CrossRef]
- Lee, S.H.; Min, D.B. Effects, quenching mechanisms, and kinetics of carotenoids in chlorophyll-sensitized photooxidation of soybean oil. J. Agric. Food Chem. 1990, 38, 1630–1634. [Google Scholar] [CrossRef]
- Rodgers, M.A.J.; Bates, A.L. Kinetic and spectroscopic features of some carotenoid triplet-states—Sensitization by singlet oxygen. Photochem. Photobiol. 1980, 31, 533–537. [Google Scholar] [CrossRef]
- Wilkinson, F.; Ho, W.T. Electronic-energy transfer from singlet molecular oxygen to carotenoids. Spectrosc. Lett. 1978, 11, 455–463. [Google Scholar] [CrossRef]
- Farmilo, A.; Wilkinson, F. On the mechanism of quenching of singlet oxygen in solution. Photochem. Photobiol. 1973, 18, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S.; Chang, Y.C.; Denny, R.W. Chemistry of singlet oxygen 10. Carotenoid quenching parallels biological protection. J. Am. Chem. Soc. 1970, 92, 5216–5218. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S.; Denny, R.W. Chemistry of singlet oxygen .7. Quenching by beta-carotene. J. Am. Chem. Soc. 1968, 90, 6233–6235. [Google Scholar] [CrossRef]
- Foote, C.S. Quenching of Singlet Oxygen; Academic Press: New York, NY, USA, 1979; Volume 5, pp. 139–171. [Google Scholar]
- Murasecco-Suardi, P.; Oliveros, E.; Braun, A.M.; Hansen, H.J. Singlet-oxygen quenching by carotenoids—Steady-state luminescence experiments. Helv. Chim. Acta 1988, 71, 1005–1010. [Google Scholar] [CrossRef]
- Krasnovsky, A.A. Photo-luminescence of singlet oxygen in pigment solutions. Photochem. Photobiol. 1979, 29, 29–36. [Google Scholar] [CrossRef]
- Mathews-Roth, M.M.; Wilson, T.; Fujimori, E.; Krinsky, N.I. Carotenoid chromophore length and protection against photosensitization. Photochem. Photobiol. 1974, 19, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Li, T.L.; King, J.M.; Min, Y.B. Quenching mechanisms and kinetics of carotenoids in riboflavin photosensitized singlet oxygen oxidation of vitamin D-2. J. Food Biochem. 2000, 24, 477–492. [Google Scholar] [CrossRef]
- Oliveros, E.; Braun, A.M.; Aminiansaghafi, T.; Sliwka, H.R. Quenching of singlet oxygen (1Dg) by carotenoid derivatives—Kinetic analysis by near-infrared luminescence. New J. Chem. 1994, 18, 535–539. [Google Scholar]

| Peak | Carotenoid | OH/Epoxy | tR (min) | λmax (nm) a | % III/II a,b | % AB/AII a,c | [M + H]+ (m/z) |
|---|---|---|---|---|---|---|---|
| 1 | all-trans-violaxanthin | 2/2 | 18 | 416/440/465 (416/440/465) | 50 (98) | 0 | 601 |
| 2 | all-trans-lutein | 2/0 | 23 | 420/443/469 (421/445/474) | 25 (60) | 0 | 569 |
| 3 | all-trans-zeaxanthin | 2/0 | 28 | 424/448/475 (424/449/476) | 25 (25) | 0 | 569 |
| 4 | not identified (cis-xanthophyll with 4 oxygens) | 4 | 32 | 330/422/444/469 | 43 | 14 | 601 |
| 5 | 5,6-epoxy-α-carotene suspected | 0/1 | 43 | 421/445/470 (418/441/469) * | 0 (10) * | 0 | 553 |
| 6 | all-trans-β-cryptoxanthin | 1/0 | 52 | 426/449/476 (425/449/476) | 36 (25) | 0 | 553 |
| 7 | not identified (mixture of monoxygenated xanthophyll and carotene) | ? | 62 | 330/423/444/470 | 10 | 18 | mixture 537/553 |
| 8 | cis-isomere of β-carotene, position 13 (supposed) | 0/0 | 67 | 332/421/443/470 (338/420/444/470) * | 16 (12) * | 20 (47) * | 537 |
| 9 | all-trans-β-carotene | 0/0 | 74 | 426/449/476 (425/450/477) | 23 (25) | 0 | 537 |
| 10 | cis-isomere of β-carotene, position 9 (supposed) | 0/0 | 75 | 331/424/445/469 (330/420/444/472) * | 11 (20) * | 26 (18) * | 537 |
| 11 | all-trans-lycopene | 0/0 | 86 | 444/470/502 (444/470/501) | 66 (65) | 0 | 537 |
| Carotenoids | kQ 109 M−1·s−1 (Solvent) | 1O2 Production | Method of Detection of 1O2 Quenching | References |
|---|---|---|---|---|
| lycopene | 17 (C6H6) | Phenazine | 1O2 emission | [38] |
| 18 (C6H5CH3) | Phenazine | 1O2 emission | [38] | |
| 19(C6H14) | Phenazine | 1O2 emission | [38] | |
| 9.0 (CHCl3) | NDPO2 | 1O2 emission | [39] | |
| 19 (CHCl3) | Phenazine | 1O2 emission | [38] | |
| 14 (CCl4) | Phenazine | 1O2 emission | [38] | |
| 13.8 (EtOH/CHCl3/D2O 50/50/1) | EP-1 | DPBF | [40] | |
| 31(EtOH/CHCl3/D2O 50/50/1) | NDPO2 | 1O2 emission | [41,42] | |
| 8.8 (EtOH/CHCl3/D2O 50/50/1) | DMNO2 | 1O2 emission | [43] | |
| 17.5(EtOH/CHCl3/H2O 50/50/1) | Phenazine | 1O2 emission | [38] | |
| 31 (EtOH/CHCl3/H2O 50/50/1) | NDPO2 | 1O2 emission | [41] | |
| 0.13 (ascorbic acid in methanol) | 1-NN | 1O2 emission | [44] | |
| 23–25 (reverse micelle (RM)) | RB | DMA | [45] | |
| 8.8 (CHCl3) | DMNO2 | 1O2 emission | [43] | |
| 9 (EtOH/CHCl3/H2O 50/50/1) | NDPO2 | 1O2 emission | [46] | |
| 6.93 (soybean oil) | Chlorophyll | Headspace oxygen depletion by gas chromatography | [47] | |
| β-carotene | 13.0 (C6H6) | Phenazine | 1O2 emission | [38] |
| 13.8 (C6H6) | Anthracene/Naphthalene | Radiolysis/1O2 emission | [48] | |
| 12.5–14 (C6H6) | Anthracene/Naphthalene | Radiolysis/1O2 emission | [49] | |
| 13 (C6H6) | Anthracene | DPBF | [50] | |
| 14 (toluene) | Phenazine | 1O2 emission | [38] | |
| 14 (C6H14) | Phenazine | 1O2 emission | [38] | |
| 5.0 (CHCl3) | NDPO2 | 1O2 emission | [39] | |
| 14.0 (EtOH/CHCl3/H2O 50/50/1) | NDPO2 | 1O2 emission | [41] | |
| 12 (EtOH/CHCl3/H2O: 50/50/1) | Phenazine | 1O2 emission | [38] | |
| 10.8 (EtOH/CHCl3/D2O 50/50/1) | EP-1 | DPBF | [40] | |
| 4.2 (EtOH/CHCl3/D2O 50/50/1) | NDPO2 | 1O2 emission | [41,42] | |
| 8.4 (EtOH/CHCl3/D2O 50/50/1) | DMNO2 | 1O2 emission | [43] | |
| 30 (MeOH/C6H6 1/4) | MB | 2-methyl-2-penten | [51] | |
| 5 (MeOH/C6H6 1/4) | Anthracene/Naphthalene | Radiolysis/1O2 emission | [52] | |
| 13 (MeOH/C6H6 1/4) | Anthracene/Naphthalene | Radiolysis/1O2 emission | [53] | |
| 10.9 ± 0.5 (THF) | TPP | 1O2 emission | [36] | |
| 2.3 (DPPC) | RB and PBA | 1O2 emission | [33] | |
| 9.9 (CCl4) | Phenazine | 1O2 emission | [38] | |
| 5.9 (CCl4) | 1H-P + RB | 1O2 emission | [54] | |
| 0.7 (CCl4) | Porphyrin | 1O2 emission | [55] | |
| 11 (CHCl3) | Phenazine | 1O2 emission | [38] | |
| 8.1 (CHCl3) | DMNO2 | 1O2 emission | [43] | |
| 23 (C6H6/MeOH: 3/2) | MB/RB | 1O2 emission | [56] | |
| 1.5 (CD3OD) | 1H-P/RB | 1O2 emission | [54] | |
| 0.35 (Ascorbic acid in MeOH) | 1-NN | 1O2 emission | [44] | |
| 5 (H2O/ (CH3)2CO 12/88) | Riboflavin | GC with thermal conductivity | [57] | |
| 12.67 (reverse micelle (RM)) | RB | DMA | [45] | |
| 5 (EtOH/CHCl3/D2O 50/50/1) | NDPO2 | 1O2 emission | [46] | |
| Lutein | 11.0 (C6H6) | TPP | 1O2 emission | [58] |
| 16 (C6H6) | Phenazine | 1O2 emission | [38] | |
| 8.0 (EtOH/CH2Cl2/H2O; 50:50:1) | NDPO2 | 1O2 emission | [41] | |
| 0.11 (DPPC) | RB and PBA | 1O2 emission | [33] | |
| 9.24 (EtOH/CHCl3/D2O 50/50/1) | EP-1 | DPBF | [40] | |
| 2.4 (EtOH/CHCl3/D2O 50/50/1) | NDPO2 | 1O2 emission | [42] | |
| 21 (MeOH/C6H6 1/4) | Anthracene/Naphthalene | Radiolysis/1O2 emission | [53] | |
| 1.3 (ascorbic acid in methanol) | 1-NN | 1O2 emission | [44] | |
| 10-33 reverse micelle (RM) | RB | DMA | [45] | |
| 5.72 (soybean oil) | Chlorophyll | Headspace oxygen depletion by gas chromatography | [47] | |
| β-cryptoxanthin | 7.31 (EtOH/CHCl3/D2O 50/50/1) | EP-1 | DPBF | [40] |
| 1.8–6 (EtOH/CHCl3/D2O 50/50/1) | NDPO2 | 1O2emission | [41,42] | |
| Zeaxanthin | 12.6 (C6H6) | Phenazine | 1O2emission | [38] |
| 12 (C6H6) | Phenazine | 1O2emission | [38] | |
| 2.8 (C6H6) | Anthracene/Naphthalene | Radiolysis/1O2 emission | [48] | |
| 10 (EtOH/CHCl3/D2O 50/50/1) | NDPO2 | 1O2emission | [41] | |
| 0.23 (DPPC) | RB and PBA | 1O2emission | [33] | |
| 10.5 (EtOH/CHCl3/D2O 50/50/1) | EP-1 | DPBF | [40] | |
| 3.0 (EtOH/CHCl3/D2O 50/50/1) | NDPO2 | 1O2emission | [41] | |
| 10 (EtOH/CHCl3/H2O 50/50/1) | NDPO2 | 1O2emission | [41] | |
| 0.77 (ascorbic acid in methanol) | 1-NN | Radiolysis/1O2 emission | [44] | |
| 6.79 (soybean oil) | Chlorophyll | Headspace oxygen depletion by gas chromatography | [47] | |
| Violaxanthin | 16 (C6H6) | Phenazine | 1O2 emission | [38] |
| 9 reverse micelle (RM) | RB | DMA | [45] |
| Carotenoids | Nb of C=C and OH Group | Kq (M−1·s−1) |
|---|---|---|
| β-carotene in hexane | 11 C=C | 1.1 × 109 |
| β-cryptoxanthin | 11 C=C and 1 OH | 1.6 × 109 |
| lycopene | 13 C=C | 1.1 × 109 |
| Lutein | 11 C=C and 2 OH | 8.0 × 108 |
| Zeaxanthin | 11 C=C and 2 OH | 6.0 × 107 |
| (Peak 4) | - | 7.2 × 107 |
| 5,6-epoxy-α-carotene | 10 C=C and 1 OH, 2 epoxy | 3.8 × 107 |
| Violaxanthin | 9 C=C, 2 OH and 2 epoxy group | 5.8 × 107 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaghdoudi, K.; Ngomo, O.; Vanderesse, R.; Arnoux, P.; Myrzakhmetov, B.; Frochot, C.; Guiavarc’h, Y. Extraction, Identification and Photo-Physical Characterization of Persimmon (Diospyros kaki L.) Carotenoids. Foods 2017, 6, 4. https://doi.org/10.3390/foods6010004
Zaghdoudi K, Ngomo O, Vanderesse R, Arnoux P, Myrzakhmetov B, Frochot C, Guiavarc’h Y. Extraction, Identification and Photo-Physical Characterization of Persimmon (Diospyros kaki L.) Carotenoids. Foods. 2017; 6(1):4. https://doi.org/10.3390/foods6010004
Chicago/Turabian StyleZaghdoudi, Khalil, Orleans Ngomo, Régis Vanderesse, Philippe Arnoux, Bauyrzhan Myrzakhmetov, Céline Frochot, and Yann Guiavarc’h. 2017. "Extraction, Identification and Photo-Physical Characterization of Persimmon (Diospyros kaki L.) Carotenoids" Foods 6, no. 1: 4. https://doi.org/10.3390/foods6010004
APA StyleZaghdoudi, K., Ngomo, O., Vanderesse, R., Arnoux, P., Myrzakhmetov, B., Frochot, C., & Guiavarc’h, Y. (2017). Extraction, Identification and Photo-Physical Characterization of Persimmon (Diospyros kaki L.) Carotenoids. Foods, 6(1), 4. https://doi.org/10.3390/foods6010004






