Functional Starters for Functional Yogurt
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Analysis
| Stain | pH | Prot (%) | Casein (%) | WSEs (%) | Fat (g/100 g) | Lactic acid (g/L) |
|---|---|---|---|---|---|---|
| 1 Day | ||||||
| CNT | 4.19 ± 0.01 | 3.28 ± 0.11 | 2.45 ± 0.11 | 0.15 ± 0.01 | 2.68 ± 0.17 | 3.98 ± 0.35 |
| LpWCFS1 | 4.07 * ± 0.02 | 3.43 ± 0.11 | 2.41 ± 0.11 | 0.19 ± 0.01 | 3.75 * ± 0.39 | 4.43 ± 0.65 |
| Lp8328 | 4.28 * ± 0.01 | 3.35 ± 0.23 | 2.59 ± 0.16 | 0.19 ± 0.01 | 4.05 * ± 0.13 | 4.65 ± 1.04 |
| 14 Days | ||||||
| CNT | 4.25 ± 0.01 | 3.03 ± 0.00 | 2.42 ± 0.00 | 0.11 ± 0.01 | 4.43 ± 0.10 | 4.90 ± 0.81 |
| LpWCFS1 | 4.18 * ± 0.01 | 2.95 ± 0.11 | 2.34 ± 0.11 | 0.10 ± 0.00 | 4.48 ± 0.10 | 5.05 ± 0.04 |
| Lp8328 | 4.13 * ± 0.01 | 3.19 ** ± 0.00 | 2.27 ± 0.14 | 0.12 ± 0.00 | 4.45 ± 0.06 | 5.43 ± 0.51 |
| 28 Days | ||||||
| CNT | 4.22 ± 0.01 | 1.99 ± 0.11 | 1.61 ± 0.07 | 0.10 ± 0.00 | 4.3 ± 0.20 | 5.14 ± 0.23 |
| LpWCFS1 | 4.17 * ± 0.01 | 2.87 * ± 0.00 | 2.24 * ± 0.02 | 0.10 ± 0.00 | 4.4 ± 0.00 | 5.46 ± 0.62 |
| Lp8328 | 4.21 ± 0.01 | 2.95 * ± 0.11 | 2.13 * ± 0.11 | 0.12 ± 0.00 | 4.3 ± 0.20 | 5.44 ± 0.26 |
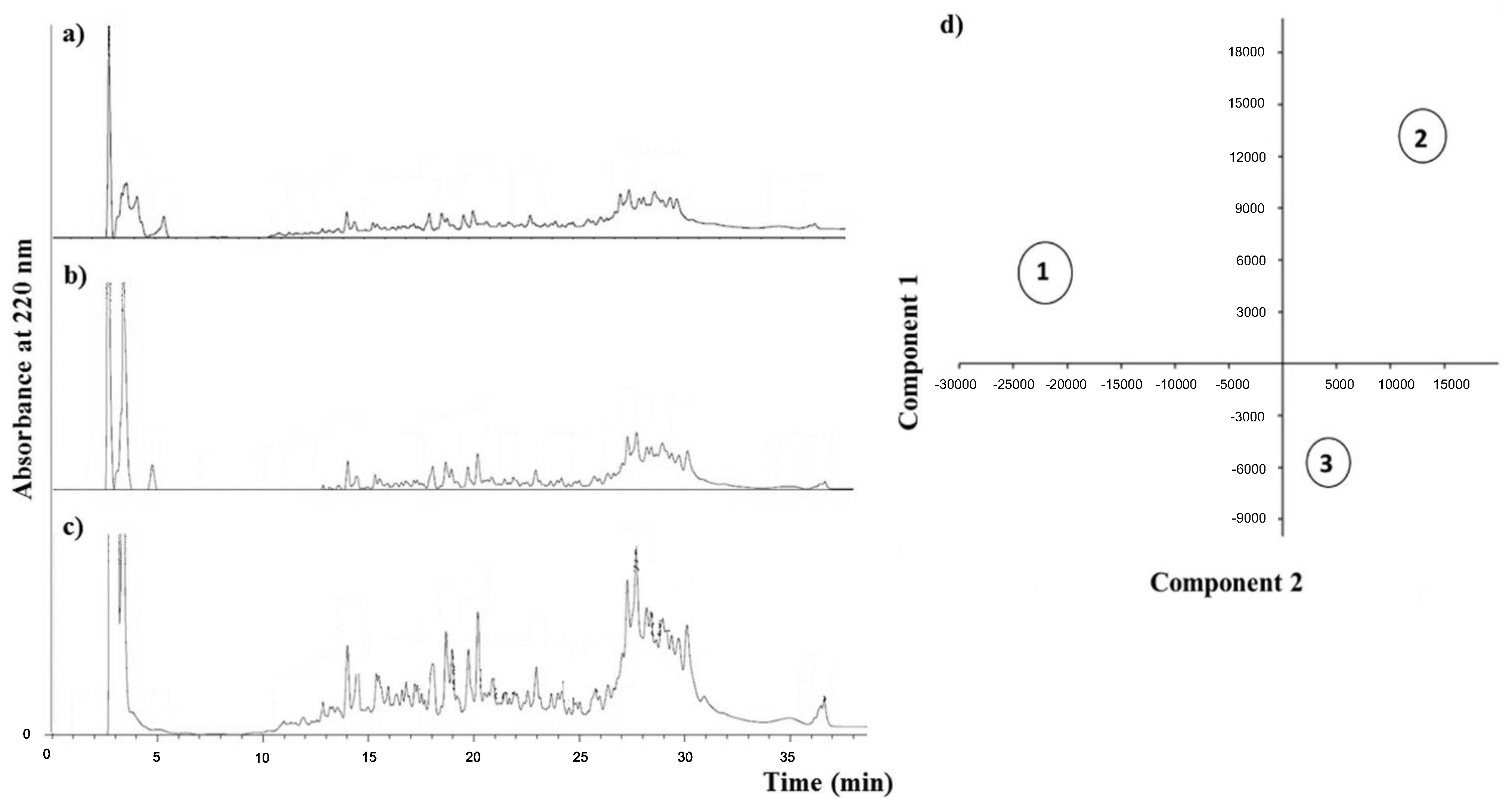
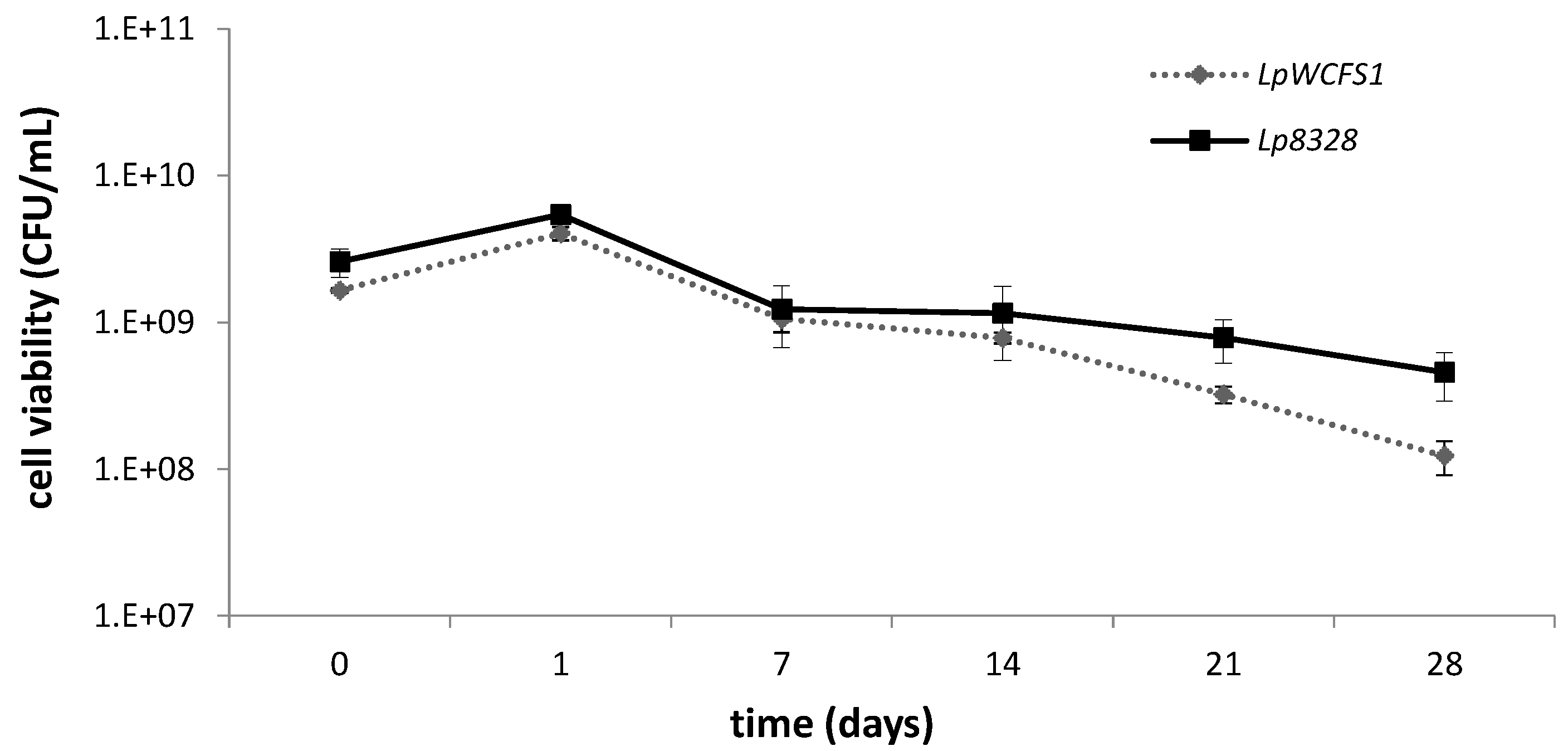
2.2. Viability of Lactobacillus plantarum Strains
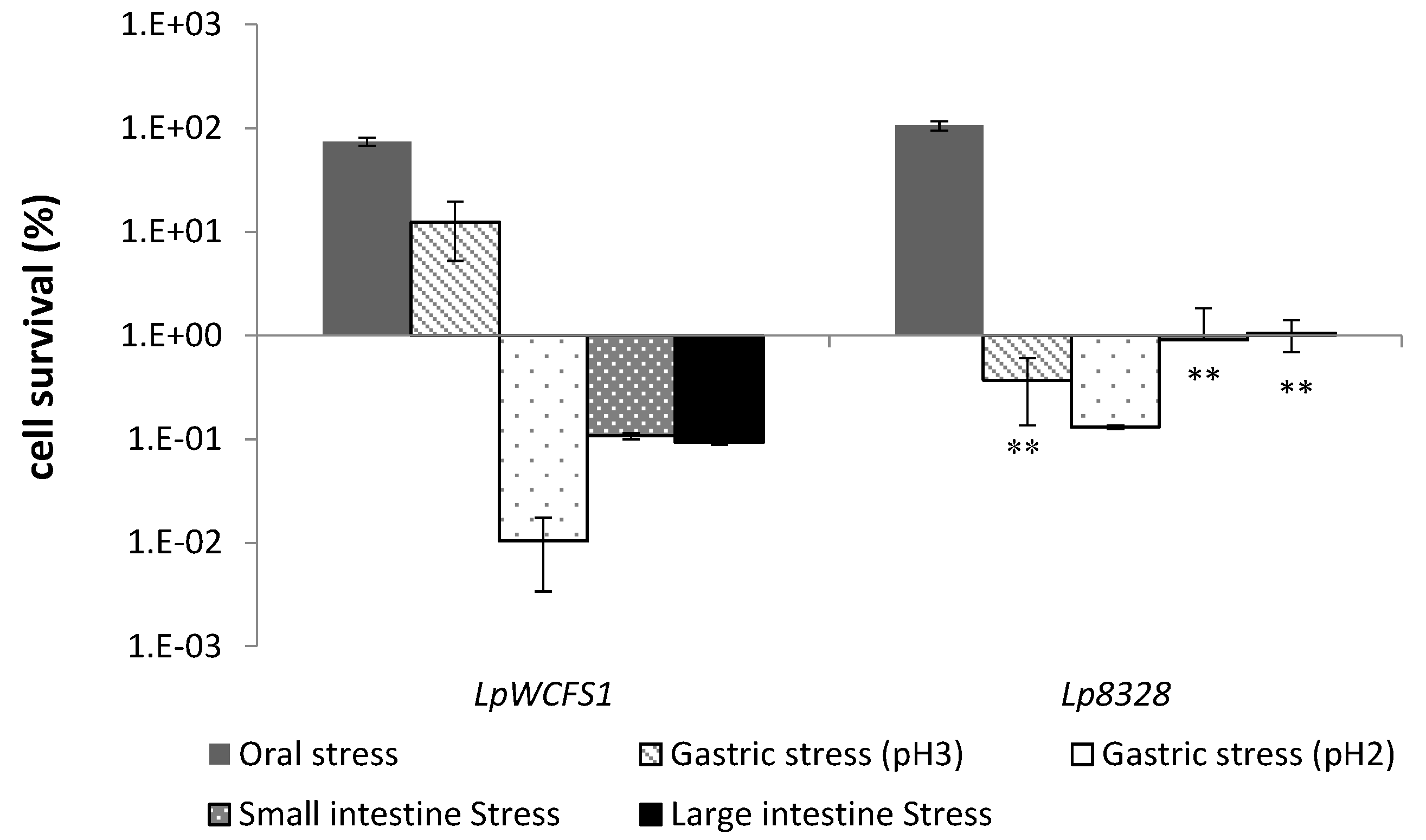
2.3. Oro-Gastrointestinal Tolerance Assay
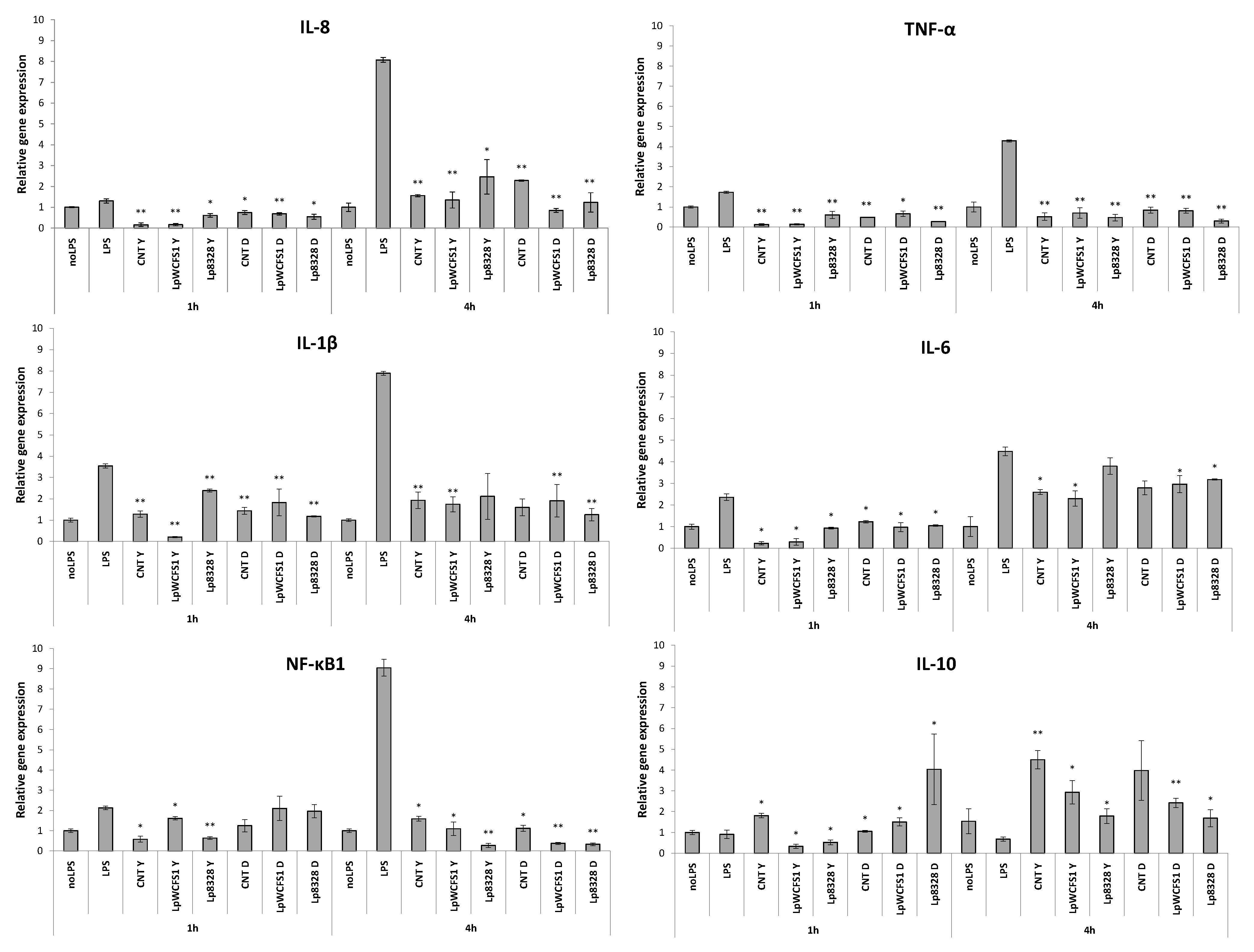
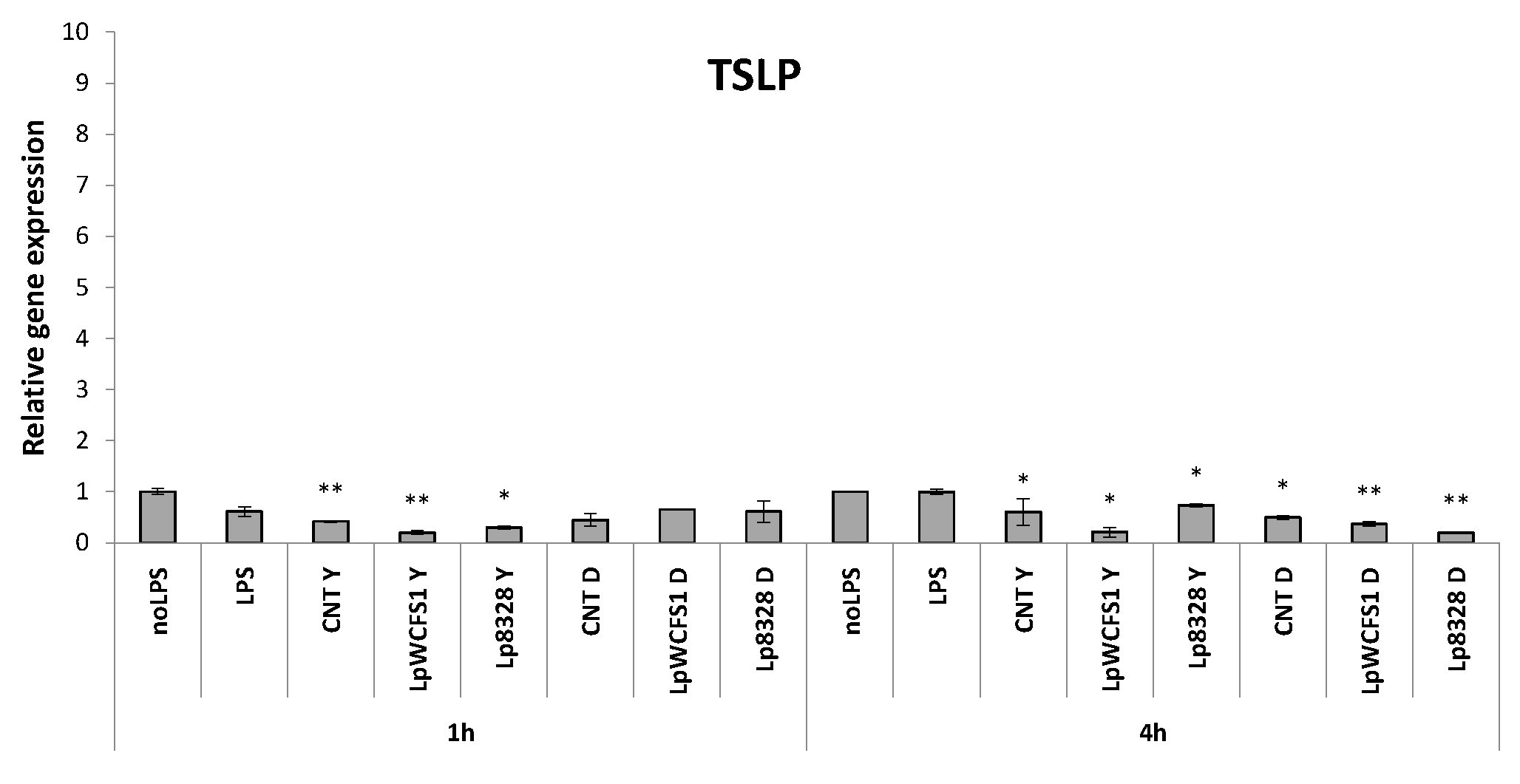
2.4. Stimulation of THP-1 Cells with Lactobacillus plantarum Strains
3. Experimental Section
3.1. Bacterial Strains, Human Cells and Growth Conditions
3.2. Yogurt Fermentation
3.3. Chemical Analysis
3.4. Microbiological Analysis
3.5. Oro-Gastrointestinal Tolerance Assay
3.6. Stimulation of THP-1 Cells with Lactobacilli
3.7. Propidium Monoazide Treatment and Microbial DNA Extraction
3.8. THP-1 RNA Extraction and Reverse Transcription
3.9. Q-PCR Analysis
| Oligonucleotide | Name | Sequence (5′–3′) |
|---|---|---|
| IL-1βF | Interleukin 1β | ATGATGGCTTATTACAGTGGCAA |
| IL-1βR | GTCGGAGATTCGTAGCTGGA | |
| NF-κB1F | Nuclear factor kappa B | GGTGCGGCTCATGTTTACAG |
| NF-κB1R | GATGGCGTCTGATACCACGG | |
| IL-10F | Interleukin 10 | GACTTTAAGGGTTACCTGGGTTG |
| IL-10R | TCACATGCGCCTTGATGTCTG | |
| TSLPF | Thymic stromal lymphopoietin | ATGTTCGCCATGAAAACTAAGGC |
| TSLPR | GCGACGCCACAATCCTTGTA | |
| GAPDHF | Glyceraldehyde phosphate dehydrogenase | CGACCACTTTGTCAAGCTCA |
| GAPDHR | AGGGGTCTACATGGCAACTG | |
| β-actF | β-Actin | AAAGACCTGTACGCCAACAC |
| β-actR | CATACTCCTGCTTGCTGATCC | |
| IL-6F | Interleukin 6 | TACCCCCAGGAGAAGATTCC |
| IL-6R | TTTTCTGCCAGTGCCTCTTT | |
| IL-8F | Interleukin 8 | TGTGGAGAAGTTTTTGAAGAGGG |
| IL-8R | CCAGGAATCTTGTATTGCATCTGG | |
| TNF-αF | Tumor necrosis factor α | AACCTCCTCTCTGCCATCAA |
| TNF-αR | ATGTTCGTCCTCCTCACAGG |
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prado, F.C.; Parada, J.L.; Pandey, A.; Soccol, C.R. Trends in non-dairy probiotic beverages. Food Res. Int. 2008, 41, 111–123. [Google Scholar] [CrossRef]
- Gorbach, S.L. Probiotics in the third millennium. Dig. Liver Dis. 2002, 34, S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for the Evaluation of Probiotics in Food. Food and Agriculture Organization of United Nations and World Health Organization Working Group Report. 2002. Available online: ftp://ftp.fao.org/es/esn/food/wgreport2.pdf (accessed on 14 May 2014).
- Vinderola, G.; Matar, C.; Perdigon, G. Role of intestinal epithelial cells in immune effects mediated by gram-positive probiotic bacteria: Involvement of toll-like receptors. Clin. Diagn. Lab. Immunol. 2005, 12, 1075–1084. [Google Scholar] [PubMed]
- Holzapfel, W.H.; Haberer, P.; Snel, J.; Schillinger, U.; Huis in’t Veld, J.H.J. Overview of gut flora and probiotics. Int. J. Food Microbial. 1998, 41, 85–101. [Google Scholar] [CrossRef]
- Marteau, P.R.; de Vrese, M.; Cellier, C.J.; Schrezenmeir, J. Protection from gastrointestinal diseases with the use of probiotics. Am. J. Clin. Nutr. 2001, 73, 430s–436s. [Google Scholar] [PubMed]
- Patel, R.M.; Lin, P.W. Developmental biology of gut-probiotic interaction. Gut Microbes 2010, 1, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Coico, R.; Sunshine, G.; Benjamini, E. Immunology: A Short Course; Wiley-Liss: New York, NY, USA, 2003. [Google Scholar]
- Jung, H.C.; Eckmann, L.; Yang, S.-K.; Panja, A.; Fierer, J.; Morzycka-Wroblewska, E.; Kagnoff, M.F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 1995, 95, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Delcenserie, V.; Martel, D.; Lamoureux, M.; Amiot, J.; Boutin, Y.; Roy, D. Immunomodulatory effects of probiotics in the intestinal tract. Curr. Issues Mol. Biol. 2008, 10, 37–54. [Google Scholar] [PubMed]
- Maccaferri, S.; Klinder, A.; Brigidi, P.; Cavina, P.; Costabile, A. Potential probiotic Kluyveromyces marxianus B0399 modulates the immune response in Caco-2 cells and peripheral blood mononuclear cells and impacts the human gut microbiota in an in vitro colonic model system. Appl. Environ. Microbial. 2012, 78, 956–964. [Google Scholar] [CrossRef]
- Hart, A.; Kamm, M.A. Mechanisms of initiation and perpetuation of gut inflammation by stress. Aliment. Pharmacol. Ther. 2002, 16, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.J.; Husmann, R.J.; Villamar, M.; Winship, T.R.; Buck, R.H.; Zuckermann, F.A. Lactobacillus rhamnosus HN001 attenuates allergy development in a pig model. PLoS One 2011, 6, e16577. [Google Scholar] [CrossRef] [PubMed]
- Mullin, J.M.; Valenzano, M.C.; Verrecchio, J.J.; Kothari, R. Age-and diet-related increase in transepithelial colon permeability of Fischer 344 rats. Dig. Dis. Sci. 2002, 47, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Wichers, H. Immunomodulation by food: Promising concept for mitigating allergic disease? Anal. Bioanal. Chem. 2009, 395, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Haller, D.; Bode, C.; Hammes, W.P.; Pfeifer, A.M.; Schiffrin, E.J.; Blum, S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 2000, 47, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Effect of dairy probiotic combinations on in vitro gastrointestinal tolerance, intestinal epithelial cell adhesion and cytokine secretion. J. Funct. Foods 2014, 8, 18–25. [Google Scholar] [CrossRef]
- Grimoud, J.; Durand, H.; de Souza, S.; Monsan, P.; Ouarné, F.; Theodorou, V.; Roques, C. In vitro screening of probiotics and synbiotics according to anti-inflammatory and anti-proliferative effects. Int. J. Food Microbial. 2010, 144, 42–50. [Google Scholar] [CrossRef]
- Pelto, L.; Isolauri, E.; Lilius, E.M.; Nuutila, J.; Salminen, S. Probiotic bacteria down-regulate the milk-induced inflammatory response in milk hypersensitive subjects but have an immunostimulatory effect in healthy subjects. Clin. Exp. Allergy 1998, 28, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Asai, Y.; Tamai, R.; Makimura, Y.; Sakamoto, H.; Hashikawa, S.; Yasuda, K. Natural killer cell activities of synbiotic Lactobacillus casei ssp. casei in conjunction with dextran. Clin. Exp. Immunol. 2006, 143, 103–109. [Google Scholar] [CrossRef]
- Chandan, R.C.; Kilara, A. Manufacturing Yogurt and Fermented Milks, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- Mollet, B. Genetically improved starter strains: Opportunities for the dairy industry. Int. Dairy J. 1999, 9, 11–15. [Google Scholar] [CrossRef]
- Papadimitriou, C.G.; Vafopoulou-Mastrojiannaki, A.; Silva, S.V.; Gomes, A.M.; Malcata, F.X.; Alichanidis, E. Identification of peptides in traditional and probiotic sheep milk yoghurt with angiotensin I-converting enzyme (ACE)-inhibitory activity. Food Chem. 2007, 105, 647–656. [Google Scholar] [CrossRef]
- Bautista, E.S.; Dahiya, R.S.; Speck, M.L. Identification of compounds causing symbiotic growth of Streptococcus thermophilus and Lactobacillus bulgaricus in milk. J. Dairy Res. 1966, 33, 299–307. [Google Scholar] [CrossRef]
- Rajagopal, S.N.; Sandine, W.E. Associative growth and proteolysis of Streptococcus thermophilus and Lactobacillus bulgaricus in skim milk. J. Dairy Sci. 1990, 73, 894–899. [Google Scholar] [CrossRef]
- Àlvarez, G.; González, M.; Isabal, S.; Blanc, V.; León, R. Method to quantify live and dead cells in multi-species oral biofilm by real-time PCR with propidium monoazide. AMB Express 2013, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Shortt, C. The probiotics century: Historical and current perspectives. Trends Food Sci. Technol. 1999, 10, 411–417. [Google Scholar] [CrossRef]
- Arena, M.P.; Russo, P.; Capozzi, V.; López, P.; Fiocco, D.; Spano, G. Probiotic abilities of riboflavin-overproducing Lactobacillus strains: A novel promising application of probiotics. Appl. Microbiol. Biotechnol. 2014, 98, 7569–7581. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Caggianiello, G.; Fiocco, D.; Russo, P.; Torelli, M.; Spano, G.; Capozzi, V. Barley β-glucans-containing food enhances probiotic performances of beneficial bacteria. Int. J. Mol. Sci. 2014, 15, 3025–3039. [Google Scholar] [CrossRef] [PubMed]
- Bove, P.; Russo, P.; Capozzi, V.; Gallone, A.; Spano, G.; Fiocco, D. Lactobacillus plantarum passage through an oro-gastro-intestinal tract simulator: Carrier matrix effect and transcriptional analysis of genes associated to stress and probiosis. Microbiol. Res. 2013, 168, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. In vitro analysis of gastrointestinal tolerance and intestinal cell adhesion of probiotics in goat’s milk ice cream and yogurt. Food Res. Int. 2012, 49, 619–625. [Google Scholar] [CrossRef]
- Danis, V.A.; Franic, G.M.; Rathjen, D.A.; Brooks, P.M. Effects of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-2, interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNF-alpha) and IL-8 the production of immunoreactive IL-1 and TNF-alpha by human monocytes. Clin. Exp. Immunol. 1991, 85, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, K.A. Regulation of interleukin-8 gene expression. J. Interf. Cytokine Res. 1999, 19, 429–438. [Google Scholar] [CrossRef]
- DeNichilo, M.O.; Stewart, A.G.; Vadas, M.A.; Lopez, A.F. Granulocyte-macrophage colony-stimulating factor is a stimulant of platelet-activating factor and superoxide anion generation by human neutrophils. J. Biol. Chem. 1991, 266, 4896–4902. [Google Scholar] [PubMed]
- Ferrante, A. Activation of neutrophils by interleukins-l and -2 and tumor necrosis factors. Immunol. Ser. 1992, 57, 417–436. [Google Scholar] [PubMed]
- Sims, J.E.; Smith, D.E. The IL-1 family: Regulations of immunity. Immunology 2010, 10, 89–102. [Google Scholar] [PubMed]
- Riedel, C.U.; Foata, F.; Philippe, D.; Adolfsson, O.; Eikmanns, B.J.; Blum, S. Anti-inflammatory effects of bifidobacteria by inhibition of LPS-induced NF-kappaB activation. World J. Gastroenterol. 2006, 12, 3729–3735. [Google Scholar] [PubMed]
- Erickson, K.L.; Hubbard, N.E. Probiotic immunomodulation in health and disease. J. Nutr. 2000, 130, 403S–409S. [Google Scholar] [PubMed]
- Candela, M.; Perna, F.; Carnevali, P.; Vitali, B.; Ciati, R.; Gionchetti, P.; Rizzello, F.; Campieri, M.; Brigidi, P. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: Adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int. J. Food Microbial. 2008, 125, 286–292. [Google Scholar] [CrossRef]
- Cui, H.H.; Chen, C.L.; Wang, J.D.; Yang, Y.J.; Cun, Y.; Wu, J.B.; Liu, Y.H.; Dan, H.L.; Jian, Y.T.; Chen, X.Q. Effects of probiotic on intestinal mucosa of patients with ulcerative colitis. World J. Gastroenterol. 2004, 10, 1521–1525. [Google Scholar] [PubMed]
- Taylor, B.C.; Zaph, C.; Troy, A.E.; Du, Y.; Guild, K.J.; Comeau, M.R.; Artis, D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 2009, 206, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, J.; Wu, Y.; Li, J. The regulation of thymic stromal lymphopoietin in gut immune homeostasis. Dig. Dis. Sci. 2011, 56, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Comeau, M.R.; Ziegler, S.F. The influence of TSLP on the allergic response. Mucosal Immunol. 2010, 3, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Takai, T. TSLP expression: Cellular sources, triggers, and regulatory mechanisms. Allergol. Int. 2012, 61, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Kleerebezem, M.; Boekhorst, J.; van Kranenburg, R.; Molenaar, D.; Kuipers, O.P.; Leer, R.; Tarchini, R.; Peters, S.A.; Sandbrink, H.M.; Fiers, M.W.; et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 2003, 100, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Rosburg, V.; Boylston, T.; White, P. Viability of Bifidobacteria strains in yogurt with added oat beta‐glucan and corn starch during cold storage. J. Food Sci. 2010, 75, C439–C444. [Google Scholar] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- British Standards Institution. Determination of Fat Content of Milk and Milk Products (Gerber Method); British Standards Institution: London, UK, 1989. [Google Scholar]
- Kuchroo, C.N.; Fox, P.F. Soluble nitrogen in Cheddar cheese: Comparison of extraction procedures. Milchwissenschaft 1982, 37, 331–335. [Google Scholar]
- Vreeburg, R.A.; Bastiaan-Net, S.; Mes, J.J. Normalization genes for quantitative RT-PCR in differentiated Caco-2 cells used for food exposure studies. Food Funct. 2011, 2, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Quigley, L.; O’Sullivan, O.; Beresford, T.P.; Paul Ross, R.; Fitzgerald, G.F.; Cotter, P.D. A comparison of methods used to extract bacterial DNA from raw milk and raw milk cheese. J. Appl. Microbiol. 2012, 113, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Bove, P.; Gallone, A.; Russo, P.; Capozzi, V.; Albenzio, M.; Spano, G.; Fiocco, D. Probiotic features of Lactobacillus plantarum mutant strains. Appl. Microbiol. Biotechnol. 2012, 96, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Fiocco, D.; Collins, M.; Muscariello, L.; Hols, P.; Kleerebezem, M.; Msadek, T.; Spano, G. The Lactobacillus plantarum ftsH gene is a novel member of the CtsR stress response regulon. J. Bacteriol. 2009, 191, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Spandidos, A.; Wang, X.; Wang, H.; Seed, B. PrimerBank: A resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucl. Acids Res. Database Issue 2010, 38, 792–799. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Paleontología Electrónica 2001, 4, 1–9. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arena, M.P.; Caggianiello, G.; Russo, P.; Albenzio, M.; Massa, S.; Fiocco, D.; Capozzi, V.; Spano, G. Functional Starters for Functional Yogurt. Foods 2015, 4, 15-33. https://doi.org/10.3390/foods4010015
Arena MP, Caggianiello G, Russo P, Albenzio M, Massa S, Fiocco D, Capozzi V, Spano G. Functional Starters for Functional Yogurt. Foods. 2015; 4(1):15-33. https://doi.org/10.3390/foods4010015
Chicago/Turabian StyleArena, Mattia P., Graziano Caggianiello, Pasquale Russo, Marzia Albenzio, Salvatore Massa, Daniela Fiocco, Vittorio Capozzi, and Giuseppe Spano. 2015. "Functional Starters for Functional Yogurt" Foods 4, no. 1: 15-33. https://doi.org/10.3390/foods4010015
APA StyleArena, M. P., Caggianiello, G., Russo, P., Albenzio, M., Massa, S., Fiocco, D., Capozzi, V., & Spano, G. (2015). Functional Starters for Functional Yogurt. Foods, 4(1), 15-33. https://doi.org/10.3390/foods4010015







