Valorization and Environmental Impacts of Pecan Waste: A Critical Review
Abstract
1. Introduction
2. Methods
3. Pecan Shells and Utilizations
3.1. Pecan Growth Areas and Other Basic Growth Conditions
3.2. Current Issues and Pecan Shell Composition
3.3. Pecan Waste Valorization
3.4. Alignment with the United Nations Sustainable Development Goals
3.5. Bio-Energy Production Applications
3.6. Bioplastics and Other Sustainable Materials
3.7. Pecan Shells for Functional Foods
3.8. Activated Carbon from Pecan Shells and Its Applications
3.9. Soil Microbes Improvement from Pecan Shells (Figure 5)
3.10. Pecan Shell Biochar Application in Soil
4. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Larez, F.L.; Tapia-Hernández, J.A.; Quintana, D.S.Z.; Moreno-Vásquez, M.J.; Rodríguez-Félix, F.; Del-Toro-Sánchez, C.L. Valorization of three pecan nutshell cultivars by ultrasound assisted extraction of polyphenolic compounds and its potential free radical inhibition. Review 2022, preprint. [Google Scholar] [CrossRef]
- Zhang, R.; Peng, F.; Li, Y. Pecan production in China. Sci. Hortic. 2015, 197, 719–727. [Google Scholar] [CrossRef]
- Jimenez, M.E.C.; Gabilondo, J.; Bodoira, R.M.; Laverde, L.M.A.; Santagapita, P.R. Extraction of bioactive compounds from pecan nutshell: An added-value and low-cost alternative for an industrial waste. Food Chem. 2024, 453, 139596. [Google Scholar] [CrossRef]
- Fronza, D.; Hamann, J.J.; Both, V.; Anese, R.D.O.; Meyer, E.A. Pecan cultivation: General aspects. Cienc. Rural 2018, 48, e20170179. [Google Scholar] [CrossRef]
- Santana-Méridas, O.; González-Coloma, A.; Sánchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- Hidalgo, A.I.C.; Aguirre, M.R.; Valenzuela, E.; Gomez, J.Y.V.; Dávila, A.C.; Varma, R.S.; Sánchez, V.H.R. Sustainable application of pecan nutshell waste: Greener synthesis of Pd-based nanocatalysts for electro-oxidation of methanol. Int. J. Hydrogen Energy 2016, 41, 23329–23335. [Google Scholar] [CrossRef]
- Ng, C.; E Marshall, W.; Rao, R.M.; Bansode, R.R.; Losso, J.N. Activated carbon from pecan shell: Process description and economic analysis. Ind. Crop. Prod. 2003, 17, 209–217. [Google Scholar] [CrossRef]
- Panta, S.; Zhou, B.; Zhu, L.; Maness, N.; Rohla, C.; Costa, L.; Ampatzidis, Y.; Fontainer, C.; Kaur, A.; Zhang, L. Selecting non-linear mixed effect model for growth and development of pecan nut. Sci. Hortic. 2023, 309, 111614. [Google Scholar] [CrossRef]
- Lang, J. How to Properly Water Your Trees. Available online: https://www.arborday.org/perspectives/how-properly-water-your-trees#:~:text=How%20Much,just%20use%20your%20best%20guess (accessed on 11 December 2025).
- Wang, J.; Miller, D.R.; Sammis, T.W.; Gutschick, V.P.; Simmons, L.J.; Andales, A.A. Energy balance measurements and a simple model for estimating pecan water use efficiency. Agric. Water Manag. 2007, 91, 92–101. [Google Scholar] [CrossRef]
- Wells, L. Pecan Water Requirements and Irrigation Scheduling. In UGA Cooperative Extension Circular; University of Georgia Department of Horticulture: Athens, GA, USA, 2016. [Google Scholar]
- Parker, M.; Sorensen, K.; Brock, J. Growing Pecans in North Carolina (Revised AG-81). In North Carolina Cooperative Extension Service; NC State University: Raleigh, NC, USA, 2016; Available online: http://content.ces.ncsu.edu/growing-pecans-in-north-carolina (accessed on 30 July 2025).
- Shapiro-Ilan, D.I.; Cottrell, T.E.; Jackson, M.A.; Wood, B.W. Control of Key Pecan Insect Pests Using Biorational Pesticides. J. Econ. Entomol. 2013, 106, 257–266. [Google Scholar] [CrossRef]
- Dunford, N.T.; Gumus, Z.P.; Gur, C.S. Chemical Composition and Antioxidant Properties of Pecan Shell Water Extracts. Antioxidants 2022, 11, 1127. [Google Scholar] [CrossRef]
- Prasad, R.B.N. WALNUTS AND PECANS. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 6071–6079. [Google Scholar] [CrossRef]
- Yemmireddy, V.K.; Cason, C.; Moreira, J.; Adhikari, A. Effect of pecan variety and the method of extraction on the antimicrobial activity of pecan shell extracts against different foodborne pathogens and their efficacy on food matrices. Food Control. 2020, 112, 107098. [Google Scholar] [CrossRef]
- Littlefield, B. Characterization of Pecan Shells for Value-added Applications. Master’s Thesis, Auburn University, Auburn, AL, USA, 2010. [Google Scholar]
- United Nations. The Sustainable Development Goals Report. 2025. Available online: https://unstats.un.org/sdgs/report/2025 (accessed on 15 December 2025).
- La Peña, M.M.-D.; Rábago-Panduro, L.M.; Martín-Belloso, O.; Welti-Chanes, J. Challenges and Benefits of Using Pecan Kernels, Derivatives, and Byproducts as Alternative Ingredients in Food Product Development. Food Rev. Int. 2023, 39, 2530–2542. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Alves, J.L.F.; da Silva, J.C.G.; Di Domenico, M.; Arias, S.; Pacheco, J.G.A.; Marangoni, C.; Machado, R.A.F.; Bolzan, A. Prospecting pecan nutshell pyrolysis as a source of bioenergy and bio-based chemicals using multicomponent kinetic modeling, thermodynamic parameters estimation, and Py-GC/MS analysis. Renew. Sustain. Energy Rev. 2022, 153, 111753. [Google Scholar] [CrossRef]
- Grande, L.; Pedroarena, I.; Korili, S.A.; Gil, A. Hydrothermal Liquefaction of Biomass as One of the Most Promising Alternatives for the Synthesis of Advanced Liquid Biofuels: A Review. Materials 2021, 14, 5286. [Google Scholar] [CrossRef]
- Kundariya, N.; Mohanty, S.S.; Varjani, S.; Ngo, H.H.; Wong, J.W.C.; Taherzadeh, M.J.; Chang, J.-S.; Ng, H.Y.; Kim, S.-H.; Bui, X.-T. A review on integrated approaches for municipal solid waste for environmental and economical relevance: Monitoring tools, technologies, and strategic innovations. Bioresour. Technol. 2021, 342, 125982. [Google Scholar] [CrossRef]
- Mariana, M.; Hps, A.K.; Mistar, E.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J. Water Process. Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Searchinger, T.; Heimlich, R.; Houghton, R.A.; Dong, F.; Elobeid, A.; Fabiosa, J.; Tokgoz, S.; Hayes, D.; Yu, T.-H. Use of U.S. Croplands for Biofuels Increases Greenhouse Gases Through Emissions from Land-Use Change. Science 2008, 319, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Ale, S.; Femeena, P.V.; Mehan, S.; Cibin, R. Environmental impacts of bioenergy crop production and benefits of multifunctional bioenergy systems. In Bioenergy with Carbon Capture and Storage; Elsevier: Amsterdam, The Netherlands, 2019; pp. 195–217. [Google Scholar] [CrossRef]
- Nadányi, R.; Džuganová, M.; Ház, A.A. Optimized Lignin Recovery From Black Liquor For Enhanced Mechanical Properties of Acrylonitrile Butadiene Rubber Composites. Acta Fac. Xylologiae Zvolen 2024, 66, 51. [Google Scholar] [CrossRef]
- Orue, A.; Eceiza, A.; Arbelaiz, A. The use of alkali treated walnut shells as filler in plasticized poly(lactic acid) matrix composites. Ind. Crop. Prod. 2020, 145, 111993. [Google Scholar] [CrossRef]
- Mennani, M.; Kasbaji, M.; Benhamou, A.A.; Boussetta, A.; Kassab, Z.; El Achaby, M.; Grimi, N.; Moubarik, A. The potential of lignin-functionalized metal catalysts—A systematic review. Renew. Sustain. Energy Rev. 2024, 189, 113936. [Google Scholar] [CrossRef]
- Sánchez-Acosta, D.; Rodriguez-Uribe, A.; Álvarez-Chávez, C.R.; Mohanty, A.K.; Misra, M.; López-Cervantes, J.; Madera-Santana, T.J. Physicochemical Characterization and Evaluation of Pecan Nutshell as Biofiller in a Matrix of Poly(lactic acid). J. Polym. Environ. 2019, 27, 521–532. [Google Scholar] [CrossRef]
- Agustin-Salazar, S.; Ricciulli, M.; Ambrogi, V.; Cerruti, P.; Scarinzi, G. Thermomechanical Properties and Biodegradation Behavior of Itaconic Anhydride-Grafted PLA/Pecan Nutshell Biocomposites. Polymers 2022, 14, 5532. [Google Scholar] [CrossRef]
- Raya-Morquecho, E.M.; Aguilar-Zarate, P.; Sepúlveda, L.; Michel, M.R.; Iliná, A.; Aguilar, C.N.; Ascacio-Valdés, J.A. Ellagitannins and Their Derivatives: A Review on the Metabolization, Absorption, and Some Benefits Related to Intestinal Health. Microbiol. Res. 2025, 16, 113. [Google Scholar] [CrossRef]
- De La Rosa, L.A.; Alvarez-Parrilla, E.; Shahidi, F. Phenolic Compounds and Antioxidant Activity of Kernels and Shells of Mexican Pecan (Carya illinoinensis). J. Agric. Food Chem. 2011, 59, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Harper, P. A Review of the Dietary Intake, Bioavailability and Health Benefits of Ellagic Acid (EA) with a Primary Focus on Its Anti-Cancer Properties. Cureus 2023, 15, e43156. [Google Scholar] [CrossRef]
- Cason, C.; Yemmireddy, V.K.; Moreira, J.; Adhikari, A. Antioxidant Properties of Pecan Shell Bioactive Components of Different Cultivars and Extraction Methods. Foods 2021, 10, 713. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Rahmani, A.H. Unlocking the Pharmacological Potential of Myricetin Against Various Pathogenesis. Int. J. Mol. Sci. 2025, 26, 4188. [Google Scholar] [CrossRef]
- Huang, C.Y. Pecan shell by-products—Phenolic compound contents and antimicrobial properties. AIMS Agric. Food 2020, 5, 218–232. [Google Scholar]
- Mateș, L.; Banc, R.; Zaharie, F.A.; Rusu, M.E.; Popa, D.-S. Mechanistic Insights into the Biological Effects and Antioxidant Activity of Walnut (Juglans regia L.) Ellagitannins: A Systematic Review. Antioxidants 2024, 13, 974. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic Potential Through Its Antioxidant Property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.; Roberts, T. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Kosker, A.R.; Özogul, F.; Durmus, M.; Ucar, Y.; Ayas, D.; Regenstein, J.M.; Özogul, Y. Tetrodotoxin levels in pufferfish (Lagocephalus sceleratus) caught in the Northeastern Mediterranean Sea. Food Chem. 2016, 210, 332–337. [Google Scholar] [CrossRef]
- Ultee, A.; Slump, R.A.; Steging, G.; Smid, E.J. Antimicrobial Activity of Carvacrol toward Bacillus cereus on Rice. J. Food Prot. 2000, 63, 620–624. [Google Scholar] [CrossRef]
- Wang, X.W.; Tan, B.Z.; Sun, M.; Ho, B.; Ding, J.L. Thioredoxin-like 6 protects retinal cell line from photooxidative damage by upregulating NF-κB activity. Free. Radic. Biol. Med. 2008, 45, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhao, Y.; Chen, X.; Zheng, Y.; Liu, W.; Liu, X. Taxifolin, a novel food, attenuates acute alcohol-induced liver injury in mice through regulating the NF-κB-mediated inflammation and PI3K/Akt signalling pathways. Pharm. Biol. 2021, 59, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, Y.; Li, Y.; Zhang, Z.; Liang, Y. Antioxidant and prooxidant activities of phenolic acids commonly existed in vegetables and their relationship with structures. Food Sci. Technol. 2022, 42, e07622. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Beltrán-Velasco, A.I.; Rubio-Zarapuz, A.; Martínez-Guardado, I.; Valcárcel-Martín, R.; Tornero-Aguilera, J.F. Functional and Therapeutic Roles of Plant-Derived Antioxidants in Type 2 Diabetes Mellitus: Mechanisms, Challenges, and Considerations for Special Populations. Antioxidants 2025, 14, 725. [Google Scholar] [CrossRef]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, S.; Semde, R.; Teng, H.; Shi, M.; Huang, L.; Lou, X.; Jia, B.; Zhu, H.; Zhao, Y. Protocatechuic Acid Improves Alzheimer’s Disease by Regulating the Cholinergic Synaptic Signaling Pathway. Chem. Biodivers. 2025, 22, e202402771. [Google Scholar] [CrossRef]
- Trevisan, G.; Rossato, M.F.; Hoffmeister, C.; Müller, L.G.; Pase, C.; Córdova, M.M.; Rosa, F.; Tonello, R.; Hausen, B.S.; Boligon, A.A.; et al. Antinociceptive and antiedematogenic effect of pecan ( Carya illinoensis ) nut shell extract in mice: A possible beneficial use for a by-product of the nut industry. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 401–410. [Google Scholar] [CrossRef]
- El Hawary, S.S.; Saad, S.; El Halawany, A.M.; Ali, Z.Y.; El Bishbishy, M. Phenolic content and anti-hyperglycemic activity of pecan cultivars from Egypt. Pharm. Biol. 2016, 54, 788–798. [Google Scholar] [CrossRef]
- FloresCordova, M.; Snchez, E.; MuozMrquez, E.; OjedaBarrios, D.; SotoParra, J.; PreciadoRangel, P. Phytochemical composition and antioxidant capacity in Mexican pecan nut. Emir. J. Food Agric. 2017, 29, 346–350. [Google Scholar] [CrossRef]
- Hilbig, J.; Policarpi, P.d.B.; Grinevicius, V.M.A.d.S.; Mota, N.S.R.S.; Toaldo, I.M.; Luiz, M.T.B.; Pedrosa, R.C.; Block, J.M. Aqueous extract from pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell show activity against breast cancer cell line MCF-7 and Ehrlich ascites tumor in Balb-C mice. J. Ethnopharmacol. 2018, 211, 256–266. [Google Scholar] [CrossRef]
- do Prado, A.C.P.; da Silva, H.S.; da Silveira, S.M.; Barreto, P.L.M.; Vieira, C.R.W.; Maraschin, M.; Ferreira, S.R.S.; Block, J.M. Effect of the extraction process on the phenolic compounds profile and the antioxidant and antimicrobial activity of extracts of pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell. Ind. Crop. Prod. 2014, 52, 552–561. [Google Scholar] [CrossRef]
- Vazquez-Flores, A.; Wong-Paz, J.; Lerma-Herrera, M.; Martinez-Gonzalez, A.; Olivas-Aguirre, F.; Aguilar, C.; Wall-Medrano, A.; Gonzalez-Aguilar, G.; Alvarez-Parrilla, E.; de la Rosa, L. Proanthocyanidins from the kernel and shell of pecan (Carya illinoinensis): Average degree of polymerization and effects on carbohydrate, lipid, and peptide hydrolysis in a simulated human digestive system. J. Funct. Foods 2017, 28, 227–234. [Google Scholar] [CrossRef]
- Lerma-Herrera, M.A.; Núñez-Gastélum, J.A.; Ascacio-Valdés, J.; Aguilar, C.N.; Rodrigo-García, J.; Díaz-Sánchez, A.G.; Alvarez-Parrilla, E.; de la Rosa, L.A. Estimation of the Mean Degree of Polymerization of Condensed Tannins from the Kernel and Shell of Carya illinoinensis by HPLC/MS and Spectrophotometric Methods. Food Anal. Methods 2017, 10, 3023–3031. [Google Scholar] [CrossRef]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Rajoka, M.S.R.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.; Simal-Gandara, J. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, L.A.; Vazquez-Flores, A.A.; Alvarez-Parrilla, E.; Rodrigo-García, J.; Medina-Campos, O.N.; Ávila-Nava, A.; González-Reyes, S.; Pedraza-Chaverri, J. Content of major classes of polyphenolic compounds, antioxidant, antiproliferative, and cell protective activity of pecan crude extracts and their fractions. J. Funct. Foods 2014, 7, 219–228. [Google Scholar] [CrossRef]
- Rodrigues, N.P.; Pechina, B.D.R.; Sarkis, J.R. A comprehensive approach to pecan nut valorization: Extraction and characterization of soluble and insoluble-bound phenolics. J. Am. Oil Chem. Soc. 2022, 99, 843–854. [Google Scholar] [CrossRef]
- Vattem, D.A.; Shetty, K. BIOLOGICAL FUNCTIONALITY OF ELLAGIC ACID: A REVIEW. J. Food Biochem. 2005, 29, 234–266. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Dolan, L.; Matulka, R.; Worn, J.; Nizio, J. Safety studies conducted on pecan shell fiber, a food ingredient produced from ground pecan shells. Toxicol. Rep. 2016, 3, 87–97. [Google Scholar] [CrossRef]
- Smolskaya, S.; Logashina, Y.A.; Andreev, Y.A. Escherichia coli Extract-Based Cell-Free Expression System as an Alternative for Difficult-to-Obtain Protein Biosynthesis. Int. J. Mol. Sci. 2020, 21, 928. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.I.; Dubey, N.; Ganeshpurkar, A. Hepatoprotective potential of vanillic acid against isoniazid-rifampicin-induced liver toxicity. Asp. Mol. Med. 2025, 5, 100087. [Google Scholar] [CrossRef]
- Alasalvar, C.; Huang, G.; Bolling, B.W.; Jantip, P.; Pegg, R.B.; Wong, X.K.; Chang, S.K.; Pelvan, E.; de Camargo, A.C.; Mandalari, G.; et al. Upcycling commercial nut byproducts for food, nutraceutical, and pharmaceutical applications: A comprehensive review. Food Chem. 2025, 467, 142222. [Google Scholar] [CrossRef]
- Bezerra, F.D.S.; Koblitz, M.G.B. Extraction of Phenolic Compounds from Agro-Industrial By-Products Using Natural Deep Eutectic Solvents: A Review of Green and Advanced Techniques. Separations 2025, 12, 150. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Da Silva, J.; Da Silva, A.F.V.; Cesca, K.; Ribeiro, P.R.V.; De Brito, E.S.; Ferreira, S.R.S. Efficient and sustainable recovery of bioactive compounds from cashew nut testa shell (Anacardium occidentale L.) using pressurized liquid extraction. In Analytical and Bioanalytical Chemistry; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Niandou, M.A.S.; Novak, J.M.; Bansode, R.R.; Yu, J.; Rehrah, D.; Ahmedna, M. Selection of Pecan Shell–Based Activated Carbons for Removal of Organic and Inorganic Impurities from Water. J. Environ. Qual. 2013, 42, 902–911. [Google Scholar] [CrossRef]
- Bansode, R. Treatment of Organic and Inorganic Pollutants in Municipal Wastewater by Agricultural By-Product Based Granular Activated Carbons (GAC). Master’s Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2002. [Google Scholar] [CrossRef]
- Goncharov, G.; Soktoev, B.; Farkhutdinov, I.; Matveenko, I. Heavy metals in urban soil: Contamination levels, spatial distribution and human health risk assessment (the case of Ufa city, Russia). Environ. Res. 2024, 257, 119216. [Google Scholar] [CrossRef]
- Bansode, R.R.; Losso, J.N.; Marshall, W.E.; Rao, R.M.; Portier, R.J. Pecan shell-based granular activated carbon for treatment of chemical oxygen demand (COD) in municipal wastewater. Bioresour. Technol. 2004, 94, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Appleman, T.D.; Higgins, C.P.; Quiñones, O.; Vanderford, B.J.; Kolstad, C.; Zeigler-Holady, J.C.; Dickenson, E.R. Treatment of poly- and perfluoroalkyl substances in U.S. full-scale water treatment systems. Water Res. 2014, 51, 246–255. [Google Scholar] [CrossRef]
- Johns, M.M.; Marshall, W.E.; Toles, C.A. The effect of activation method on the properties of pecan shell-activated carbons. J. Chem. Technol. Biotechnol. 1999, 74, 1037–1044. [Google Scholar] [CrossRef]
- Herrera, E.; Lindemann, W. Nitrogen Movement in the Soil-Pecan Tree System. New Mexico State University Cooperative Extension Service Guide H-651. Las Cruces, NM. 2001. Available online: https://pubs.nmsu.edu/_h/H651.pdf (accessed on 30 July 2025).
- Wang, Z.; Zhou, M.; Liu, H.; Huang, C.; Ma, Y.; Ge, H.X.; Ge, X.; Fu, S. Pecan agroforestry systems improve soil quality by stimulating enzyme activity. PeerJ 2022, 10, e12663. [Google Scholar] [CrossRef] [PubMed]
- Idowu, O.J.; Sanogo, S.; Brewer, C.E. Short-Term Impacts of Pecan Waste By-Products on Soil Quality in Texturally Different Arid Soils. Commun. Soil Sci. Plant Anal. 2017, 48, 1781–1791. [Google Scholar] [CrossRef]
- Zhou, J.; Hong, W.; Feng, J.; Song, L.; Li, X.; Xu, S.; Zhou, S. Effects of applying peanut shell and its biochar on the microbial activity and community structure of dryland red soil. Heliyon 2023, 9, e12604. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Jackson, L.E. Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol. Biochem. 2003, 35, 29–36. [Google Scholar] [CrossRef]
- Saye, L.M.G.; Navaratna, T.A.; Chong, J.P.J.; O’Malley, M.A.; Theodorou, M.K.; Reilly, M. The Anaerobic Fungi: Challenges and Opportunities for Industrial Lignocellulosic Biofuel Production. Microorganisms 2021, 9, 694. [Google Scholar] [CrossRef]
- Zhang, Y.; Idowu, O.; Brewer, C. Using Agricultural Residue Biochar to Improve Soil Quality of Desert Soils. Agriculture 2016, 6, 10. [Google Scholar] [CrossRef]
- Martínez-Casillas, D.; Mascorro-Gutiérrez, I.; Arreola-Ramos, C.; Villafán-Vidales, H.; Arancibia-Bulnes, C.; Ramos-Sánchez, V.; Cuentas-Gallegos, A. A sustainable approach to produce activated carbons from pecan nutshell waste for environmentally friendly supercapacitors. Carbon 2019, 148, 403–412. [Google Scholar] [CrossRef]
- Neira-Vielma, A.A.; Meléndez-Ortiz, H.I.; García-López, J.I.; Sanchez-Valdes, S.; Cruz-Hernández, M.A.; Rodríguez-González, J.G.; Ramírez-Barrón, S.N. Green Synthesis of Silver Nanoparticles Using Pecan Nut (Carya illinoinensis) Shell Extracts and Evaluation of Their Antimicrobial Activity. Antibiotics 2022, 11, 1150. [Google Scholar] [CrossRef]
- Busscher, W.J.; Novak, J.M.; Evans, D.E.; Watts, D.W.; Niandou, M.A.S.; Ahmedna, M. Influence of Pecan Biochar on Physical Properties of a Norfolk Loamy Sand. Soil Sci. 2010, 175, 10–14. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, F.; Yu, J.; Liu, Z. Feedstock-Induced Changes in the Physicochemical Characteristics of Biochars Produced from Different Types of Pecan Wastes. Forests 2024, 15, 366. [Google Scholar] [CrossRef]
- Khurshid, K.; Nawaz, R.; Khalid, A.; Mahmood, S.; Qadeer, S.; Hanafiah, M.M.; Anjum, M. Optimization of Heavy Metals Removal Through Pecan Shell-Based Modified Activated Carbon from Pharmaceutical Wastewater and Evaluation of the Treated Effluent for Irrigating Pisum sativum. Int. J. Environ. Res. 2025, 19, 60. [Google Scholar] [CrossRef]
- Pereira, E.I.P.; Suddick, E.C.; Six, J. Carbon Abatement and Emissions Associated with the Gasification of Walnut Shells for Bioenergy and Biochar Production. PLoS ONE 2016, 11, e0150837. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, Q.; Peng, F.; Yu, J. Biochar Loaded with a Bacterial Strain N33 Facilitates Pecan Seedling Growth and Shapes Rhizosphere Microbial Community. Plants 2024, 13, 1226. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, S.S.; Withana, P.A.; Lim, J.Y.; You, S.; Chang, S.X.; Wang, F.; Rhee, J.H.; Ok, Y.S. Carbon negative biochar systems contribute to sustainable urban green infrastructure: A critical review. Green Chem. 2024, 26, 10634–10660. [Google Scholar] [CrossRef]
- Dueñas, J.V. Antioxidant Properties and Applications of Pecan (Carya illinoinensis (Wangenh.) K. Koch). Ph.D. Thesis, UPC Universitat Politècnica de Catalunya, Barcelona, Spain, 2021. Available online: https://upcommons.upc.edu/entities/publication/9abe4078-6cb6-4651-a464-002cad8712e1 (accessed on 30 July 2025).
- Carrasco, J.C., Jr. Sustainable Disposal Of Pecan Shells. Master’s Thesis, The University of Texas at El Paso, El Paso, TX, USA, 2025. [Google Scholar]





| Document Inclusion Criteria | Document Exclusion Criteria |
|---|---|
| Article Published In English | Non-English publications |
| Peer-reviewed original research articles and reviews | Theses, dissertations, conference abstracts, book chapters, non-peer-reviewed material |
| Studies published between 2005 and 2025 | Studies published before 2005 |
| Studies related to pecan or walnut shell valorization, biochar, activated carbon, extraction of phenolics, or circular bioeconomy | Studies unrelated to nutshell valorization, extraction, or environmental applications |
| Articles reporting extraction techniques, adsorption properties, physicochemical characterization, bioactive compound yields, or environmental applications | Articles that focus solely on agronomic traits, food chemistry unrelated to shell valorization, or non-material uses |
| Bioactive Compounds | Group | Structure | Representative Functions | Typical Extraction Yield (%) | Bioavailability | Industrial Application Potential | Extraction Reference |
|---|---|---|---|---|---|---|---|
| Benzoic acid | Hydroxybenzoic acids |  | Regulate gut functions | 0.2–1.0% | High (well absorbed, rapid metabolism) | Food preservative, antimicrobial agent | [14,31] |
| Ellagic acid | Hydroxybenzoic acids |  | Against oxidation-linked chronic diseases such as cancer and cardiovascular diseases | 0.1–0.5% | Low (poor solubility, slow absorption) | Nutraceuticals, anti-aging cosmetics | [32,33] |
| Gallic acid | Hydroxybenzoic acids |  | Antioxidant, anticancer, antimicrobial, gastrointestinal, cardiovascular, metabolic, neuropsychological and miscellaneous diseases | 1–5% | Moderate–High | Natural antioxidant, pharmaceuticals | [34,35] |
| p-Hydroxy benzoic acid | Hydroxybenzoic acids |  | Antioxidant, anti-inflammatory, and intestinal barrier-repairing effects | 0.2–1.2% | Moderate | Cosmetic preservatives (parabens precursor) | [33,36] |
| Protocatechuic acid | Hydroxybenzoic acids |  | Antioxidant, anti-inflammatory, Neuroprotective properties | 0.3–2.0% | Moderate | Anti-inflammatory and neuroprotective ingredients | [33,36] |
| Pyrogallic acid | Hydroxybenzoic acids |  | Reducing agent (such as metal), antiseptic properties | 0.1–0.3% | Moderate | Hair dyes, inks, metal processing | [32,37] |
| Vanillic acid | Hydroxybenzoic acids |  | Antioxidant, anti-inflammatory, and anti-cancer | 0.2–1.5% | Moderate | Flavoring, fragrance industry | [38] |
| 2-Hydroxycinnamic acid | Hydroxycinnamic acid derivatives |  | Antioxidant, anti-inflammatory, and antimicrobial agent | 0.3–1.8% | Moderate | Food additives, antimicrobial coatings | [39,40] |
| Chlorogenic acid | Hydroxycinnamic acid derivatives |  | Regulate blood sugar, lipid metabolism, anti-inflammatory, and anti-obesity | 0.5–6% | Low-moderate | Anti-obesity supplements, cosmetics | [41] |
| Ferulic acid | Hydroxycinnamic acid derivatives | 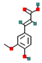 | Anti-inflammatory, antimicrobial properties | 0.4–2.5% | Moderate | UV-protective cosmetics, food antioxidants | [42,43] |
| Caffeic acid | Hydroxycinnamic acid derivatives |  | Antioxidant, anti-inflammatory, and anticancer properties | 0.4–3.0% | Moderate-High | Natural preservatives, skincare products | [44,45] |
| Hesperetin | Flavones | 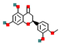 | Antioxidant, anti-inflammatory, and neuroprotective properties | 0.05–0.3% | Low | Functional beverages, supplements | [46,47] |
| Kaempferol | Flavonols | 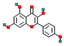 | Antioxidant, anti-inflammatory agent | 0.1–1.2% | Low (poor water solubility) | Anti-cancer research, nutraceuticals | [48,49] |
| Quercetin | Flavonols |  | Antioxidant, anti-inflammatory agent | 0.2–2.0% | Low–Moderate (improves with glycosides) | Nutraceuticals, anti-allergy formulations | [50,51] |
| Rutin | Flavonols |  | Antioxidant, anti-inflammatory agent | 0.1–1.0% | Low | Vascular supplements, pharmaceuticals | [52] |
| Naringenin | Flavanones |  | Antioxidant, anti-inflammatory, anti-cancer, anti-diabetic, and neuroprotective effects | 0.05–0.4% | Low | Diabetes-related supplements | [53] |
| Catechin | Monomeric flavan-3-ols |  | Antioxidant, neuroprotective, anti-cancer | 0.3–3.8% | High | Functional beverages, pharmaceuticals | [54] |
| Epicatechin | Monomeric flavan-3-ols |  | Antioxidant, nitric oxide production, improve blood flow, enhance muscle growth | 0.3–4.0% | High | Sports nutrition, cardiovascular supplements | [55] |
| Epicatechin gallate | Monomeric flavan-3-ols |  | Antioxidant, anti-inflammatory, cardiovascular health, cancer chemoprevention, and neuroprotective properties | 0.1–1.2% | Moderate | Cardioprotective formulations | [55] |
| Epigallocatechin | Monomeric flavan-3-ols |  | Antioxidant, anti-inflammatory, anti-carcinogenic, neuroprotective, and anti-fibrotic properties | 0.2–1.5% | Moderate | Cosmetic antioxidant, nutraceuticals | [56] |
| Procyanidins | Oligo and polymeric flavan-3-ols |  | Antioxidant, anti-inflammatory, cardioprotective, anti-cancer, neuroprotective, and metabolic health | 1–10% (highest yield class) | Low–Moderate (large molecules) | Cocoa extracts, cardioprotective nutraceuticals | [57] |
| Procyanidin B1 (B-type dimer) | Oligo and polymeric flavan-3-ols |  | Anti-inflammatory and anti-oxidative processes | 0.5–4% | Low | Functional foods, pharmaceuticals | [58] |
| Procyanidin B2 (B-type dimer) | Oligo and polymeric flavan-3-ols |  | Cardiovascular health, metabolic regulation, wound healing, neuroprotection, reproductive health, anti-inflammatory and antioxidant | 0.5–4% | Low | High-value nutraceuticals | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Omotonoko, J.L.Y.; Polozola, M.; Svyantek, A.; Wang, Z. Valorization and Environmental Impacts of Pecan Waste: A Critical Review. Foods 2026, 15, 168. https://doi.org/10.3390/foods15010168
Omotonoko JLY, Polozola M, Svyantek A, Wang Z. Valorization and Environmental Impacts of Pecan Waste: A Critical Review. Foods. 2026; 15(1):168. https://doi.org/10.3390/foods15010168
Chicago/Turabian StyleOmotonoko, Jean Louis Yannick, Michael Polozola, Andrej Svyantek, and Zhuoyu Wang. 2026. "Valorization and Environmental Impacts of Pecan Waste: A Critical Review" Foods 15, no. 1: 168. https://doi.org/10.3390/foods15010168
APA StyleOmotonoko, J. L. Y., Polozola, M., Svyantek, A., & Wang, Z. (2026). Valorization and Environmental Impacts of Pecan Waste: A Critical Review. Foods, 15(1), 168. https://doi.org/10.3390/foods15010168








