The Effect of Oral Intake of Low-Temperature-Processed Whey Protein Concentrate on Colitis and Gene Expression Profiles in Mice
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Whey Protein
2.2. Animals and Diets
2.3. Histopathological Experiments
| Parameters | Scores | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Extent of damage to colon structure | normal | mild | moderate | extensive |
| Crypt atrophy | not present | mild | moderate | extensive |
| Degree of cellular infiltration | normal | mild | moderate | extensive |
| Extent of muscle thickening | normal | mild | moderate | extensive |
| Extent of crypt abscess | not present | mild | moderate | extensive |
| Goblet cell depletion | normal | mild | moderate | extensive |
2.4. Western Blotting
2.5. DNA Microarray and RT-QPCR
| Gene symbol | GenBank accession | Primer BankID | Primer sequence (5ʹ–3ʹ) | Amplicon size |
|---|---|---|---|---|
| Forward primer/reverse primer | ||||
| Gbp1 | NM_010259 | 6753948a1 | ACAACTCAGCTAACTTTGTGGG TGATACACAGGCGAGGCATATTA | 183 |
| Gbp2 | NM_010260 | 6753950a1 | CTGCACTATGTGACGGAGCTA GAGTCCACACAAAGGTTGGAAA | 115 |
| IFNgr2 | NM_008338 | 6680373a1 | TCCTCGCCAGACTCGTTTTC GTCTTGGGTCATTGCTGGAAG | 115 |
| GAPDH | NM_008084 | 6679937a1 | AGGTCGGTGTGAACGGATTTG GGGGTCGTTGATGGCAACA | 123 |
3. Results and Discussion
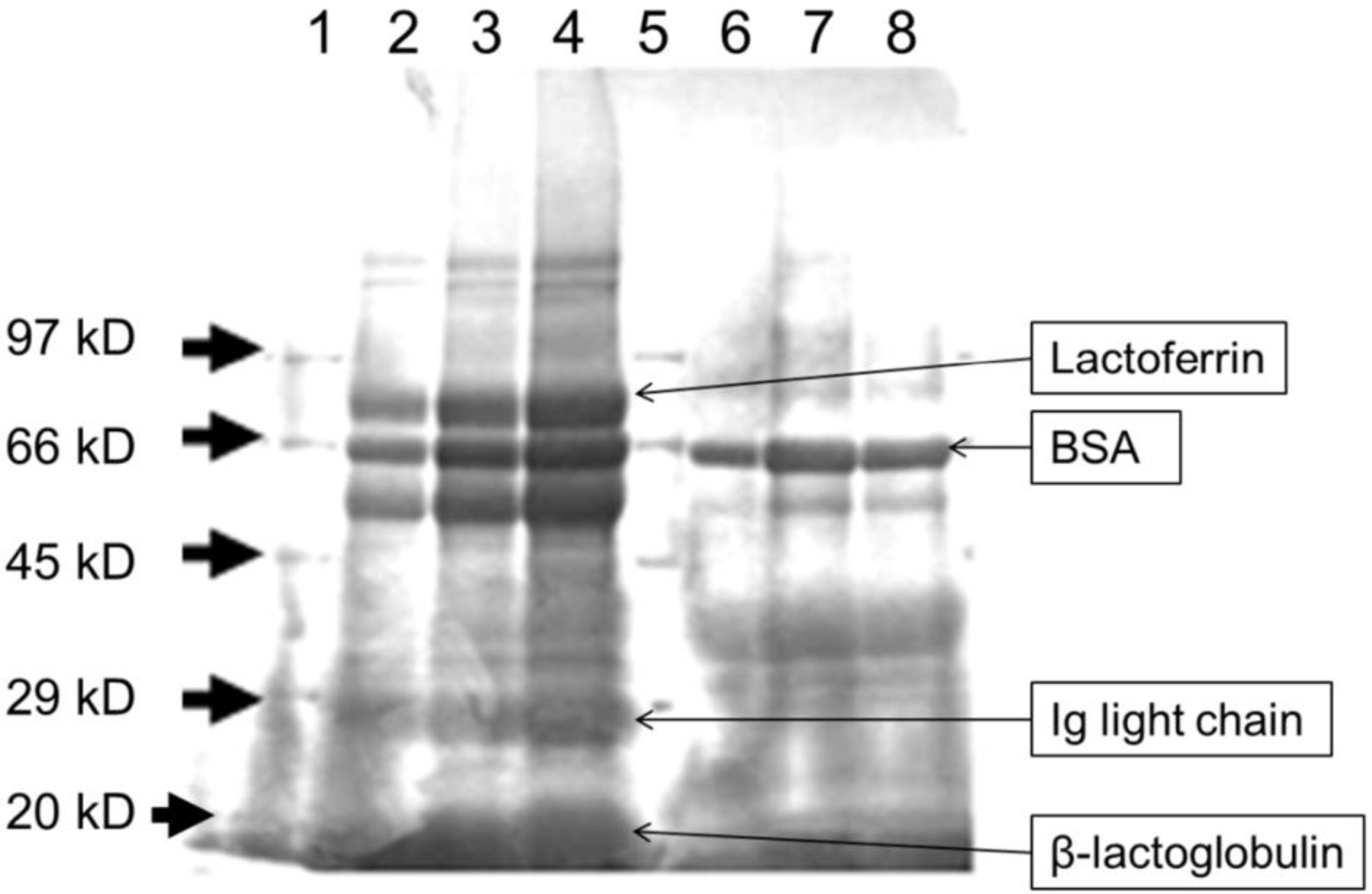
3.1. Heat Treatment Affects the Characteristics of Whey Proteins
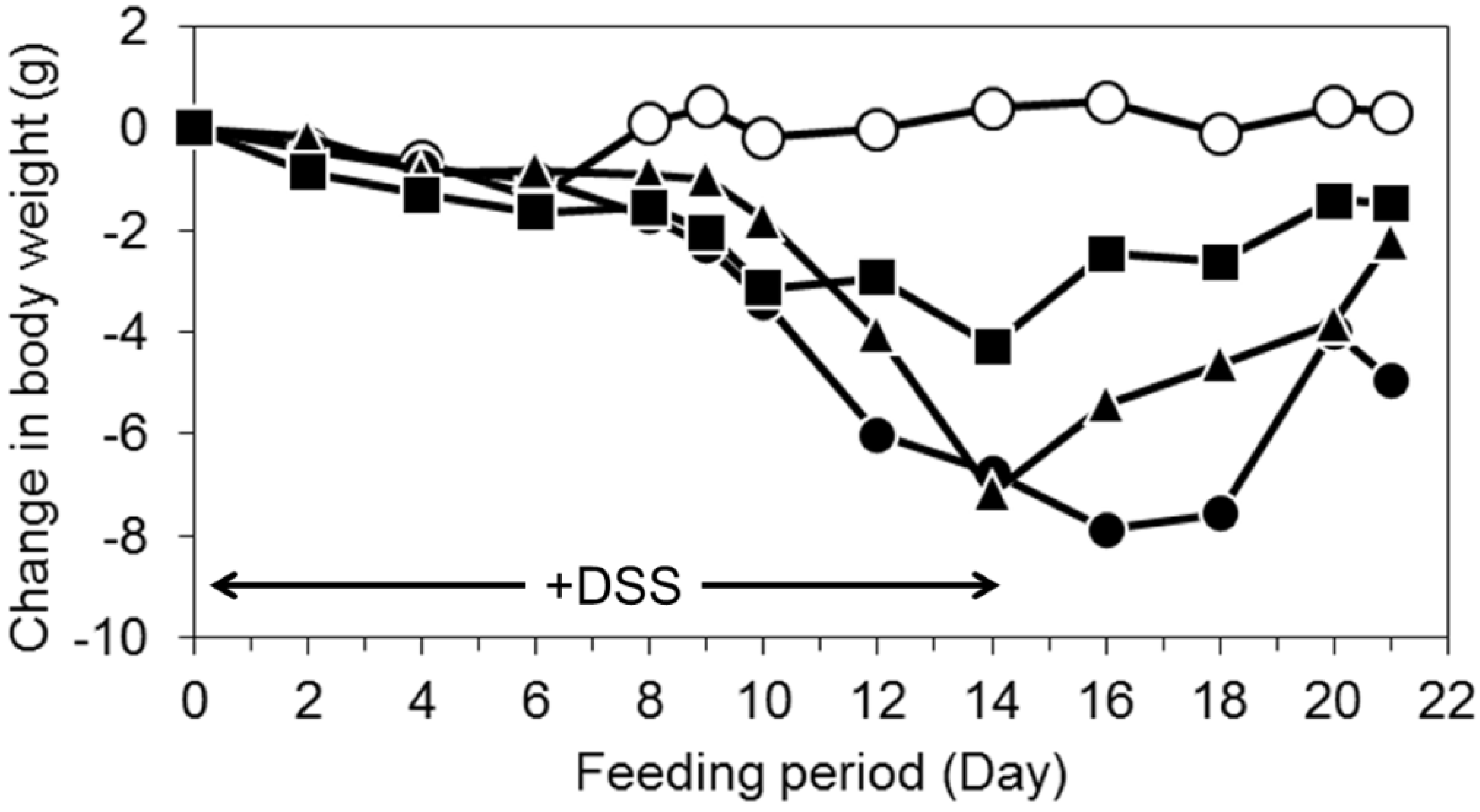
3.2. Oral Intake of LWPC Protects against DSS-Induced Colitis in Mice and Enhances Recovery from Colitis
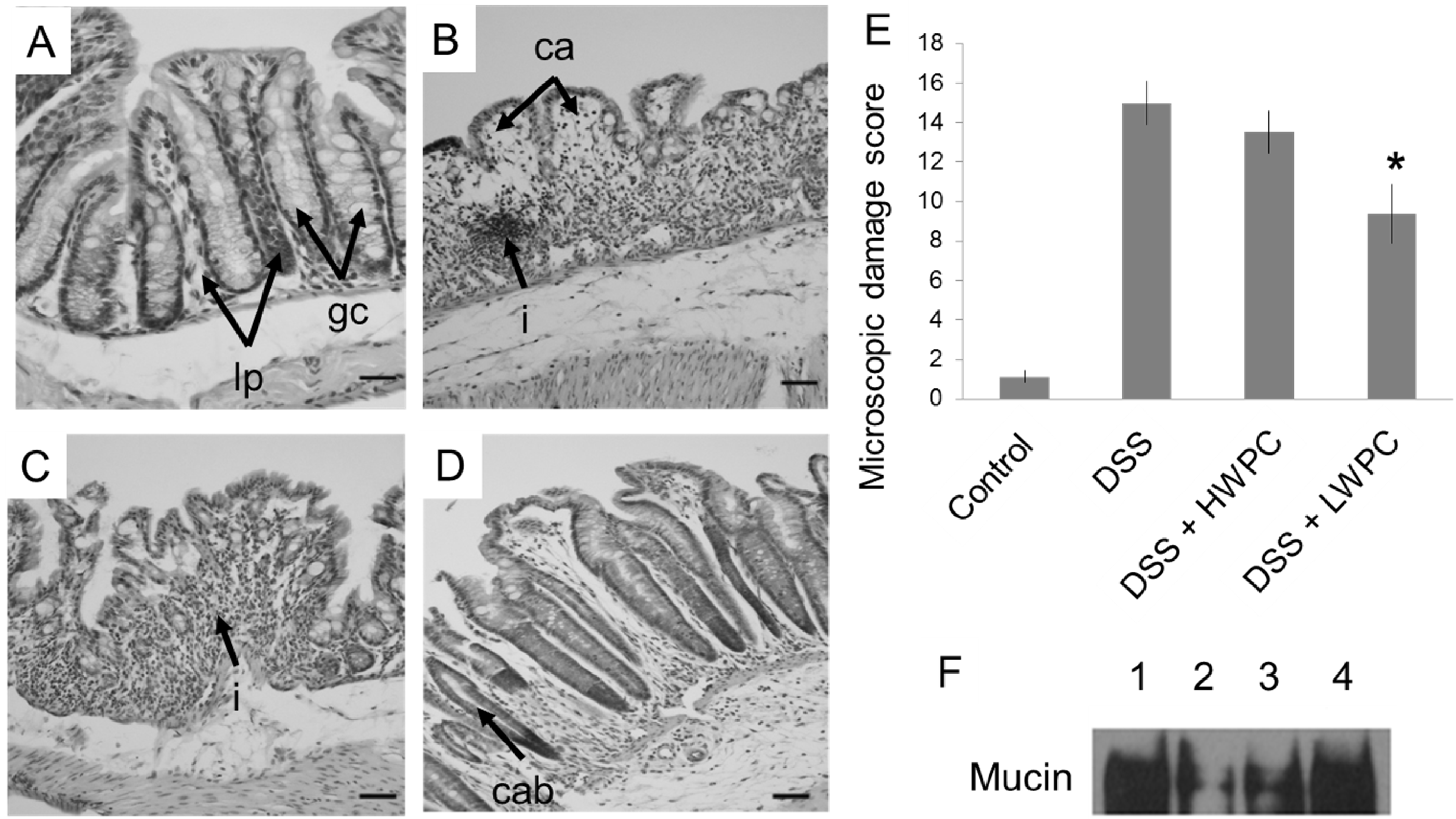
3.3. LWPC Protects the Intestinal Epithelium against DSS-Induced Inflammation
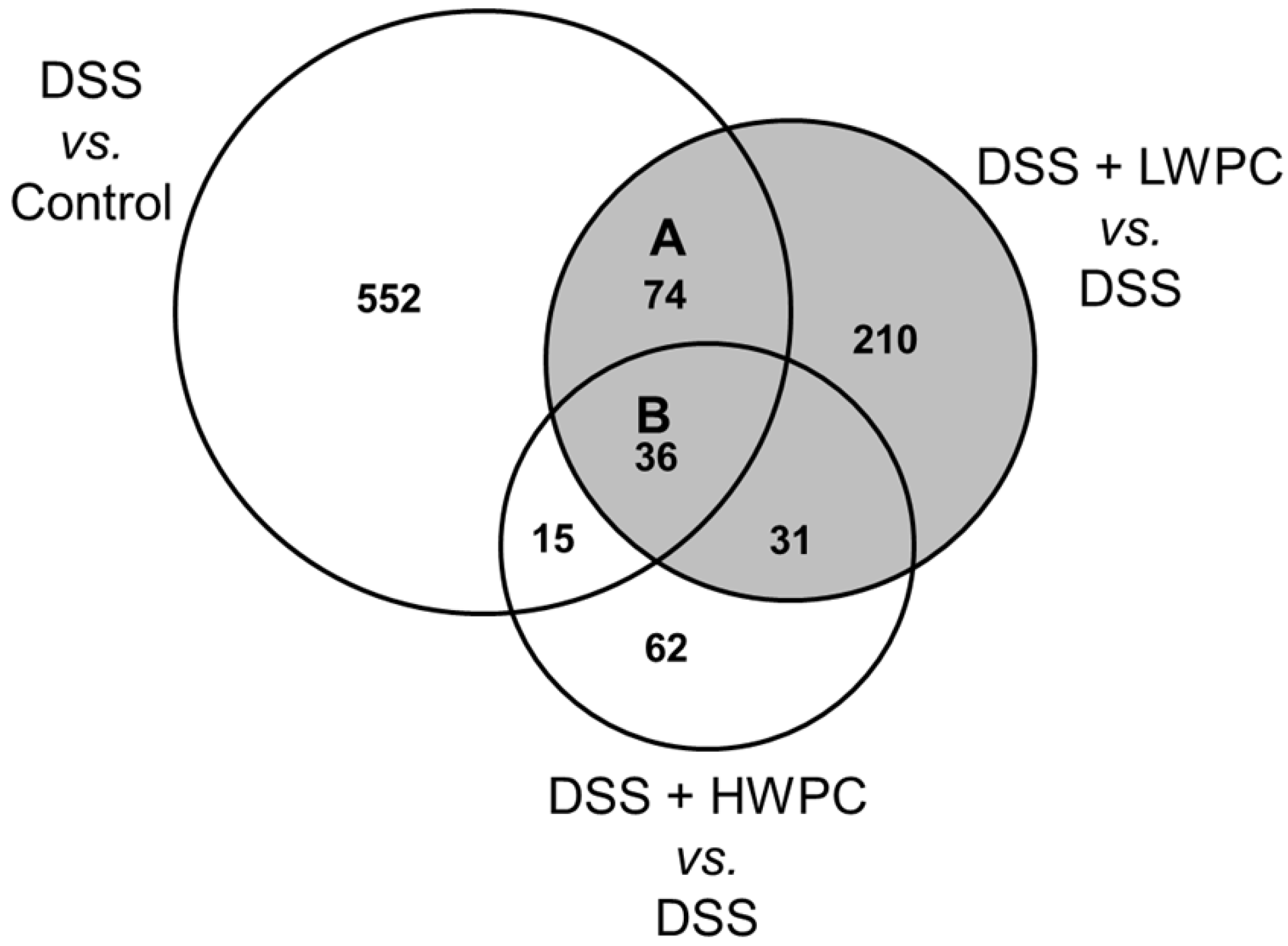
3.4. The Effect of LWPC on Gene Expression under DSS-Induced Inflammation in the Colon
| Treatment group | Number of genes differentially regulated (fold change vs. control >2) | ||
|---|---|---|---|
| Upregulated | Downregulated | Total | |
| DSS | 348 | 328 | 677 |
| DSS + LWPC | 213 | 138 | 351 |
| DSS + HWPC | 113 | 31 | 144 |
| GO biological process | Number of genes (fold change > 2) | % * | p-Value ** |
|---|---|---|---|
| GO:0006955 Immune response | 12 | 12.2 | 3.1 × 10−4 |
| GO:0006869 Lipid transport | 5 | 5.1 | 4.6 × 10−3 |
| GO:0010876 Lipid localization | 5 | 5.1 | 6.0 × 10−3 |
| GO:0015918 Sterol transport | 3 | 3.1 | 7.4 × 10−3 |
| GO:0030301 Cholesterol transport | 3 | 3.1 | 7.4 × 10−3 |
| GO:0006954 Inflammatory response | 6 | 6.1 | 9.0 × 10−3 |
| GO:0048593 Camera-type eye morphogenesis | 3 | 3.1 | 3.0 × 10−2 |
| GO:0042159 Lipoprotein catabolic process | 2 | 2.0 | 3.4 × 10−2 |
| GO:0034754 Cellular hormone metabolic process | 3 | 3.1 | 3.5 × 10−2 |
| GO:0010817 Regulation of hormone levels | 4 | 4.1 | 3.6 × 10−2 |
| GenBank Accession No. | Gene symbol | Gene description | Regulation byDSS and LWPC | |
|---|---|---|---|---|
| NM_013459 | Cfd | Complement factor D (adipsin) | ↓ | ↑ |
| NM_001159564 | Itgb6 | Integrin beta 6 | ↑ | ↓ |
| NM_001276445 | Tlr1 | Toll like receptor 1 | ↑ | ↓ |
| NM_012012 | Exo1 | Exonuclease 1 | ↑ | ↓ |
| NM_133829 | Mfsd6 | Major facilitator S domain containing 6 | ↑ | ↓ |
| NM_008599 | Cxcl9 | Chemokine (c-x-c motif) ligand 9 | ↑ | ↓ |
| NM_021443 | Ccl8 | Chemokine (c-cl motif) ligand 8 | ↑ | ↓ |
| NM_011315 | Saa3 | Serum amyloid A3 | ↑ | ↓ |
| NM_010259 | Gbp1 | Guanylate binding protein 1 | ↑ | ↓ |
| NM_010260 | Gbp2 | Guanylate binding protein 2 | ↑ | ↓ |
| NM_145545 | Gbp6 | Guanylate binding protein 6 | ↑ | ↓ |
| NM_001039701 | Il1rn | Interleukin 1 receptor antagonist | ↑ | ↑ |
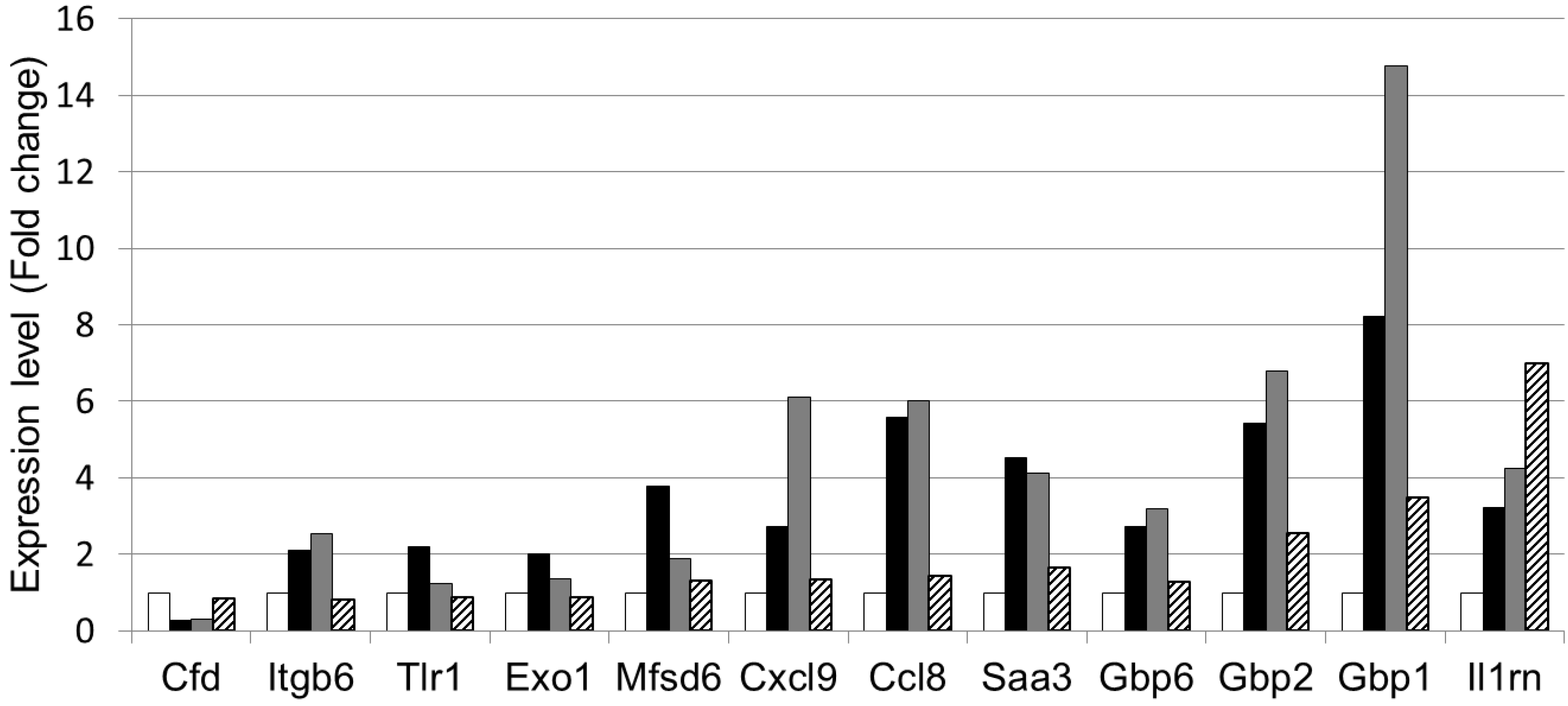
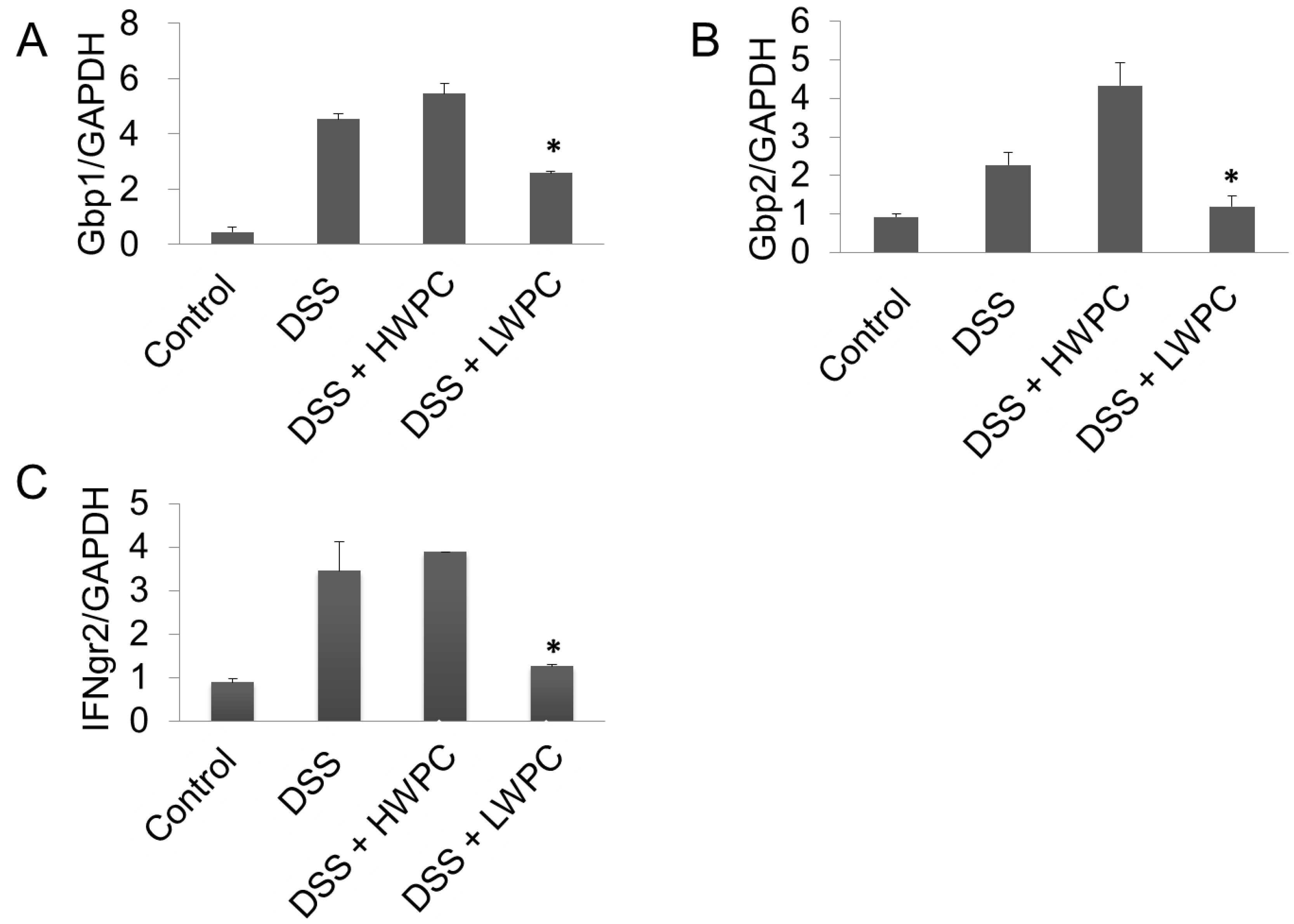
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hugot, J.P. Genetic origin of IBD. Inflamm. Bowel Dis. 2004, 10, S11–S15. [Google Scholar] [CrossRef]
- MacDermott, R.P. Alterations of the mucosal immune system in inflammatory bowel disease. J. Gastroenterol. 1996, 31, 907–916. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Fried, M.; Krabshuis, J.H.; Cohen, H.; Eliakim, R.; Fedail, S.; Gearry, R.; Goh, K.L.; Hamid, S.; Khan, A.G.; et al. World gastroenterology organization practice guidelines for the diagnosis and management of IBD in 2010. Inflamm. Bowel Dis. 2010, 16, 112–124. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Tuskey, A.; Dassopoulos, T.; Harris, M.L.; Brant, S.R. Rising hospitalization rates in inflammatory bowel disease in the Unites States between 1998–2004. Inflam. Bowel Dis. 2007, 13, 1529–1535. [Google Scholar] [CrossRef]
- Rogler, G. Gastrointestinal and liver adverse effects of drugs used for IBD. Best Pract. Res. Clin. Gastroentrol. 2010, 24, 157–165. [Google Scholar] [CrossRef]
- Young, D.; Ibuki, M.; Nakamori, T.; Fan, M.; Mine, Y. Soy-derived di & tripeptides alleviates colon and ileum inflammation in pigs with dextran sulfate sodium-induced colitis. J. Nutr. 2012, 142, 363–368. [Google Scholar] [CrossRef]
- Hwang, J.W.; Lee, S.J.; Kim, Y.S.; Kim, E.K.; Ahn, C.B.; Jeon, Y.J.; Moon, S.H.; Jeon, B.T.; Park, P.J. Purification and characterization of a novel peptide with inhibitory effects on colitis induced mice by dextran sulfate sodium from enzymatic hydrolysis of Crassotea gigas. Fish Shellfish Immonol. 2012, 33, 993–999. [Google Scholar] [CrossRef]
- Fox, P.F.; McSweeney, P.L.H. Dairy Chemistry and Biochemistry; Springer: Berlin, Germany, 1998; pp. 186–187. [Google Scholar]
- McCarthy, R.; Mills, S.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from casein and whey proteins. In Milk and Dairy Products as Functional Foods; Kanekanian, A., Ed.; John Wiley & Sons: Oxford, UK, 2014; pp. 23–32. [Google Scholar]
- Korhonen, H.; Pihlanto, A. Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum. Curr. Pharm. Des. 2007, 13, 829–843. [Google Scholar] [CrossRef]
- Gill, H.S.; Cross, M.L. Anticancer properties of bovine milk. Br. J. Nutr. 2000, 84, S161–S166. [Google Scholar]
- Cornish, J.; Callon, K.E.; Naot, D.; Palmano, K.P.; Banovic, T.; Bava, U. Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology 2004, 145, 4366–4374. [Google Scholar] [CrossRef]
- Krissansen, G.W. Emerging health properties of whey proteins and their clinical implications. J. Am. Col. Nutr. 2007, 26, 713s–723s. [Google Scholar] [CrossRef]
- McIntosh, G.H.; Royle, P.J.; Leu, R.K.L.; Regester, G.O.; Johnson, M.A.; Grinsted, R.L.; Kenward, R.S.; Smithers, G.W. Whey proteins as functional food ingredients? Int. Dairy J. 1998, 8, 425–434. [Google Scholar] [CrossRef]
- Chatterton, D.E.W.; Smithers, G.; Roupas, P.; Brodkorb, A. Bioactivity of β-lactoglobulin and α- lactalbumin—Technological implications for processing. Int. Dairy J. 2006, 16, 1229–1240. [Google Scholar] [CrossRef]
- Maqueda, D.M.; Miralles, B.; Teresa, S.D.P.; Reveron, I.; Munoz, R.; Recio, I. Food derived peptide stimulate mucin sectretion and gene expression in intestinal cells. J. Agric. Food Chem. 2012, 60, 8600–8605. [Google Scholar] [CrossRef] [Green Version]
- Posadas, R.L.; Requena, P.; Gonzalez, R.; Suarez, M.D.; Zarsuelo, A.; Medina, F.S.; Augustin, O.M. Bovine glycomacropeptide has intestinal anti-inflammatory effect in rats with dextran sulfate-induced colitis. J. Nutr. 2010, 140, 2014–2019. [Google Scholar] [CrossRef]
- Li, Y.; Ostergaard, M.V.; Jiang, P.; Chatterton, D.E.W.; Thymann, T.; Kvistgaard, A.S.; Sangild, P.T. Whey protein processing influences formula induced gut maturation in preterm pigs. J. Nutr. 2013, 143, 1934–1942. [Google Scholar] [CrossRef]
- Elfstrand, L.; Mansson, H.C.; Paulsson, M.; Nyberg, L.; Akesson, B. Immunoglobins, growth factors and growth hormones in bovine colostrum and the effects of processing. Int. Dairy J. 2002, 12, 879–887. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition AdHoc Writing Committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar]
- Lien, E.L.; Boyle, F.G.; Wrenn, J.M.; Perry, R.W.; Thompson, C.A.; Borzelleca, J.F. Comparison of AIN-76A and AIN-93G diets: A 13-week study in rats. Food Chem. Toxicol. 2001, 30, 385–392. [Google Scholar]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar]
- McCafferty, D.M.; Sihota, E.; Muscara, M.; Wallace, J.L.; Sharkey, K.A.; Kubes, P. Spontaneously developing chronic colitis in IL-10/iNOS double-deficient mice. Am. J. Phys. Gast. Liver Phys. 2000, 279, G90–G99. [Google Scholar]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protocols 2009, 4, 44–57. [Google Scholar]
- DAVID Bioinformatics Resources 6.7. Available online: http://david.abcc.ncifcrf.gov/ (accessed on 15 June 2012).
- The Gene Ontology. Available online: http://www.geneontology.org (accessed on 15 June 2012).
- Spandidos, A.; Wang, X.; Wang, H.; Seed, B. PrimerBank: A resource for human and mouse PCR primer pairs for gene expression detection and quantification. Nuc. Acids Res. 2009, 38, D792–D799. [Google Scholar] [CrossRef]
- PrimerBank. Available online: http://pga.mgh.harvard.edu/primerbank/ (accessed on 2 May 2013).
- Jimenez, X.T.; Cuenca, A.A.; Jurado, A.T.; Corona, A.A.; Urista, C.R.M. Traditional methods for whey protein isolation and concentration: Effects on nutritional properties and biological activities. J. Mex. Chem. Soc. 2012, 56, 369–377. [Google Scholar]
- Morin, P.; Pouliot, Y.; Florez, R.J. A comparative study of the fractionation of regular buttermilk and whey buttermilk by microfiltration. J. Food. Eng. 2006, 77, 521–528. [Google Scholar] [CrossRef]
- Jovanovic, S.; Barac, M.; Macej, O.; Vucic, T.; Lacnjevac, C. SDS-PAGE analysis of soluble proteins in reconstituted milk exposed to different heat treatments. Sensors 2007, 7, 371–383. [Google Scholar] [CrossRef]
- Lin, S.; Sun, J.; Cao, D.; Cao, J.; Jiang, W. Distinction of differently heat treated bovine milks by native-PAGE fingerprinting of their whey proteins. Food Chem. 2010, 121, 803–808. [Google Scholar] [CrossRef]
- Tavares, T.G.; Malcata, X.F. Whey proteins as source of bioactive peptides against hypertension. In Bioactive Food Peptides in Health and Disease; Hernandez-Ledesma, B., Hsieh, C., Eds.; Intech: Rijeka, Croatia, 2013. [Google Scholar]
- Yanaba, K.; Yoshizaki, A.; Asano, Y.; Kadono, T.; Tedder, T.F.; Sato, S. IL-10-producing B10 cells inhibit intestinal injury in a mouse model. Am. J. Pathol. 2011, 178, 735–743. [Google Scholar] [CrossRef]
- Cook, M.D.; Martin, S.A.; Williams, C.; Whitlock, K.; Wallig, M.A.; Pence, B.D.; Woods, J.A. Forced treadmill exercise training exacerbates inflammation and cause mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav. Immun. 2013, 33, 46–56. [Google Scholar] [CrossRef]
- Sprong, R.C.; Schonewille, A.J.; van der Meer, R. Dietary cheese whey protein protects rats against mild dextran sulfate sodium-induced colitis: Role of mucin and microbiota. J. Dairy Sci. 2010, 93, 1364–1371. [Google Scholar] [CrossRef]
- Gersemann, M.; Becker, S.; Nuding, S.; Antoni, L.; Ott, G.; Fritz, P.; Oue, N.; Yasui, W.; Wehkamp, J.; Stange, E.F. Olfactomedin-4 is a glycoprotein secreted into mucus in active IBD. J. Crohns Colitis 2012, 6, 425–434. [Google Scholar] [CrossRef]
- Bounous, G.; Gold, P. The biological activity of undenatured dietary whey proteins: Role of glutathione. Ciln. Invest. Med. 1991, 14, 296–309. [Google Scholar]
- Hongsprabhas, P.; Kerdchouay, P.; Sakulsom, P. Loweirng the maillard reaction products (MRPs) in heated whey protein products and their cytotoxicity in human cell models by whey protein hydrolysates. Food. Res. Int. 2011, 44, 748–754. [Google Scholar] [CrossRef]
- Lubeseder-Martellato, C.; Guenzi, E.; Jörg, A.; Töpolt, K.; Naschberger, E.; Kremmer, E.; Zietz, C.; Tschachler, E.; Hutzler, P.; Schwemmle, M.; et al. Guanylate-binding protein-1 expression is selectively induced by inflammatory cytokines and is an activation marker of endothelial cells during inflammatory diseases. Am. J. Pathol. 2002, 161, 1749–1759. [Google Scholar] [CrossRef]
- Mirpuri, J.; Brazil, J.C.; Berardinelli, A.J.; Nasr, T.R.; Cooper, K.; Schooner, M.; Lin, P.W.; Parkos, C.A.; Louis, N.A. Commensal Escherichia coli reduces epithelial apoptosis through IFN-αA mediated induction of GBP-1 in human and murine models of developing intestine. J. Immunol. 2010, 184, 7186–7195. [Google Scholar] [CrossRef]
- Guenzi, E.; Töpolt, K.; Cornali, E.; Lubeseder-Martellato, C.; Jörg, A.; Matzen, K.; Zietz, C.; Kremmer, E.; Nappi, F.; Schwemmle, M.; et al. The helical domain of GBP-1 mediates the inhibition of endothelial cell proliferation by inflammatory cytokines. EMBO J. 2001, 20, 5568–5577. [Google Scholar] [CrossRef]
- Britzen-Laurent, N.; Lipnik, K.; Ocker, M.; Naschberger, E.; Schellerer, V.S.; Croner, R.S.; Vieth, M.; Waldner, M.; Steinberg, P.; Hohenadl, C.; Stürzl, M. GBP-1 acts as a tumor suppressor in colorectal cancer cells. Carcinogenesis 2013, 34, 153–162. [Google Scholar] [CrossRef]
- Gorbachev, A.V.; Kobayashi, H.; Kudo, D.; Tannenbaum, C.S.; Finke, J.H.; Shu, S.; Farber, J.M.; Fairchild, R.L. CXC chemokine ligand 9/monokine induced by IFN-gamma production by tumor cells is critical for T cell-mediated suppression of cutaneous tumors. J. Immunol. 2007, 178, 2278–2286. [Google Scholar] [CrossRef]
- Wang, F.; Graham, W.V.; Wang, Y.; Witkowski, E.D.; Schwarz, B.T.; Turner, J.R. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 2005, 166, 409–419. [Google Scholar] [CrossRef]
- Kominsky, D.J.; Campbell, E.L.; Ehrentraut, S.F.; Wilson, K.E.; Kelly, C.J.; Glover, L.E.; Collins, C.B.; Bayless, A.J.; Saeedi, B.; Colgan, S.P.; Bruder, D. IFN-γ mediated of an apical IL-10 receptor on polarized intestinal epithelia. J. Immunol. 2014, 193, 1267–1276. [Google Scholar]
- Riley, J.K.; Taked, K.; Akira, S.; Schreiber, R.D. Interleukin-10 receptor signaling through the JAK- STAT pathway; requirement for two distinct receptor-derived signals for anti-inflammatory action. J. Biol. Chem. 1999, 274, 16513–16521. [Google Scholar]
- Diegelmann, I.; Olzsal, T.; Goke, B.; Blumberg, R.S.; Brand, S. A novel role for interleukin-27(IL-27) as mediator of intestinal epithelial barrier protection mediated via differential signal transducer activator of transcription (STAT) protein signaling and induction of antibacterial and anti-inflammatory proteins. J. Biol. Chem. 2012, 287, 286–295. [Google Scholar]
- Piccolomini, A.F.; Iskandar, M.M.; Lands, L.C.; Kubow, S. High hydrostatic pressure pre-treatment of whey proteins enhances whey protein hydrolysate inhibition of oxidative stress and IL-8 secretion in intestinal epithelial cells. Food Nutr. Res. 2012, 56. [Google Scholar] [CrossRef]
- Rusu, D.; Drouin, R.; Pouliot, Y.; Gauthier, S.; Poubelle, P.E. A bovine whey protein extract can enhance innate immunity by priming normal human blood neutrophils. J. Nutr. 2008, 139, 386–393. [Google Scholar] [CrossRef]
- Attaallah, W.; Yilmaz, A.M.; Erdoğan, N.; Yalçin, A.S.; Aktan, A.O. Whey protein versus whey protein hydrolyzate for the protection of azoxymethane and dextran sodium sulfate induced colonic tumors in rats. Path. Oncol. Res. 2012, 18, 817–822. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar]
- Shin, K.; Horigome, A.; Yamauchi, K.; Yaeshima, T.; Iwatsuki, K. Orally administered lactoperoxidase ameliorates dextran sulfate sodium-induced colitis in mice by up-regulating colonic interleukin-10 and maintaining peripheral regulatory T cells. Int. Immunopharmacol. 2009, 9, 1387–1393. [Google Scholar] [CrossRef]
- Hering, N.A.; Andres, A.; Fromm, A.; Tol, E.A.; Amasheh, M.; Mankertz, J.; Fromm, M.; Schulzke, D. Transforming growth factor-β, a whey protein component, strengthens the intestinal barrier by upregulating claudin-4 in HT-29/B6 cells. J. Nutr. 2011, 141, 783–789. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jayatilake, S.; Arai, K.; Kumada, N.; Ishida, Y.; Tanaka, I.; Iwatsuki, S.; Ohwada, T.; Ohnishi, M.; Tokuji, Y.; Kinoshita, M. The Effect of Oral Intake of Low-Temperature-Processed Whey Protein Concentrate on Colitis and Gene Expression Profiles in Mice. Foods 2014, 3, 351-368. https://doi.org/10.3390/foods3020351
Jayatilake S, Arai K, Kumada N, Ishida Y, Tanaka I, Iwatsuki S, Ohwada T, Ohnishi M, Tokuji Y, Kinoshita M. The Effect of Oral Intake of Low-Temperature-Processed Whey Protein Concentrate on Colitis and Gene Expression Profiles in Mice. Foods. 2014; 3(2):351-368. https://doi.org/10.3390/foods3020351
Chicago/Turabian StyleJayatilake, Sharmila, Katsuhito Arai, Nanami Kumada, Yoshiko Ishida, Ichiro Tanaka, Satoru Iwatsuki, Takuji Ohwada, Masao Ohnishi, Yoshihiko Tokuji, and Mikio Kinoshita. 2014. "The Effect of Oral Intake of Low-Temperature-Processed Whey Protein Concentrate on Colitis and Gene Expression Profiles in Mice" Foods 3, no. 2: 351-368. https://doi.org/10.3390/foods3020351
APA StyleJayatilake, S., Arai, K., Kumada, N., Ishida, Y., Tanaka, I., Iwatsuki, S., Ohwada, T., Ohnishi, M., Tokuji, Y., & Kinoshita, M. (2014). The Effect of Oral Intake of Low-Temperature-Processed Whey Protein Concentrate on Colitis and Gene Expression Profiles in Mice. Foods, 3(2), 351-368. https://doi.org/10.3390/foods3020351





