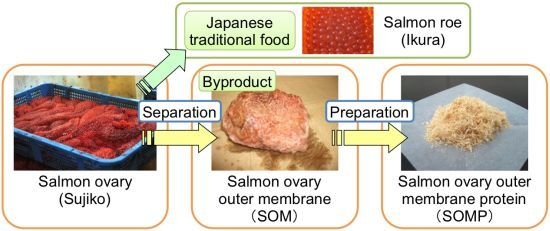
Chemical Composition of Salmon Ovary Outer Membrane and Its Protein Increases Fecal Mucins Content in C57BL/6J and Type 2 Diabetic/Obese KK-Ay Mice
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Salmon Ovary Outer Membrane Protein
2.3. Chemical Analysis
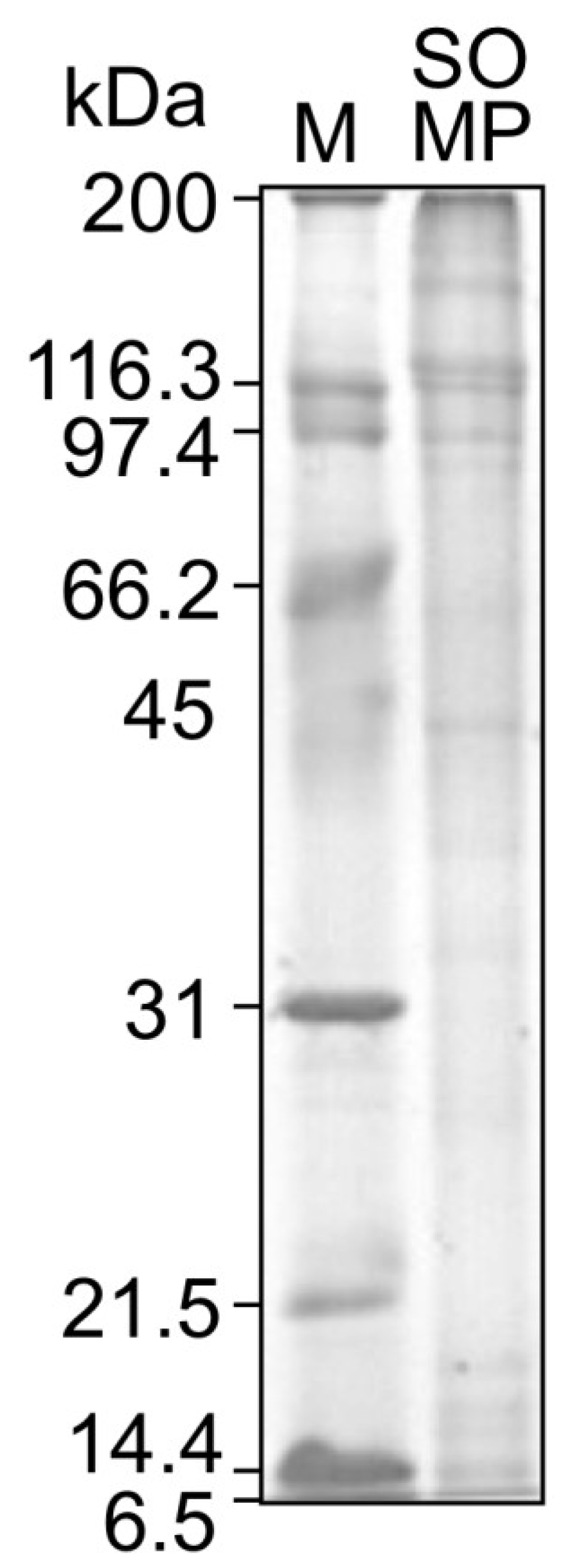
2.4. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
2.5. In Vitro Digestion
2.6. Protein Digestibility
2.7. Animal Diet and Care
| Composition | C57BL/6J | KK-Ay | ||
|---|---|---|---|---|
| Control | SOMP | Control | SOMP | |
| g/kg | ||||
| Casein † | 258 | 129 | 230 | 115 |
| Salmon ovary outer membrane protein ‡ | - | 129 | - | 115 |
| Dextrinized corn starch | 60.2 | 60.2 | 92.1 | 92.1 |
| Corn starch | 181.286 | 181.286 | 277.386 | 277.386 |
| Sucrose | 100 | 100 | 100 | 100 |
| Cellulose | 50 | 50 | 50 | 50 |
| AIN93G mineral mixture | 35 | 35 | 35 | 35 |
| AIN93 vitamin mixture | 10 | 10 | 10 | 10 |
| l-Cystine | 3 | 3 | 3 | 3 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 |
| Lard | 230 | 230 | 130 | 130 |
| Soybean oil | 70 | 70 | 70 | 70 |
| tert-Butylhydroquinone | 0.014 | 0.014 | 0.014 | 0.014 |
2.8. Analysis of Serum and Fecal Biochemical Compositions
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of SOM and SOMP
| Composition | SOM † | SOMP | ||
|---|---|---|---|---|
| Crude protein (g/100 g) | 61.9 | 86.9 | ||
| Crude lipid (g/100 g) | 18.9 | 1.2 | ||
| Triacylglycerol (g/100 g lipid) | 40.1 | - | ||
| Phospholipids (g/100 g lipid) | 52.0 | - | ||
| Total cholesterol (g/100 g lipid) ‡ | 5.8 | - | ||
| Fatty acid composition (%) | ||||
| C16:0 | 14.7 | - | ||
| C16:1 n-9 | 5.1 | - | ||
| C18:0 | 3.4 | - | ||
| C18:1 n-9 | 16.8 | - | ||
| C20:4 n-6 | 1.2 | - | ||
| C20:5 n-3 (EPA) | 11.1 | - | ||
| C22:6 n-3 (DHA) | 18.8 | - | ||
| Moisture (g/100 g) | 7.1 | 8.0 | ||
| Ash (g/100 g) | 5.9 | 1.6 | ||
3.2. Amino Acid Composition and Molecular Weight of SOMP
| Amino acid | SOMP | Casein |
|---|---|---|
| g/kg protein | ||
| Alanine | 60 | 44 |
| Arginine | 72 | 28 |
| Aspartic acid † | 118 | 68 |
| Glutamic acid ‡ | 114 | 184 |
| Glycine | 104 | 31 |
| Histidine | 23 | 26 |
| Hydroxyproline | 62 | ND |
| Isoleucine | 32 | 54 |
| Leucine | 46 | 92 |
| Lysine | 59 | 69 |
| Methionine | 21 | 25 |
| Phenylalanine | 39 | 39 |
| Proline | 50 | 123 |
| Serine | 52 | 62 |
| Threonine | 79 | 44 |
| Tyrosine | 33 | 39 |
| Valine | 35 | 72 |
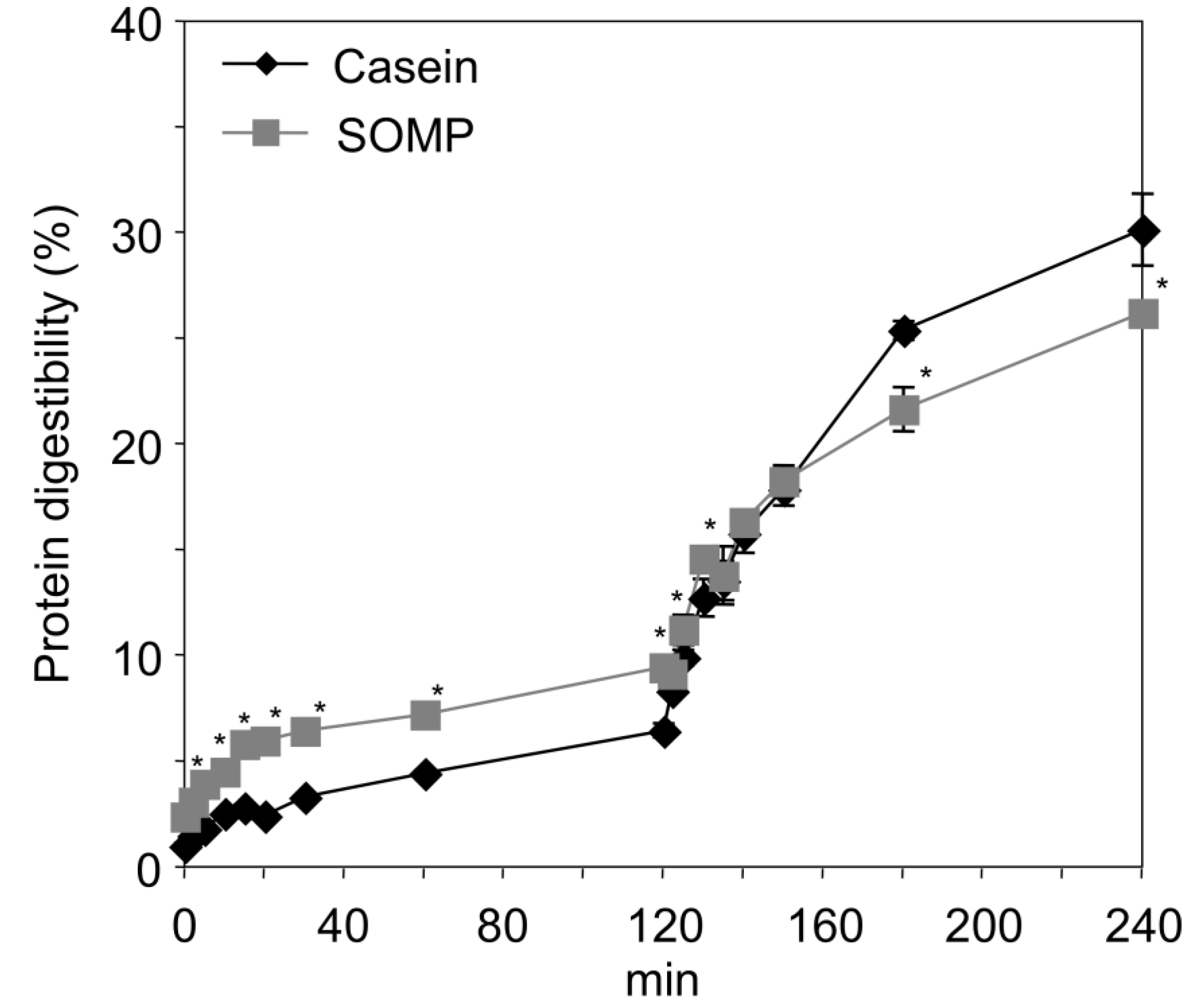
3.3. In Vitro Digestion of SOMP
3.4. Effects of SOMP on Growth Parameters and Organ Weights
| C57BL/6J | KK-Ay | |||
|---|---|---|---|---|
| Control | SOMP | Control | SOMP | |
| Growth parameters | ||||
| Initial BW (g) | 18.0 ± 0.7 | 18.2 ± 0.5 | 18.1 ± 1.9 | 18.4 ± 2.1 |
| Final BW (g) | 28.2 ± 1.9 | 26.9 ± 1.4 | 43.5 ± 2.0 | 42.2 ± 5.4 |
| BW gain (g/day) | 0.28 ± 0.07 | 0.25 ± 0.05 | 0.91 ± 0.33 | 0.85 ± 0.17 |
| Food intake (g/day) | 3.41 ± 0.65 | 3.15 ± 0.68 | 5.70 ± 0.35 | 5.39 ± 0.32 |
| Food efficiency (g/g) † | 0.082 ± 0.018 | 0.080 ± 0.016 | 0.107 ± 0.010 | 0.114 ± 0.030 |
| Water intake (mL/day) | 2.94 ± 1.14 | 3.31 ± 1.28 | 35.0 ± 11.8 | 35.0 ± 11.8 |
| Relative organ weight (g/100 g BW) | ||||
| Liver weight | 4.01 ± 0.30 | 3.94 ± 0.27 | 5.66 ± 0.45 | 5.85 ± 0.39 |
| Kidney | 1.19 ± 0.09 | 1.27 ± 0.06 | 1.53 ± 0.11 | 1.54 ± 0.13 |
| Spleen | 0.24 ± 0.03 | 0.24 ± 0.02 | 0.29 ± 0.06 | 0.26 ± 0.03 |
| Epididymal WAT | 3.99 ± 0.76 | 3.87 ± 0.42 | 4.88 ± 0.42 | 4.66 ± 0.40 |
| Perirenal and retroperitoneal WAT | 1.19 ± 0.17 | 1.33 ± 0.20 | 2.22 ± 0.38 | 2.20 ± 0.27 |
| BAT | 0.48 ± 0.04 | 0.45 ± 0.04 | 0.74 ± 0.11 | 0.67 ± 0.10 |
3.5. Effects of SOMP on Serum and Fecal Biochemical Parameters
| C57BL/6J | KK- Ay | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | SOMP | Control | SOMP | |||||||||
| Serum | ||||||||||||
| TG (mg/dL) | 51.3 ± 29.5 | 44.6 ± 9.4 | 649 ± 320 | 457 ± 158 | ||||||||
| NEFA (μEq/L) | 1866 ± 520 | 1881 ± 353 | 4034 ± 1052 | 3691 ± 1064 | ||||||||
| PL (mg/dL) | 345 ± 27 | 318 ± 22 | 371 ± 34 | 384 ± 50 | ||||||||
| Cholesterol (mg/dL) | 177 ± 13 | 168 ± 13 | 182 ± 18 | 196 ± 19 | ||||||||
| AST (IU/L) | - | 121.1 ± 48.4 | 87.6 ± 13.7 | |||||||||
| ALT (IU/L) | - | 42.5 ± 4.5 | 34.0 ± 2.9 * | |||||||||
| Feces | ||||||||||||
| Dry weight (g/day) | 1.75 ± 0.16 | 1.63 ± 0.18 | 2.67 ± 0.77 | 2.84 ± 0.78 | ||||||||
| Fatty acids (mg/day) | 0.81 ± 0.06 | 0.50 ± 0.06 * | 0.16 ± 0.04 | 0.23 ± 0.08 | ||||||||
| Nitrogen (mg/day) | 35.1 ± 4.7 | 43.0 ± 7.0 * | 47.2 ± 7.0 | 72.3 ± 17.1 * | ||||||||
| NH3 (mg/day) | 2.39 ± 0.68 | 2.58 ± 0.33 | 3.48 ± 0.70 | 2.40 ± 0.71 * | ||||||||
| Mucins (μg/day) | 119 ± 30 | 174 ± 54 * | 186 ± 42 | 269 ± 75 * | ||||||||
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- FAO (2011) Global Datasets: Capture Production 1950–2011. Available online: http://www.fao.org/fishery/statistics/software/fishstat/en (accessed on 16 April 2013).
- Hayashi, H.; Tanaka, Y.; Hibino, H.; Umeda, Y.; Kawamitsu, H.; Fujimoto, H.; Amakawa, T. Beneficial effect of salmon roe phosphatidylcholine in chronic liver disease. Curr. Med. Res. Opin. 1999, 15, 177–184. [Google Scholar] [CrossRef]
- Shirouchi, B.; Nagao, K.; Inoue, N.; Ohkubo, T.; Hibino, H.; Yanagita, T. Effect of dietary omega 3 phosphatidylcholine on obesity-related disorders in obese Otsuka Long-Evans Tokushima fatty rats. J. Agric. Food Chem. 2007, 55, 7170–7176. [Google Scholar] [CrossRef]
- Dragnes, B.T.; Stormo, S.K.; Larsen, R.; Ernstsen, H.H.; Elvevoll, E.O. Utilisation of fish industry residuals: Screening the taurine concentration and angiotensin converting enzyme inhibition potential in cod and salmon. J. Food Compos. Anal. 2009, 22, 714–717. [Google Scholar]
- Senevirathne, M.; Kim, S.K. Utilization of seafood processing by-products: Medicinal applications. Adv. Food Nutr. Res. 2012, 65, 495–512. [Google Scholar] [CrossRef]
- Liaset, B.; Nortvedt, R.; Lied, E.; Espe, M. Studies on the nitrogen recovery in enzymatic hydrolysis of Atlantic salmon (Salmo salar, L.) frames by Protamex™ protease. Proc. Biochem. 2002, 37, 1263–1269. [Google Scholar] [CrossRef]
- Hosomi, R.; Fukunaga, K.; Arai, H.; Kanda, S.; Nishiyama, T.; Yoshida, M. Fish protein decreases serum cholesterol in rats by inhibition of cholesterol and bile acid absorption. J. Food Sci. 2011, 76, H116–H121. [Google Scholar] [CrossRef]
- Fukunaga, K.; Hosomi, R.; Yoshida, M. Effect of Dietary Fish Protein and Fish Oil on Azoxymethane-Induced Aberrant Crypt Foci in A/J Mice. In Omega-3 Oils: Applications in Functional Foods; Hernandez, E.M., Hosokawa, M., Eds.; AOCS Press: Urbana, IL, USA, 2011; pp. 275–294. [Google Scholar]
- Rubenstein, A.H. Obesity: A modern epidemic. Trans. Am. Clin. Climatol. Assoc. 2005, 116, 103–111. [Google Scholar]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of brownstripe red snapper (Lutjanus vitta). Food Chem. 2005, 93, 475–484. [Google Scholar] [CrossRef]
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Morimoto, Y.; Sakata, M.; Ohno, A.; Maegawa, T.; Tajima, S. Effects of Byakko-ka-ninjin-to, Bofu-tsusho-san and Gorei-san on blood glucose level, water intake and urine volume in KKAy mice (in Japanese). Yakugaku Zasshi 2002, 122, 163–168. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists), Official Methods of Analysis of the Association of Official Analytical Chemists, 16th ed.; AOAC: Arlington, VA, USA, 1995.
- Hamada, H. Effects of the preparation conditions on the physical properties of shark-skin gelatin gels. Bull. Jap. Soc. Sci. Fish. 1990, 56, 671–677. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Rouser, G.; Fkeischer, S.; Yamamoto, A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 1970, 5, 494–496. [Google Scholar] [CrossRef]
- McCreary, D.K.; Kossa, W.C.; Ramachandran, S.; Kurtz, R.R. A novel and rapid method for the preparation of methyl esters for gas chromatography: Application to the determination of the fatty acids of edible fats and oils. J. Chromatogr. Sci. 1978, 16, 329–331. [Google Scholar] [CrossRef]
- White, J.A.; Hart, R.J.; Fry, J.C. An evaluation of the Waters Pico-Tag system for the amino-acid analysis of food materials. J. Automat. Chem. 1986, 8, 170–177. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar]
- Horton, J.D.; Bashmakov, Y.; Shimomura, I.; Shimano, H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc. Natl. Acad. Sci. USA 1998, 95, 5987–5992. [Google Scholar] [CrossRef]
- Van de Kamer, J.H.; Ten Bokkel Huinink, H.; Weyers, H.A. Rapid method for the determination of fat in feces. J. Biol. Chem. 1949, 177, 347–355. [Google Scholar]
- Bovee-Oudenhoven, I.M.; Termont, D.S.; Heidt, P.J.; van der Meer, R. Increasing the intestinal resistance of rats to the invasive pathogen Salmonella enteritidis: Additive effects of dietary lactulose and calcium. Gut 1997, 40, 497–504. [Google Scholar]
- Hosomi, R.; Yoshida, M.; Fukunaga, K. Seafood consumption and components for health. Glob. J. Health Sci. 2012, 4, 72–86. [Google Scholar]
- Boukortt, F.O.; Girard, A.; Prost, J.L.; Ait-Yahia, D.; Bouchenak, M.; Belleville, J. Fish protein improves the total antioxidant status of streptozotocin-induced diabetes in spontaneously hypertensive rat. Med. Sci. Monit. 2004, 10, BR397–BR404. [Google Scholar]
- Murata, M.; Sano, Y.; Bannai, S.; Ishihara, K.; Matsushima, R.; Uchida, M. Fish protein stimulated the fibrinolysis in rats. Ann. Nutr. Metab. 2004, 48, 348–356. [Google Scholar] [CrossRef]
- Eckhoff, K.M.; Aidos, I.; Hemre, G.; Lie, O. Collagen content in farmed Atlantic salmon (Salmo salar L.) and subsequent changes in solubility during storage on ice. Food Chem. 1998, 62, 197–200. [Google Scholar] [CrossRef]
- Liu, Z.; Oliveira, A.C.; Su, Y.C. Purification and characterization of pepsin-solubilized collagen from skin and connective tissue of giant red sea cucumber (Parastichopus californicus). J. Agric. Food Chem. 2010, 58, 1270–1274. [Google Scholar] [CrossRef]
- Matmaroh, K.; Benjakul, S.; Prodpran, T.; Encarnacion, A.B.; Kishimura, H. Characteristics of acid soluble collagen and pepsin soluble collagen from scale of spotted golden goatfish (Parupeneus heptacanthus). Food Chem. 2011, 129, 1179–1186. [Google Scholar] [CrossRef]
- Reed, Z.H.; Park, J.W. Qualification and quantification of fish protein in prepared surimi crabstick. J. Food Sci. 2008, 73, C329–C334. [Google Scholar] [CrossRef]
- Al-Holy, M.A.; Rasco, B.A. Characterization of salmon (Oncorhynchus keta) and sturgeon (Acipenser transmontanus) caviar proteins. J. Food Biochem. 2006, 30, 422–428. [Google Scholar] [CrossRef]
- Harkness, M.L.; Harkness, R.D.; Venn, M.F. Digestion of native collagen in the gut. Gut 1978, 19, 240–243. [Google Scholar] [CrossRef]
- Yamatani, K.; Saeki, T.; Iwami, K.; Suzuka, T.; Kanamoto, R. Soybean resistant protein elevates fecal excretion of cholesterol and bile acids and decreases hepatic cholesterol content in comparison with soybean protein isolate. Biosci. Biotechnol. Biochem. 2009, 73, 921–922. [Google Scholar] [CrossRef]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Yamazaki, M.; Kato, N. Consumption of buckwheat protein lowers plasma cholesterol and raises fecal neutral sterols in cholesterol-Fed rats because of its low digestibility. J. Nutr. 1997, 127, 1395–1400. [Google Scholar]
- Zhaorigetu, S.; Sasaki, M.; Watanabe, H.; Kato, N. Supplemental silk protein, sericin, suppresses colon tumorigenesis in 1,2-dimethylhydrazine-treated mice by reducing oxidative stress and cell proliferation. Biosci. Biotechnol. Biochem. 2001, 65, 2181–2186. [Google Scholar] [CrossRef]
- Fujita, H.; Yamagami, T. Fermented soybean-derived Touchi-extract with anti-diabetic effect via alpha-glucosidase inhibitory action in a long-term administration study with KKAy mice. Life Sci. 2001, 70, 219–227. [Google Scholar] [CrossRef]
- Okumura, K.; Ikejima, K.; Kon, K.; Abe, W.; Yamashina, S.; Enomoto, N.; Takei, Y.; Sato, N. Exacerbation of dietary steatohepatitis and fibrosis in obese, diabetic KK-A(y) mice. Hepatol. Res. 2006, 36, 217–228. [Google Scholar] [CrossRef]
- Kon, K.; Ikejima, K.; Okumura, K.; Arai, K.; Aoyama, T.; Watanabe, S. Diabetic KK-A(y) mice are highly susceptible to oxidative hepatocellular damage induced by acetaminophen. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G329–G337. [Google Scholar] [CrossRef]
- Lin, H.C.; Visek, W.J. Colon mucosal cell damage by ammonia in rats. J. Nutr. 1991, 121, 887–893. [Google Scholar]
- Okazaki, Y.; Sitanggang, N.V.; Sato, S.; Ohnishi, N.; Inoue, J.; Iguchi, T.; Watanabe, T.; Tomotake, H.; Harada, K.; Kato, N. Burdock fermented by Aspergillus awamori elevates cecal Bifidobacterium, and reduces fecal deoxycholic acid and adipose tissue weight in rats fed a high-fat diet. Biosci. Biotechnol. Biochem. 2013, 77, 53–57. [Google Scholar] [CrossRef]
- Okazaki, Y.; Tomotake, H.; Tsujimoto, K.; Sasaki, M.; Kato, N. Consumption of a resistant protein, sericin, elevates fecal immunoglobulin A, mucins, and cecal organic acids in rats fed a high-fatdiet. J. Nutr. 2011, 141, 1975–1981. [Google Scholar] [CrossRef]
- Belley, A.; Keller, K.; Göttke, M.; Chadee, K. Intestinal mucins in colonization and host defense against pathogens. Am. J. Trop. Med. Hyg. 1999, 60, 10–15. [Google Scholar]
- Velcich, A.; Yang, W.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maeda, H.; Hosomi, R.; Chiba, U.; Fukunaga, K. Chemical Composition of Salmon Ovary Outer Membrane and Its Protein Increases Fecal Mucins Content in C57BL/6J and Type 2 Diabetic/Obese KK-Ay Mice. Foods 2013, 2, 415-429. https://doi.org/10.3390/foods2030415
Maeda H, Hosomi R, Chiba U, Fukunaga K. Chemical Composition of Salmon Ovary Outer Membrane and Its Protein Increases Fecal Mucins Content in C57BL/6J and Type 2 Diabetic/Obese KK-Ay Mice. Foods. 2013; 2(3):415-429. https://doi.org/10.3390/foods2030415
Chicago/Turabian StyleMaeda, Hayato, Ryota Hosomi, Utako Chiba, and Kenji Fukunaga. 2013. "Chemical Composition of Salmon Ovary Outer Membrane and Its Protein Increases Fecal Mucins Content in C57BL/6J and Type 2 Diabetic/Obese KK-Ay Mice" Foods 2, no. 3: 415-429. https://doi.org/10.3390/foods2030415
APA StyleMaeda, H., Hosomi, R., Chiba, U., & Fukunaga, K. (2013). Chemical Composition of Salmon Ovary Outer Membrane and Its Protein Increases Fecal Mucins Content in C57BL/6J and Type 2 Diabetic/Obese KK-Ay Mice. Foods, 2(3), 415-429. https://doi.org/10.3390/foods2030415