Chemical Characterization of Phenol-Rich Olive Leaf Extract (Olea europaea L. cv. Ogliarola) and Its Neuro-Protective Effects on SH-SY5Y Cells from Oxidative Stress, Lipid Peroxidation, and Glycation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples and Sample Preparation
2.3. Phenols Characterization by Means of HPLC-PDA-ESI/MS
2.4. Determination of Total Phenolic Content
2.5. Antiglycation Assay
2.6. Cell Culture
2.7. Cytotoxicity Assessment by MTT Assay
2.8. Cell Cultureand Induction of Inflammatory Response
2.9. RNA Extraction, cDNA Synthesis and Quantitative Polymerase Chain Reaction (qPCR)
2.10. Determination of Malondialdehyde (MDA) Levels
2.11. Statistical Analysis
3. Results
3.1. Total Phenolic Characterization and Content
3.2. Antiglycation Activity
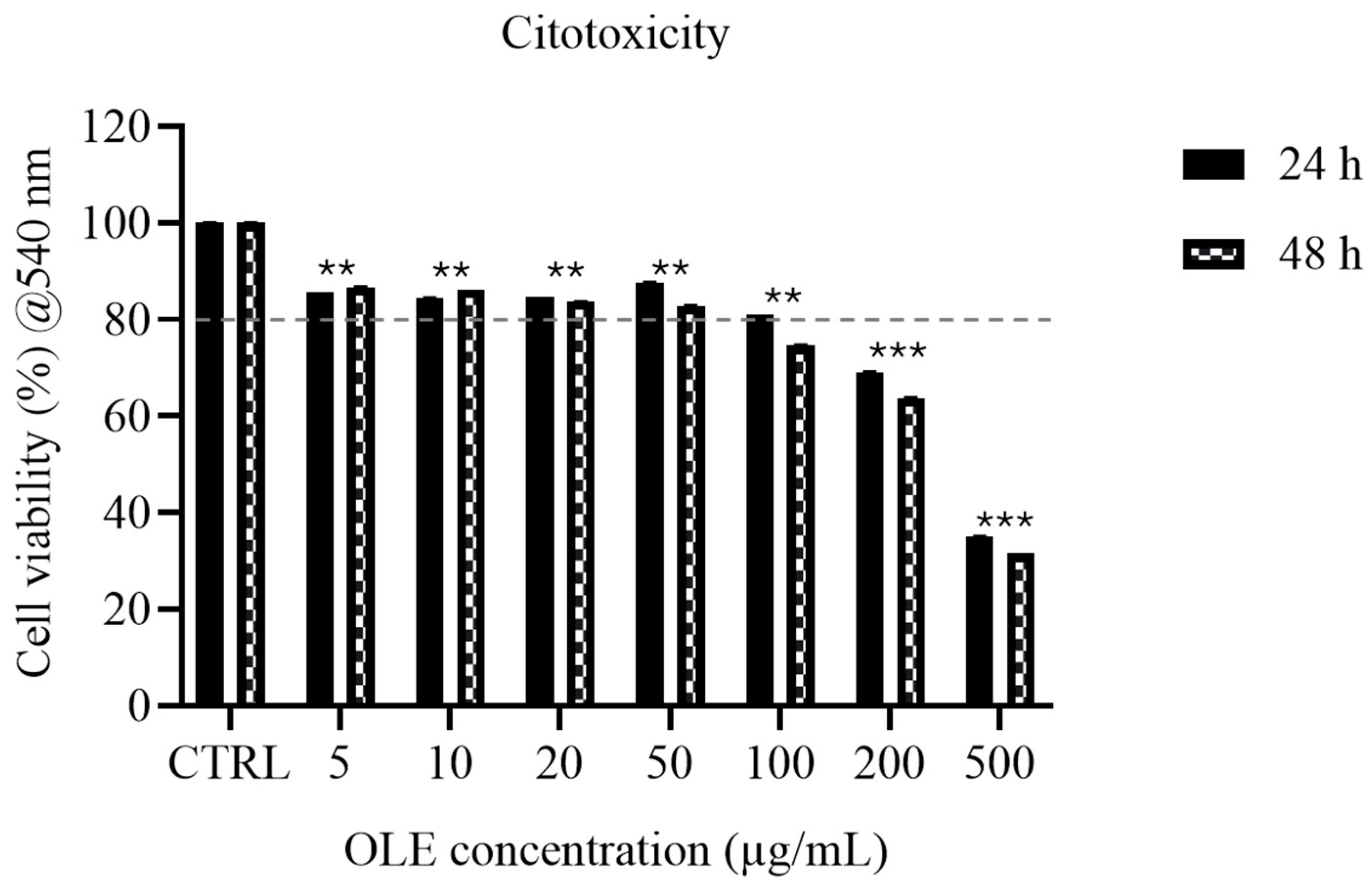
3.3. Cytotoxicity Assessment (MTT Assay)
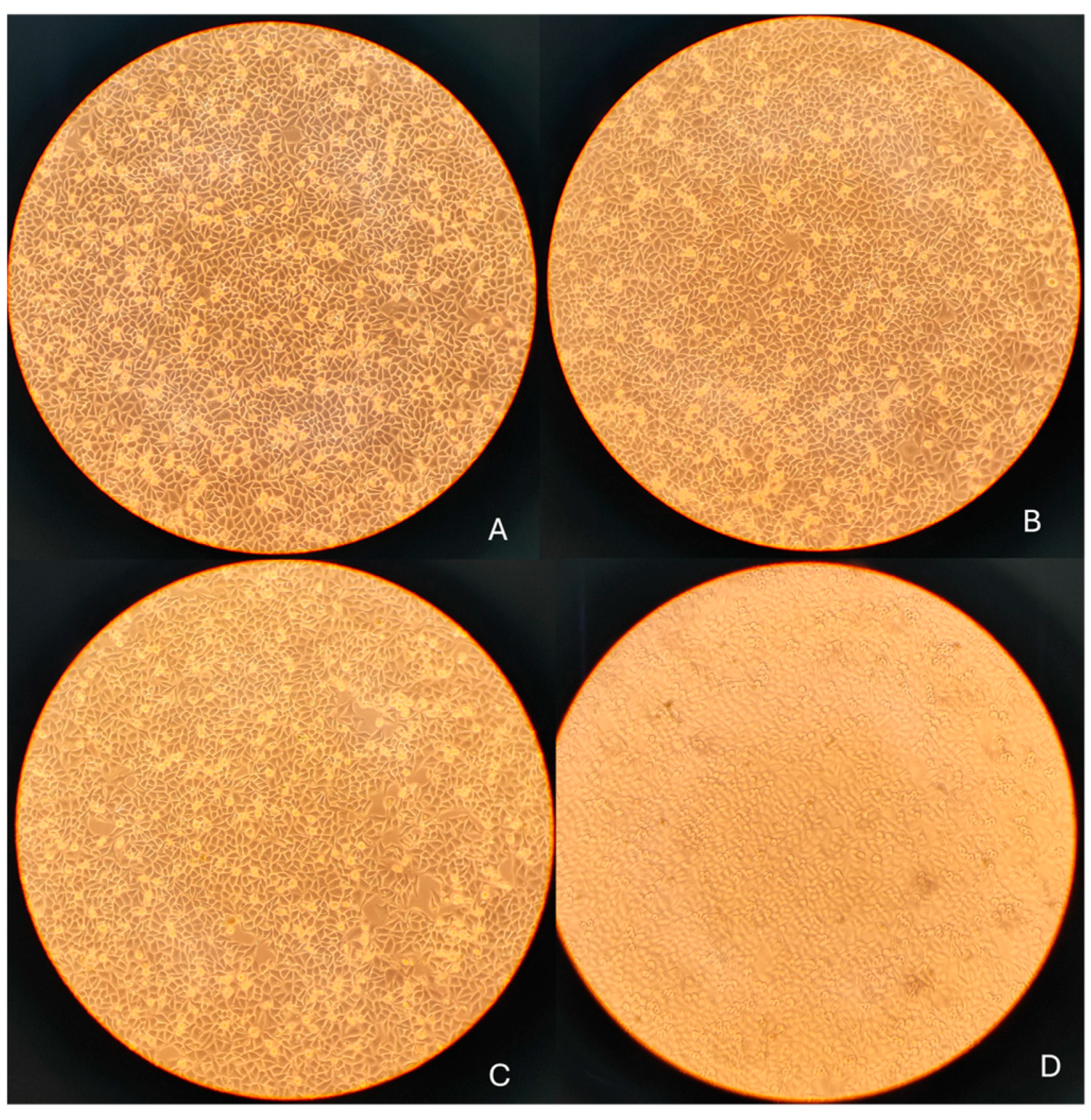
3.4. Morphological Assessment Prior to qRT-PCR
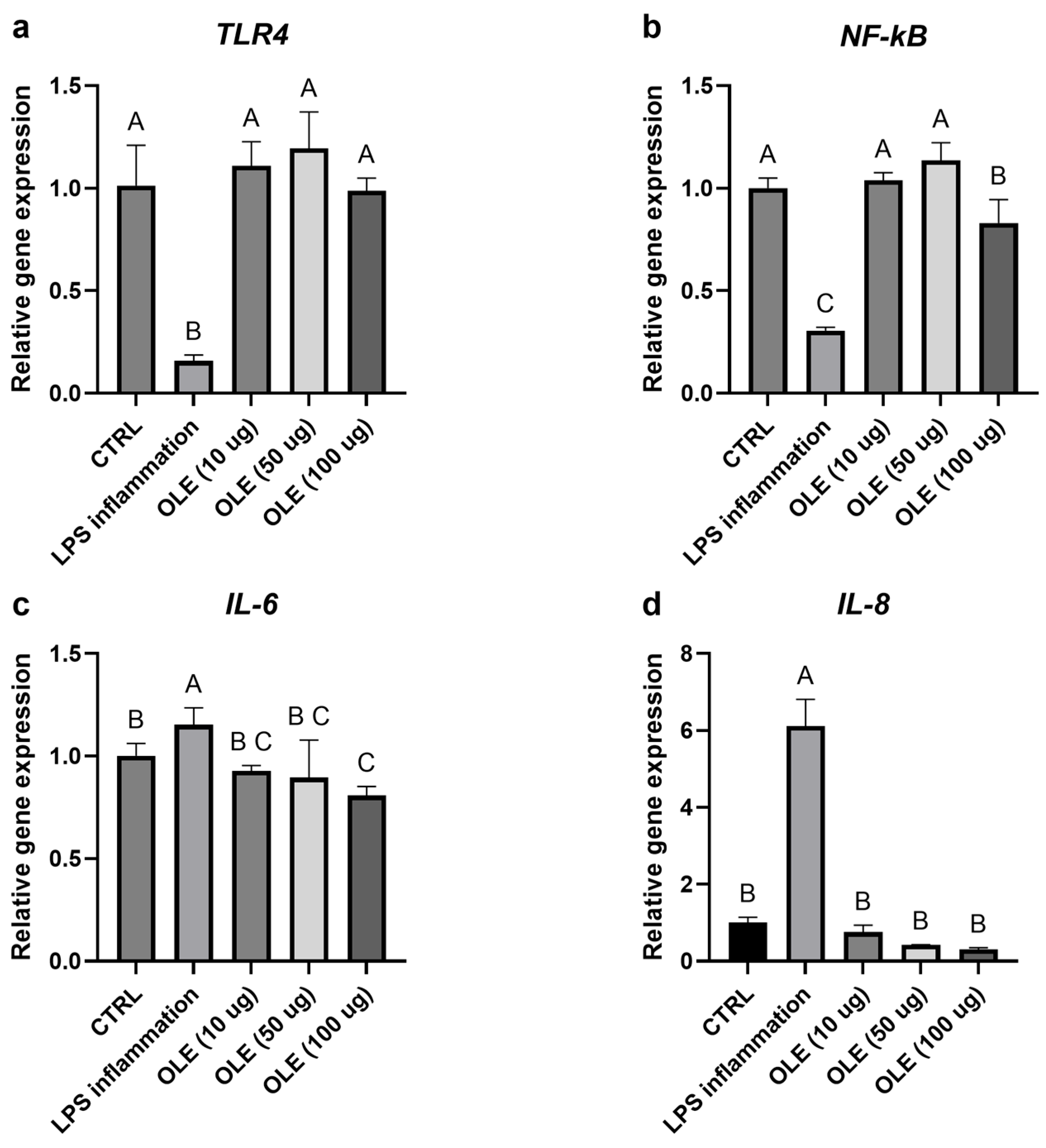
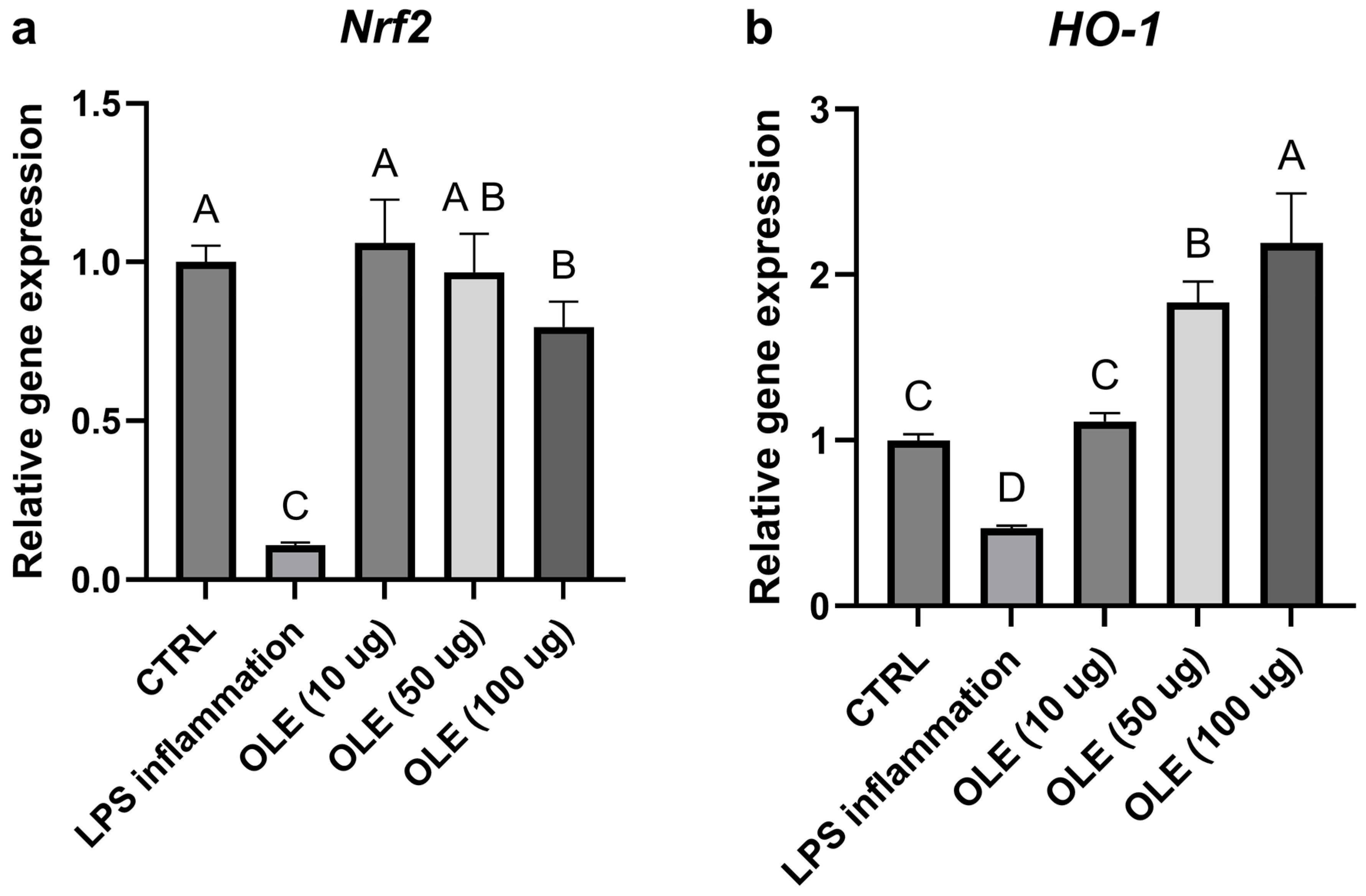
3.5. Effects on Inflammation and Oxidative Stress (qPCR)
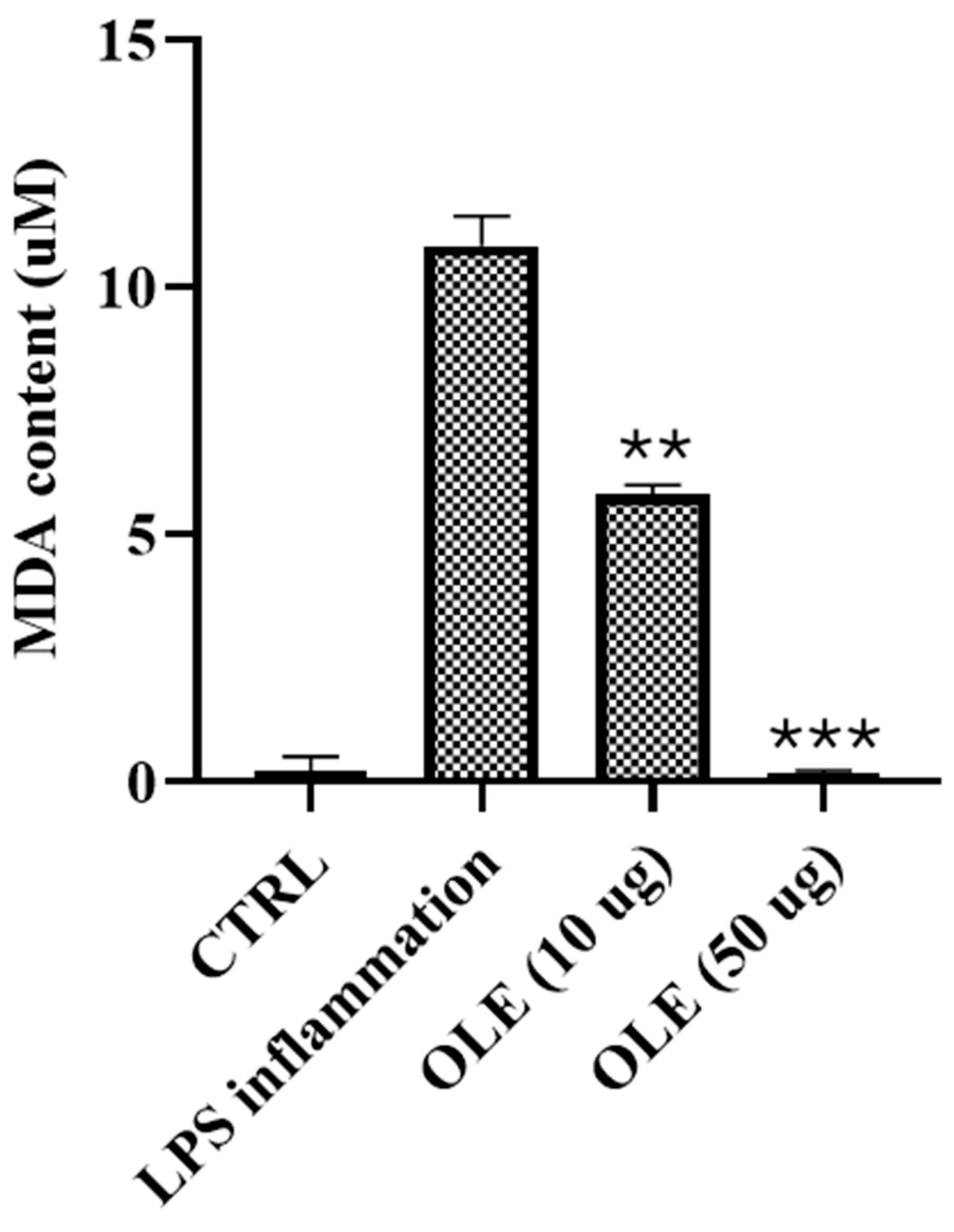
3.6. Lipid Peroxidation: Quantification of Malondialdehyde (MDA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Impellitteri, F.; Multisanti, C.R.; Riolo, K.; Zicarelli, G.; Porretti, M.; Cafeo, G.; Russo, M.; Dugo, P.; Di Bella, G.; Piccione, G.; et al. Bergamot (Citrus bergamia): A Potential New Nutraceutical Against Cellular and Physiological Alterations Induced by Emerging Contaminants in Sentinel Organisms. Antioxidants 2025, 14, 539. [Google Scholar] [CrossRef]
- Rizzo, C.; Zammuto, V.; Lo Giudice, A.; Rizzo, M.G.; Spanò, A.; Laganà, P.; Martinez, M.; Guglielmino, S.; Gugliandolo, C. Antibiofilm Activity of Antarctic Sponge-Associated Bacteria against Pseudomonas aeruginosa and Staphylococcus aureus. JMSE 2021, 9, 243. [Google Scholar] [CrossRef]
- European Commission Website, Olive Oil, an Overview of the Production and Marketing of Olive Oil in the EU. Available online: https://ec.europa.eu/info/food-farming-fisheries/plants-and-plant-products/plant-products/olive-oil (accessed on 4 November 2025).
- Otero, P.; Garcia-Oliveira, P.; Carpena, M.; Barral-Martinez, M.; Chamorro, F.; Echave, J.; Garcia-Perez, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; et al. Applications of by-products from the olive oil processing: Revalorization strategies based on target molecules and green extraction technologies. Trends Food Sci. Technol. 2021, 116, 1084–1104. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; García-García, R.; Granados, M.; Sentellas, S.; Saurina, J. Exploring polyphenol content in olive leaf waste: Effects of geographical, seasonal and varietal differences. Microchem. J. 2025, 215, 114347. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Mir-Cerdà, A.; Granados, M.; Sentellas, S.; Saurina, J. From Waste to Resource: Exploring Green Approaches for Phenolics Recovery from Olive Leaves. Antioxidants 2025, 14, 136. [Google Scholar] [CrossRef]
- Khelouf, I.; Karoui, I.J.; Lakoud, A.; Hammami, M.; Abderrabba, M. Comparative Chemical Composition and Antioxidant Activity of Olive Leaves Olea europaea L. of Tunisian and Algerian Varieties. Heliyon 2023, 9, e22217. [Google Scholar] [CrossRef]
- Palmeri, R.; Siracusa, L.; Carrubba, M.; Parafati, L.; Proetto, I.; Pesce, F.; Fallico, B. Olive Leaves, a Promising Byproduct of Olive Oil Industry: Assessment of Metabolic Profiles and Antioxidant Capacity as a Function of Cultivar and Seasonal Change. Agronomy 2022, 12, 2007. [Google Scholar] [CrossRef]
- Di Meo, M.C.; De Cristofaro, G.A.; Imperatore, R.; Rocco, M.; Giaquinto, D.; Palladino, A.; Zotti, T.; Varricchio, E. Microwave-Assisted Extraction of Olive Leaf from Five Italian Cultivars: Effects of Harvest-Time and Extraction Conditions on Phenolic Compounds and In Vitro Antioxidant Properties. ACS Food Sci. Technol. 2022, 2, 31–40. [Google Scholar] [CrossRef]
- Marx, Í.G.; Casal, S.; Rodrigues, N.; Cruz, R.; Veloso, A.C.; Pereira, J.A.; Peres, A.M. Impact of Incorporating Olive Leaves During the Industrial Extraction of cv. Arbequina Oils on the Physicochemical–Sensory Quality and Health Claim Fulfillment. Eur. Food Res. Technol. 2022, 248, 171–183. [Google Scholar] [CrossRef]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef] [PubMed]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boccardi, V.; Mancinetti, F.; Mecocci, P. Oxidative Stress, Advanced Glycation End Products (AGEs), and Neurodegeneration in Alzheimer’s Disease: A Metabolic Perspective. Antioxidants 2025, 14, 1044. [Google Scholar] [CrossRef]
- Lopez-Suarez, L.; Awabdh, S.A.; Coumoul, X.; Chauvet, C. The SH-SY5Y Human Neuroblastoma Cell Line as a Relevant In Vitro Cell Model for Investigating Neurotoxicology in Human: Focus on Organic Pollutants. Neurotoxicology 2022, 92, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.A.; Rodríguez, J.L.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.R.; Maximiliano, J.E.; Anadón, A.; Ares, I. Use of Human Neuroblastoma SH-SY5Y Cells to Evaluate Glyphosate-Induced Effects on Oxidative Stress, Neuronal Development and Cell Death Signaling Pathways. Environ. Int. 2020, 135, 105414. [Google Scholar] [CrossRef]
- Ma, W.W.; Li, C.Q.; Zhao, L.; Wang, Y.S.; Xiao, R. NF-κB-Mediated Inflammatory Damage is Differentially Affected in SH-SY5Y and C6 Cells Treated with 27-Hydroxycholesterol. Food Sci. Nutr. 2019, 7, 1685–1694. [Google Scholar] [CrossRef]
- Airapetov, M.I.; Eresko, S.O.; Rogova, A.S.; Bychkov, E.R.; Lebedev, A.A.; Shabanov, P.D. Rifampicin Inhibits TLR4 and IL1β Gene Expression and Enhances SH-SY5Y Cell Viability After Prolonged Ethanol Exposure in an In Vitro Experiment. Biomed. Chem. Res. Methods 2024, 7, e00208. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, H. Naringenin Inhibits Hydrogen Peroxide-Induced SH-SY5Y Cell Injury Through the Nrf2/HO-1 Pathway. Neurotox. Res. 2019, 36, 796–805. [Google Scholar] [CrossRef]
- Dai, H.-Y.; Chang, M.-X.; Sun, L. HOTAIRM1 Knockdown Reduces MPP+-Induced Oxidative Stress Injury of SH-SY5Y Cells by Activating the Nrf2/HO-1 Pathway. Transl. Neurosci. 2023, 14, 20220296. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xiao, R.; Zhang, Y.; Xu, D.; Wang, N.; Han, M.; Zhang, Y.; Zhang, L.; Zhou, W. DHPA Protects SH-SY5Y Cells from Oxidative Stress-Induced Apoptosis via Mitochondria Apoptosis and the Keap1/Nrf2/HO-1 Signaling Pathway. Antioxidants 2022, 11, 1794. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.Q.; Kim, J.H.; Kim, H.Y.; Kim, J.-H.; Cho, E.J. Protective Effects and Mechanisms of Pectolinarin Against H2O2-Induced Oxidative Stress in SH-SY5Y Neuronal Cells. Molecules 2023, 28, 5826. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, C.; Santoro, I.; Nardi, M.; Cassano, A.; Sindona, G. Eco-Friendly Extraction and Characterisation of Nutraceuticals from Olive Leaves. Molecules 2019, 24, 3481. [Google Scholar] [CrossRef]
- Irrera, E.; Cafeo, G.; Russo, M.; Calabrò, M.L.; Mondello, L.; Dugo, P. Streamlined and green chromatographic approach for the determination of oxygen heterocyclic compounds in foodstuffs via miniaturised extraction. Nat. Prod. Res. 2025, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dugo, L.; Russo, M.; Cacciola, F.; Mandolfino, F.; Salafia, F.; Vilmercati, A.; Fanali, C.; Casale, M.; De Gara, L.; Dugo, P.; et al. Determination of the Phenol and Tocopherol Content in Italian High-Quality Extra-Virgin Olive Oils by Using LC-MS and Multivariate Data Analysis. Food Anal. Methods 2020, 13, 1027–1041. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Oxidants and Antioxidants Part A; Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. ISBN 9780121822002. [Google Scholar]
- Pannucci, E.; Della Posta, S.; Sbrocca, I.; Cimini, S.; Fanali, C.; De Gara, L.; Dugo, L.; Santi, L. Evaluation of the antiglycative and antioxidant activities of matcha tea. Nat. Prod. Res. 2024, 1–10. [Google Scholar] [CrossRef]
- Multisanti, C.R.; Impellitteri, F.; Cannatà, G.; Cotugno, A.; Perugini, M.; Piccione, G.; Faggio, C.; Rizzo, M.G. Discovering the effects of octylisothiazolinone: Analysis of physiological changes in the Mediterranean mussel (Mytilus galloprovincialis). Ecotoxicol. Environ. Saf. 2025, 302, 118563. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Fazio, E.; De Pasquale, C.; Sciuto, E.L.; Cannatà, G.; Multisanti, C.R.; Impellitteri, F.; D’Agostino, F.G.; Guglielmino, S.P.P.; Faggio, C.; et al. Physiopathological Features in a Three-Dimensional In Vitro Model of Hepatocellular Carcinoma: Hypoxia-Driven Oxidative Stress and ECM Remodeling. Cancers 2025, 17, 3082. [Google Scholar] [CrossRef]
- Franco, D.; Leonardi, A.A.; Rizzo, M.G.; Palermo, N.; Irrera, A.; Calabrese, G.; Conoci, S. Biological response evaluation of human fetal osteoblast cells and bacterial cells on fractal silver dendrites for bone tissue engineering. Nanomaterials 2023, 13, 1107. [Google Scholar] [CrossRef]
- Shademan, B.; Yousefi, H.; Sharafkhani, R.; Nourazarian, A. LPS-Induced Neuroinflammation Disrupts Brain-Derived Neurotrophic Factor and Kinase Pathways in Alzheimer’s Disease Cell Models. Cell. Mol. Neurobiol. 2025, 45, 102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.Z.; Yu, X.H.; Tan, W.H. Baicalein inhibits macrophage lipid accumulation and inflammatory response by activating the PPARγ/LXRα pathway. Clin. Exp. Immunol. 2022, 209, 316–325. [Google Scholar] [CrossRef]
- Xiong, L.; Xie, J.; Song, C.; Liu, J.; Zheng, J.; Liu, C.; Zhang, X.; Li, P.; Wang, F. The activation of Nrf2 and its downstream regulated genes mediates the antioxidative activities of xueshuan xinmaining tablet in human umbilical vein endothelial cells. Evid. Based Complement. Altern. Med. 2015, 2015, 187265. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of malondialdehyde (MDA) by thiobarbituric acid (TBA) assay. In Plant-Microbe Interactions: Laboratory Techniques; Springer Protocols Handbooks; Springer: New York, NY, USA, 2021; pp. 103–105. ISBN 978-1-0716-1079-4. [Google Scholar]
- Gonçalves, M.; Costa, M.; Paiva-Martins, F.; Silva, P. Olive Oil Industry By-Products as a Novel Source of Biophenols with a Promising Role in Alzheimer Disease Prevention. Molecules 2024, 29, 4841. [Google Scholar] [CrossRef]
- Calvano, C.D.; Tamborrino, A. Valorization of Olive By-Products: Innovative Strategies for Their Production, Treatment and Characterization. Foods 2022, 11, 768. [Google Scholar] [CrossRef]
- Vasarri, M.; Bergonzi, M.C.; Ivanova Stojcheva, E.; Bilia, A.R.; Degl’Innocenti, D. Olea europaea L. Leaves as a Source of Anti-Glycation Compounds. Molecules 2024, 29, 4368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zafeiroudis, A.; Kamperidou, V.; Barboutis, I. Utilization of olive tree pruning residues in wood pellets. Eur. J. Wood Prod. 2024, 82, 1713–1724. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; del Mar Contreras, M.; Romero, I.; Castro, E. Towards the Integral Valorization of Olive Pomace-Derived Biomasses through Biorefinery Strategies. ChemBioEng Rev. 2024, 11, 253–277. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; del Mar Contreras, M.; Romero, I.; Castro, E. A biorefinery approach to obtain antioxidants, lignin and sugars from exhausted olive pomace. J. Ind. Eng. Chem. 2021, 96, 356–363. [Google Scholar] [CrossRef]
- Carrillo-Beltran, R.; Corpas-Iglesias, F.A.; Terrones-Saeta, J.M.; Bertoya-Sol, M. New geopolymers from industrial by-products: Olive biomass fly ash and chamotte as raw materials. Constr. Build. Mater. 2021, 272, 121924. [Google Scholar] [CrossRef]
- Gul, E.; Al Bkoor Alrawashdeh, K.; Masek, O.; Skreiberg, Ø.; Corona, A.; Zampilli, M.; Wang, L.; Samaras, P.; Yang, Q.; Zhou, H.; et al. Production and use of biochar from lignin and lignin-rich residues (such as digestate and olive stones) for wastewater treatment. J. Anal. Appl. Pyrolysis 2021, 158, 105263. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Gavrilaș, L.I.; Vodnar, D.C.; Rohn, S.; Mocan, A. Systemic health effects of oleuropein and hydroxytyrosol supplementation: A systematic review of randomized controlled trials. Antioxidants 2024, 13, 1040. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.; Aiello, A.; Rodríguez-Pérez, M.; Accardi, G.; Burgos-Ramos, E.; Silva, P. Olive oil components as novel antioxidants in neuroblastoma treatment: Exploring the therapeutic potential of oleuropein and hydroxytyrosol. Nutrients 2024, 16, 818. [Google Scholar] [CrossRef] [PubMed]
- Tambe, M.A.; de Rus Jacquet, A.; Strathearn, K.E.; Hensel, J.A.; Colón, B.D.; Chandran, A.; Yousef, G.G.; Grace, M.H.; Ferruzzi, M.G.; Wu, Q.; et al. Protective Effects of Polyphenol-Rich Extracts against Neurotoxicity Elicited by Paraquat or Rotenone in Cellular Models of Parkinson’s Disease. Antioxidants 2023, 12, 1463. [Google Scholar] [CrossRef]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of olive oil phenols in neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Charisiadis, P.; Margianni, E.; Lamari, F.N.; Gerothanassis, I.P.; Tzakos, A.G. Olive leaf extracts are a natural source of advanced glycation end product inhibitors. J. Med. Food 2013, 16, 817–822. [Google Scholar] [CrossRef]
- Chen, C. Validation of the Component Model for Prediction of Moisture Sorption Isotherms of Two Herbs and other Products. Foods 2019, 8, 191. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Forward | Reverse | Product Size (bp) | Reference |
|---|---|---|---|---|
| IL-6 | AGACAGCCACTCACCTCTTCAG | TTCTGCCAGTGCCTCTTTGCTG | 131 | [32] |
| IL-8 | TGAGAGTGATTGAGAGTGGACC | ACTTCTCCACAACCCTCTGC | 120 | This study |
| TLR4 | TATCAGAGCCTAAGCCACCT | ATTTGTCTCCACAGCCACC | 120 | This study |
| HO-1 | TGCCAGTGCCACCAAGTTCA | GATGTTGAGCAGGAACGCAG | 118 | This study |
| NF-kB | AAGCAGGAAGATGTGGTGGAG | CGTTGGGGTGTCAAGAAGTAGT | 169 | This study |
| Nrf2 | CAGCGACGGAAAGAGTATGA | TGGGCAACCTGGGAGTAG | 200 | [33] |
| GAPDH | GGAAGGTGAAGGTCGGAGTC | TGGAAGATGGTGATGGGATTT | 174 | This study |
| N° | Phenolic Compound | Concentration |
|---|---|---|
| 1 | Gallic acid | 149.7 ± 5.2 |
| 2 | Hydroxytyrosol glucoside a | 1514.1 ± 14.0 |
| 3 | Hydroxytyrosol | 786.5 ± 9.3 |
| 4 | Oleoside b | 2065.1 ± 17.9 |
| 5 | Hydroxyoleuropein b | 4792.6 ± 33.0 |
| 6 | Verbascoside | 3274.0 ± 29.4 |
| 7 | Luteolin 7-O-glucoside c | 2954.3 ± 22.6 |
| 8 | Oleuropein glucoside b | 2822.8 ± 15.9 |
| 9 | Apigenin rutinoside d | 239.1 ± 4.1 |
| 10 | Apigenin glucoside d | 2240.3 ± 16.3 |
| 11 | Luteolin 4-O-glucoside c | 789.8 ± 11.2 |
| 12 | Oleuropein | 83,331.8 ± 61.5 |
| 13 | Lucidumoside C b | 1868.2 ± 18.2 |
| Tot. | 106,828.3 ± 246.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Rizzo, M.G.; Pizziconi, B.; Riolo, K.; Cafeo, G.; Giannetto, A.; Russo, M.; Faggio, C.; Dugo, L. Chemical Characterization of Phenol-Rich Olive Leaf Extract (Olea europaea L. cv. Ogliarola) and Its Neuro-Protective Effects on SH-SY5Y Cells from Oxidative Stress, Lipid Peroxidation, and Glycation. Foods 2026, 15, 43. https://doi.org/10.3390/foods15010043
Rizzo MG, Pizziconi B, Riolo K, Cafeo G, Giannetto A, Russo M, Faggio C, Dugo L. Chemical Characterization of Phenol-Rich Olive Leaf Extract (Olea europaea L. cv. Ogliarola) and Its Neuro-Protective Effects on SH-SY5Y Cells from Oxidative Stress, Lipid Peroxidation, and Glycation. Foods. 2026; 15(1):43. https://doi.org/10.3390/foods15010043
Chicago/Turabian StyleRizzo, Maria Giovanna, Benedetta Pizziconi, Kristian Riolo, Giovanna Cafeo, Alessia Giannetto, Marina Russo, Caterina Faggio, and Laura Dugo. 2026. "Chemical Characterization of Phenol-Rich Olive Leaf Extract (Olea europaea L. cv. Ogliarola) and Its Neuro-Protective Effects on SH-SY5Y Cells from Oxidative Stress, Lipid Peroxidation, and Glycation" Foods 15, no. 1: 43. https://doi.org/10.3390/foods15010043
APA StyleRizzo, M. G., Pizziconi, B., Riolo, K., Cafeo, G., Giannetto, A., Russo, M., Faggio, C., & Dugo, L. (2026). Chemical Characterization of Phenol-Rich Olive Leaf Extract (Olea europaea L. cv. Ogliarola) and Its Neuro-Protective Effects on SH-SY5Y Cells from Oxidative Stress, Lipid Peroxidation, and Glycation. Foods, 15(1), 43. https://doi.org/10.3390/foods15010043









