Preparation, Structural Characterization and Biological Activity Study of Selenium-Rich Polysaccharides from Cyclocarya paliurus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction and Purification Process
2.3. Carbohydrate Analysis
2.4. Antioxidant Capacity Assessment
2.5. Immune Activity Assessment
2.5.1. Cell Cultures
2.5.2. Cell Viability Assay
2.5.3. Cell Phagocytosis Assay
2.5.4. Analysis of NO
2.6. Statistical Analysis
3. Results
3.1. Extraction, Separation, and Purification of CPP-1 and Se-CPP-1
3.2. Effect of Selenisation on Physicochemical Properties
3.2.1. Molecular Weight and Monosaccharide Composition Analysis
3.2.2. Determination of Particle Size, Zeta Potential, and Selenium Content
3.3. Structural Effects of Selenisation
3.4. Effect of Selenisation on Antioxidant Activity
3.5. Effect of Selenisation on Immunological Activity
3.5.1. Cell Proliferation
3.5.2. Phagocytic Activity Assay
3.5.3. Measurement of NO Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Peng, Y.; Xiang, G.; Song, W.; Feng, L.; Jiang, X.; Li, X.; He, S.; Yang, S.; Zhao, Y.; et al. Functional Characterization of Genes Related to Triterpene and Flavonoid Biosynthesis in Cyclocarya Paliurus. Planta 2024, 259, 50. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jian, Y.; Wu, Q.; Wu, J.; Sheng, W.; Jiang, S.; Shehla, N.; Aman, S.; Wang, W. Cyclocarya Paliurus (Batalin) Iljinskaja: Botany, Ethnopharmacology, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2022, 285, 114912. [Google Scholar] [CrossRef]
- Liu, W.; Deng, S.; Zhou, D.; Huang, Y.; Li, C.; Hao, L.; Zhang, G.; Su, S.; Xu, X.; Yang, R.; et al. 3,4-Seco-Dammarane Triterpenoid Saponins with Anti-Inflammatory Activity Isolated from the Leaves of Cyclocarya Paliurus. J. Agric. Food Chem. 2020, 68, 2041–2053. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Peng, Y.; Zhu, X.; Li, H.; Zhang, L.; Kong, F.; Wang, J.; Yu, D. The Phytochemicals and Health Benefits of Cyclocarya Paliurus (Batalin) Iljinskaja. Front. Nutr. 2023, 10, 1158158. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ye, J.; Zeng, J.; Chen, L.; Korpelainen, H.; Li, C. Selenium Species Transforming along Soil–Plant Continuum and Their Beneficial Roles for Horticultural Crops. Hortic. Res. 2023, 10, uhac270. [Google Scholar] [CrossRef]
- Chao, W.; Rao, S.; Chen, Q.; Zhang, W.; Liao, Y.; Ye, J.; Cheng, S.; Yang, X.; Xu, F. Advances in Research on the Involvement of Selenium in Regulating Plant Ecosystems. Plants 2022, 11, 2712. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.; Ning, Z.; Kwon, S.Y.; Li, M.-L.; Tack, F.M.G.; Kwon, E.E.; Rinklebe, J.; Yin, R. The Beneficial and Hazardous Effects of Selenium on the Health of the Soil-Plant-Human System: An Overview. J. Hazard. Mater. 2022, 422, 126876. [Google Scholar] [CrossRef]
- Schiavon, M.; Nardi, S.; Dalla Vecchia, F.; Ertani, A. Selenium Biofortification in the 21st Century: Status and Challenges for Healthy Human Nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Gui, J.-Y.; Rao, S.; Huang, X.; Liu, X.; Cheng, S.; Xu, F. Interaction between Selenium and Essential Micronutrient Elements in Plants: A Systematic Review. Sci. Total Environ. 2022, 853, 158673. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, W.; Miao, Z.; Cao, Q.; Xu, S. Role of Selenoprotein W in Participating in the Progression of Non-Alcoholic Fatty Liver Disease. Redox Biol. 2024, 71, 103114. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, N.; Li, S.; Li, S.; Zhu, S.; Cong, X.; Cheng, S.; Barba, F.J.; Zhu, Z. Polysaccharides in Selenium-Enriched Tea: Extraction Performance under Innovative Technologies and Antioxidant Activities. Foods 2022, 11, 2545. [Google Scholar] [CrossRef]
- Zhou, N.; Long, H.; Wang, C.; Zhu, Z.; Yu, L.; Yang, W.; Ren, X.; Liu, X. Characterization of Selenium-Containing Polysaccharide from Spirulina Platensis and Its Protective Role against Cd-Induced Toxicity. Int. J. Biol. Macromol. 2020, 164, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Luo, D.; Tan, K.X.; Xiang, J.Q.; Liu, W. Study of Selenium Polysaccharide from Cyclocarya Paliurus (Batal) Ijinskaj a in Blood Glucose of Diabetic Mice. Guid. J. Tradit. Chin. Med. Pharm. 2018, 24, 66–69. [Google Scholar]
- Li, J.E.; Ye, X.; Zhao, Z.; Wang, W. Structural Characterization and Antioxidant Activity of an Acetylated Cyclocarya Paliurus Polysaccharide (Ac-CPP0.1). Int. J. Biol. Macromol. 2021, 171, 112–122. [Google Scholar] [CrossRef]
- Xie, J.-H.; Zhang, F.; Wang, Z.-J.; Shen, M.-Y.; Nie, S.-P.; Xie, M.-Y. Preparation, Characterization and Antioxidant Activities of Acetylated Polysaccharides from Cyclocarya Paliurus Leaves. Carbohydr. Polym. 2015, 133, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, M.; He, J.; Chen, L.; Wang, W. In Vitro and In Vivo Immunomodulatory Activity of Acetylated Polysaccharides from Cyclocarya Paliurus Leaves. Int. J. Biol. Macromol. 2024, 259, 129174. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Shen, M.; Chen, Y.; Yu, Q.; Chen, T.; Xie, J. Alleviative Effects of Natural Plant Polysaccharides against DSS-Induced Ulcerative Colitis via Inhibiting Inflammation and Modulating Gut Microbiota. Food Res. Int. 2023, 167, 112630. [Google Scholar] [CrossRef]
- Xie, L.; Shen, M.; Huang, R.; Liu, X.; Yu, Y.; Lu, H.; Xie, J. Apoptosis of Colon Cancer CT-26 Cells Induced Polysaccharide from Cyclocarya Paliurus and Its Phosphorylated Derivative via Intrinsic Mitochondrial Passway. Food Sci. Hum. Wellness 2023, 12, 1545–1556. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, Y.; Meng, K.; Zhang, J.; Zhong, L.; Zhan, Q.; Zhao, L. Effects of Different Selenium Biofortification Methods on Pleurotus Eryngii Polysaccharides: Structural Characteristics, Antioxidant Activity and Binding Capacity In Vitro. Int. J. Biol. Macromol. 2024, 275, 133214. [Google Scholar] [CrossRef]
- Qian, L.; Du, M.; Yang, X.; Wang, Q.; Huang, S.; Ma, Y.; Sun, Y. Microanalysis Characterization and Immunomodulatory Effect for Selenium-Enriched Polysaccharide from Morchella esculenta (L.) Pers. Molecules 2023, 28, 2885. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Ding, D.; Zhang, L.; Muehlmann, L.A.; Deng, S.; Wang, X.; Li, W.; Zhang, W. Synthesis and Antioxidant Properties of Lycium Barbarum Polysaccharides Capped Selenium Nanoparticles Using Tea Extract. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, Z.; Tang, Y.; Ren, Y.; Song, Q.; Tang, Y.; Zhang, Y. Structural Characterization and Antitumor Activity of a Novel Se-Polysaccharide from Selenium-Enriched Cordyceps Gunnii. Food Funct. 2018, 9, 2744–2754. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lin, L.; Sun, B.; Zhao, M. A Comparison Study on Polysaccharides Extracted from Laminaria Japonica Using Different Methods: Structural Characterization and Bile Acid-Binding Capacity. Food Funct. 2017, 8, 3043–3052. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, T.; Mao, G.; Zhang, M.; Zheng, D.; Feng, W.; Wang, W.; Wu, X.; Yang, L. Isolation, Purification and Characterisation of Selenium-Containing Polysaccharides and Proteins in Selenium-Enriched Radix Puerariae: Se-Containing Polysaccharides and Proteins in Radix Puerariae. J. Sci. Food Agric. 2014, 94, 349–358. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Y.; Chen, Z.; Li, T.; Lu, C.; Xu, C. Impact of Molecular Weight and Chain Rigidity on the Endocytosis and Anti-Tumor Activity of Seleno-Lentinan. J. Future Foods 2025, 5, 419–427. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, H.; Yu, J.; Qu, C.; Wang, Q.; Yang, S.; Ma, S.; Ni, J. Quality Control and Immunological Activity of Lentinan Samples Produced in China. Int. J. Biol. Macromol. 2020, 159, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, Y.; Lai, Z.; Hu, X.; Wang, L.; Wang, X.; Li, Z.; Gao, M.; Yang, Y.; Wang, Q.; et al. Effect of Monosaccharide Composition and Proportion on the Bioactivity of Polysaccharides: A Review. Int. J. Biol. Macromol. 2024, 254, 127955. [Google Scholar] [CrossRef]
- Li, Z.; Nie, K.; Wang, Z.; Luo, D. Quantitative Structure Activity Relationship Models for the Antioxidant Activity of Polysaccharides. PLoS ONE 2016, 11, e0163536. [Google Scholar] [CrossRef]
- Mawhinney, M.T.; Liu, R.; Lu, F.; Maksimoska, J.; Damico, K.; Marmorstein, R.; Lieberman, P.M.; Urbanc, B. CTCF-Induced Circular DNA Complexes Observed by Atomic Force Microscopy. J. Mol. Biol. 2018, 430, 759–776. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.; Ma, L.; Zhang, Z.; Cao, L.; Liu, J.; Zeng, X. Preparation, Preliminary Characterization and Immunostimulatory Activity of Polysaccharide Fractions from the Peduncles of Hovenia Dulcis. Food Chem. 2013, 138, 41–47. [Google Scholar] [CrossRef]
- Ning, Z.; Zhai, L.; Huang, T.; Peng, J.; Hu, D.; Xiao, H.; Wen, B.; Lin, C.; Zhao, L.; Bian, Z. Identification of α-Glucosidase Inhibitors from Cyclocarya Paliurus Tea Leaves Using UF-UPLC-Q/TOF-MS/MS and Molecular Docking. Food Funct. 2019, 10, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-H.; Jin, M.-L.; Morris, G.A.; Zha, X.-Q.; Chen, H.-Q.; Yi, Y.; Li, J.-E.; Wang, Z.-J.; Gao, J.; Nie, S.-P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56, S60–S84. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Qiu, Y.; Wei, X.; Li, Z.; Hu, Z.; Gu, Y.; Zhao, Y.; Wang, Y.; Yue, T.; Yuan, Y. Characterization of Selenium-Containing Polysaccharides Isolated from Selenium-Enriched Tea and Its Bioactivities. Food Chem. 2020, 316, 126371. [Google Scholar] [CrossRef]
- Huang, F.; Sun, X.-Y.; Ouyang, J.-M. Preparation and Characterization of Selenized Astragalus Polysaccharide and Its Inhibitory Effect on Kidney Stones. Mater. Sci. Eng. C 2020, 110, 110732. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhao, Y.; Yu, J.; Ji, H.; Liu, A. Characterization of Se-Enriched Pleurotus Ostreatus Polysaccharides and Their Antioxidant Effects in Vitro. Int. J. Biol. Macromol. 2018, 111, 421–429. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Wang, J.; Guo, L.; Chu, Y.; Ma, C.; Kang, W. Structural Characterization, Chain Conformation and Immunomodulatory Activity of a Heteropolysaccharide from Inonotus Hispidus. Int. J. Biol. Macromol. 2024, 260, 129187. [Google Scholar] [CrossRef]
- Yuan, D.; Li, C.; You, L.; Dong, H.; Fu, X. Changes of Digestive and Fermentation Properties of Sargassum Pallidum Polysaccharide after Ultrasonic Degradation and Its Impacts on Gut Microbiota. Int. J. Biol. Macromol. 2020, 164, 1443–1450. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, M.; Bu, X.; Qing, Z.; Wang, L.; Xu, Y.; Yang, Y.; Bai, J. Preparation, Characterization, Antioxidant and Antiglycation Activities of Selenized Polysaccharides from Blackcurrant. RSC Adv. 2020, 10, 32616–32627. [Google Scholar] [CrossRef]
- Yang, D.; Min, L.; Zhang, R.; Zhang, H.; Xiong, K.; Zhuang, Y.; Fu, Q.; Mo, K. Antioxidant Properties and Correlation with Chemical Components in Selenium-Enriched Rape Powder. Food Sci. 2024, 45, 60–65. [Google Scholar] [CrossRef]
- Jing, Y.; Yan, M.; Zhang, H.; Liu, D.; Qiu, X.; Hu, B.; Zhang, D.; Zheng, Y.; Wu, L. Effects of Extraction Methods on the Physicochemical Properties and Biological Activities of Polysaccharides from Polygonatum Sibiricum. Foods 2023, 12, 2088. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, Y.-L.; Zhang, Y.; Li, Y.; Liang, J.; Kuang, H.-X.; Xia, Y.-G. Structure and Immunological Activity of an Arabinan-Rich Acidic Polysaccharide from Atractylodes Lancea (Thunb.) DC. Int. J. Biol. Macromol. 2022, 199, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, C.; Sun-Waterhouse, D.; Wang, J.; Neil Waterhouse, G.I.; Kang, W. Immunoregulatory Polysaccharides from Apocynum Venetum L. Flowers Stimulate Phagocytosis and Cytokine Expression via Activating the NF-κB/MAPK Signaling Pathways in RAW264.7 Cells. Food Sci. Hum. Wellness 2022, 11, 806–814. [Google Scholar] [CrossRef]
- Dong, Z.; Dong, G.; Lai, F.; Wu, H.; Zhan, Q. Purification and Comparative Study of Bioactivities of a Natural Selenized Polysaccharide from Ganoderma Lucidum Mycelia. Int. J. Biol. Macromol. 2021, 190, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Chen, J.; Qin, T.; Hu, Y.; Wang, D.; Fan, Q.; Zhang, C.; Chen, X.; Chen, X.; Liu, C.; et al. Effects of Selenylation Modification on Immune-Enhancing Activity of Garlic Polysaccharide. PLoS ONE 2014, 9, e86377. [Google Scholar] [CrossRef]
- Chen, N.; Xi, W.-J.; Hu, M.-F.; Wei, X.-Y.; Xiao, P.; Duan, J.-A. Relationship between immune regulation and structure of polysaccharides. Zhongguo Zhong Yao Za Zhi 2023, 48, 2667–2678. [Google Scholar] [CrossRef]
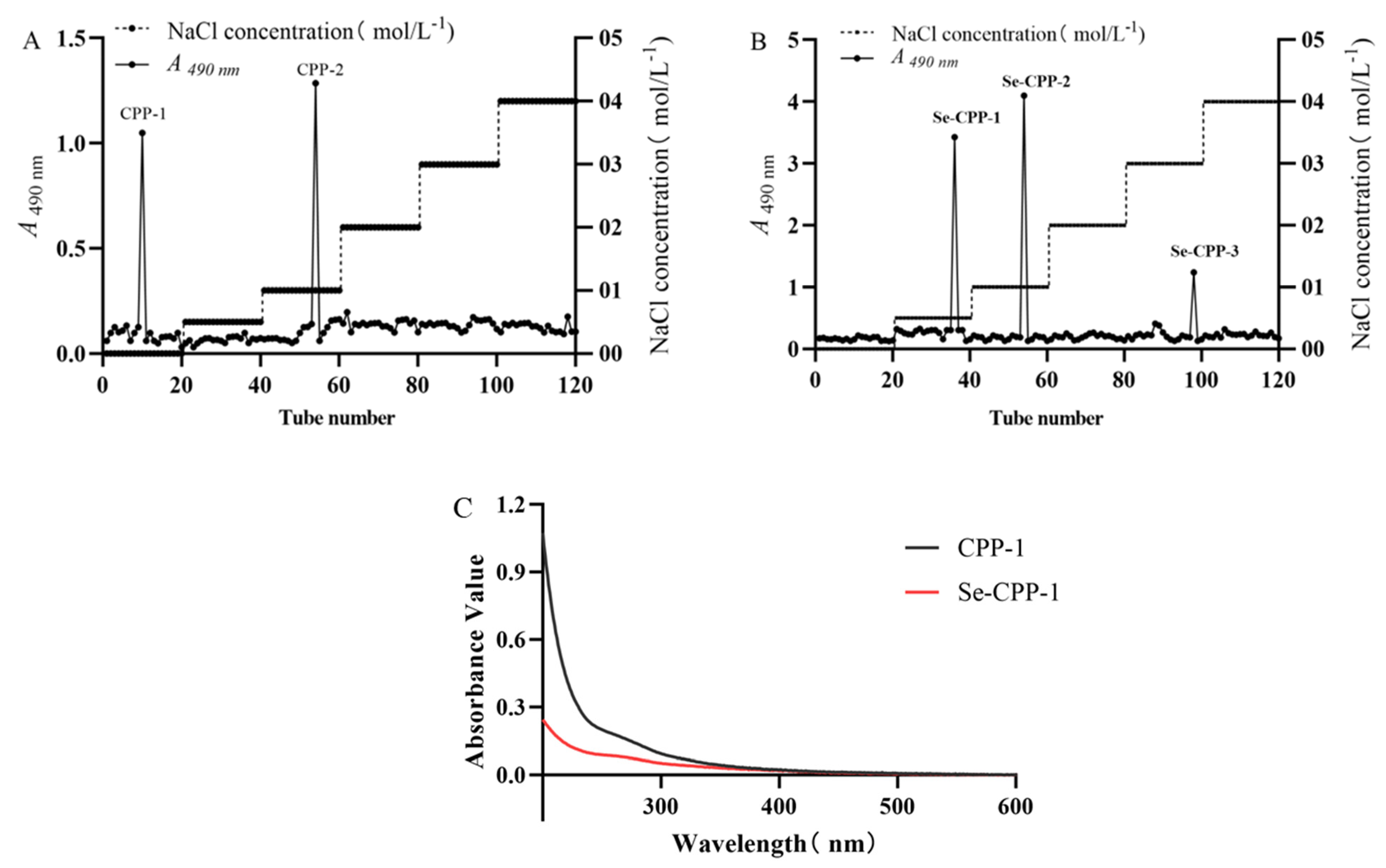

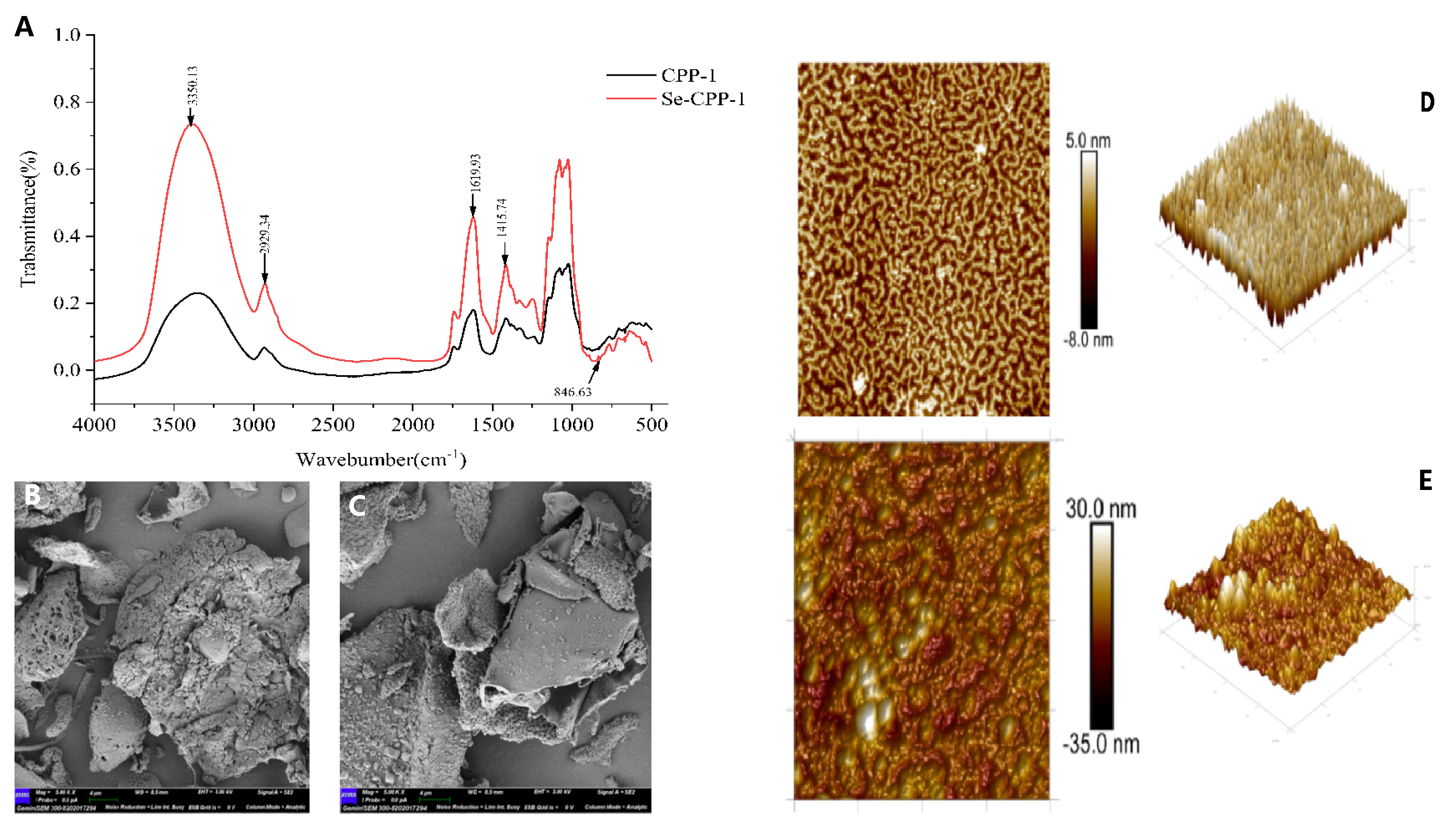
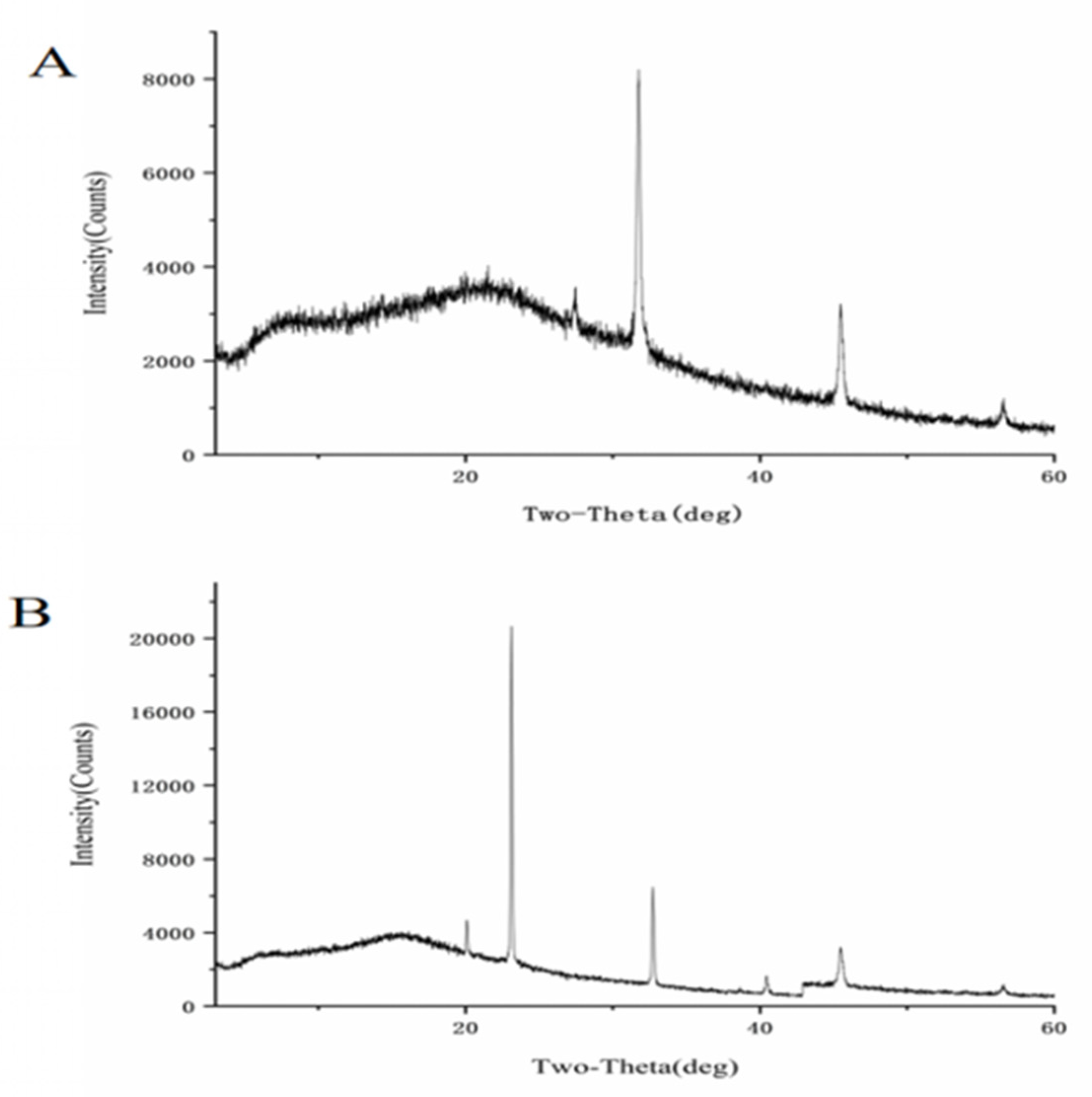

| Fraction | Percentage Composition of Monosaccharides (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fuc | Rha | Ara | Gal | Glc | Xyl | Man | Gal-UA | Glc-UA | |
| CPP-1 | 0.00 | 4.23 | 30.09 | 44.90 | 8.65 | 1.69 | 2.00 | 6.10 | 2.34 |
| Se-CPP-1 | 0.75 | 11.61 | 25.06 | 32.15 | 6.37 | 2.48 | 0.00 | 19.81 | 1.77 |
| Particle Size d/nm | Zetapotential (mV) | |
|---|---|---|
| CPP-1 | 536.96 ± 34.80 a | −21.29 ± 2.00 a |
| Se-CPP-1 | 418.22 ± 50.68 b | −43.15 ± 3.14 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Wang, Z.; Xia, Q.; Chen, J.; Lv, Q.; Zhang, S.; Cheng, S.; Chen, X.; Dong, X. Preparation, Structural Characterization and Biological Activity Study of Selenium-Rich Polysaccharides from Cyclocarya paliurus. Foods 2025, 14, 1641. https://doi.org/10.3390/foods14091641
Dong Y, Wang Z, Xia Q, Chen J, Lv Q, Zhang S, Cheng S, Chen X, Dong X. Preparation, Structural Characterization and Biological Activity Study of Selenium-Rich Polysaccharides from Cyclocarya paliurus. Foods. 2025; 14(9):1641. https://doi.org/10.3390/foods14091641
Chicago/Turabian StyleDong, Yulan, Zijue Wang, Qinghui Xia, Juan Chen, Quanwei Lv, Shaopeng Zhang, Shuiyuan Cheng, Xiaoling Chen, and Xingxing Dong. 2025. "Preparation, Structural Characterization and Biological Activity Study of Selenium-Rich Polysaccharides from Cyclocarya paliurus" Foods 14, no. 9: 1641. https://doi.org/10.3390/foods14091641
APA StyleDong, Y., Wang, Z., Xia, Q., Chen, J., Lv, Q., Zhang, S., Cheng, S., Chen, X., & Dong, X. (2025). Preparation, Structural Characterization and Biological Activity Study of Selenium-Rich Polysaccharides from Cyclocarya paliurus. Foods, 14(9), 1641. https://doi.org/10.3390/foods14091641





