Weiss or Wit: Chemical Profiling of Wheat Beers via NMR-Based Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Wheat Beer Samples
2.2. Sample Preparation
2.3. NMR Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. NMR Profiling of Wheat Beer
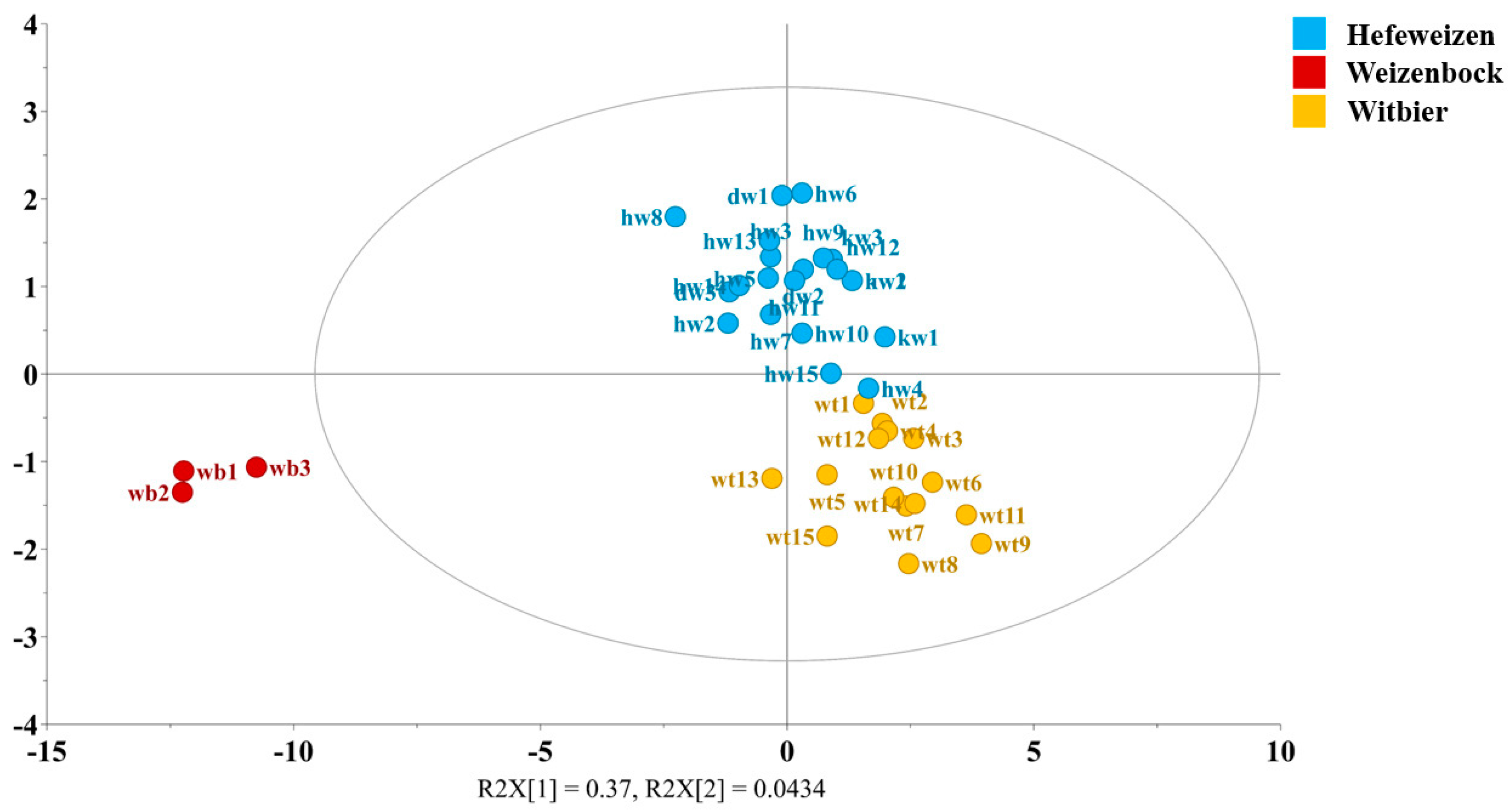
3.2. Differentiation of Hefeweizen, Weizenbock, and Witbier
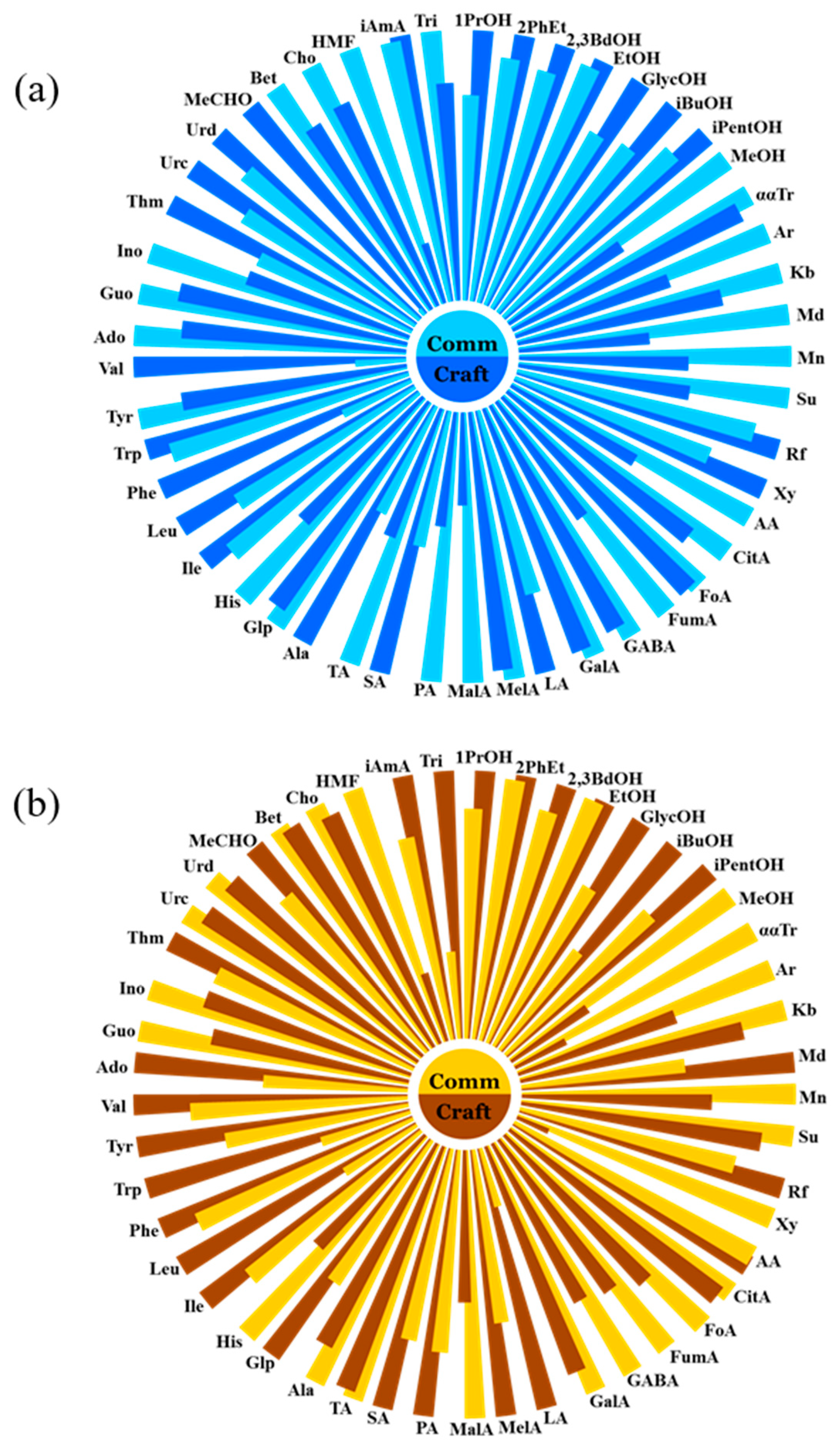
3.3. Comparative Analysis of Craft and Commercial Wheat Beers
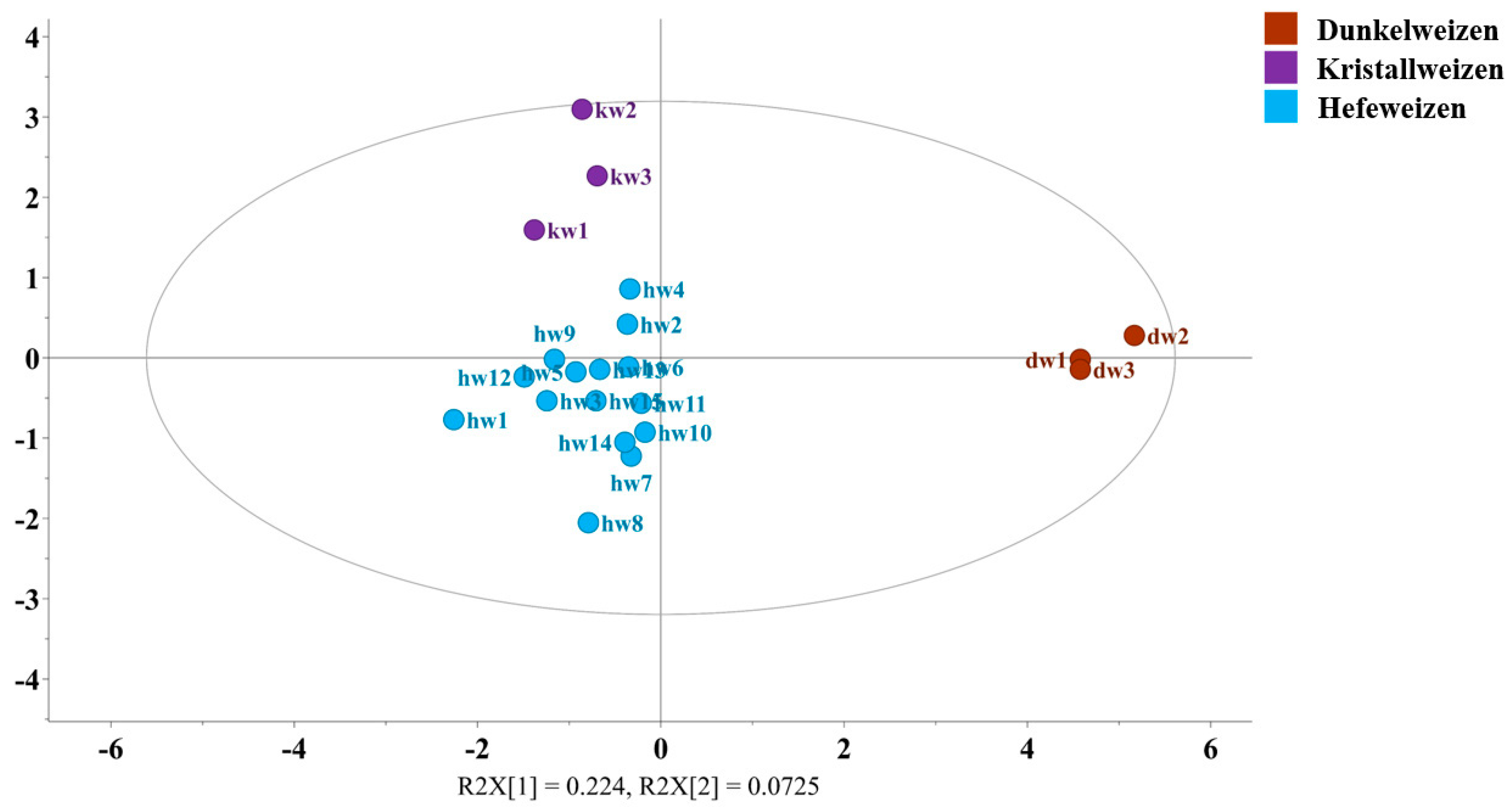
3.4. Metabolomic Differences Between Dunkelweizen, Kristallweizen, and Hefeweizen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista, Global Beer Production 1998–2023; Benchmark International: Tampa, FL, USA, 2024.
- Dineley, M. Barley, Malt And Ale in the Neolithic; Oxbow Books: Oxford, UK, 2004. [Google Scholar]
- Wang, J.; Liu, L.; Ball, T.; Yu, L.; Li, Y.; Xing, F. Revealing a 5000-year-old beer recipe in China. Proc. Natl. Acad. Sci. USA 2016, 113, 6444–6448. [Google Scholar] [CrossRef] [PubMed]
- Verberg, S. The Rise and Fall of Gruit. Brew. Hist. 2018, 174, 46–78. [Google Scholar]
- Anderson, H.E.; Liden, T.; Berger, B.K.; Zanella, D.; Ho Manh, L.; Wang, S.; Schug, K.A. Profiling of Contemporary Beer Styles Using Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry, Multivariate Analysis, and Machine Learning Techniques. Anal. Chim. Acta 2021, 1172, 338668. [Google Scholar] [CrossRef]
- Exactitude Consultancy. Wheat Beer Market Size, Status & Forecast to 2032; Exactitude Consultancy: Pune, India, 2025. [Google Scholar]
- Bamforth, C. Beer: Tap into the Art and Science of Brewing, 4th ed.; Oxford University Press: Oxford, UK, 2024. [Google Scholar]
- De Flaviis, R.; Santarelli, V.; Grilli, S.; Sacchetti, G. An Integrative Multi-Omics Approach Aimed to Gain Insight on the Effect of Composition, Style, Yeast, and Wheat Species on Wheat Craft Beer Flavour. Food Chem. 2024, 441, 138387. [Google Scholar] [CrossRef] [PubMed]
- De Flaviis, R.; Santarelli, V.; Sacchetti, G. Tracking Wheat Variety and Origin by the Shape Analysis of the Volatiles Fingerprint of Wheat Kernels and Wheat Beers. Appl. Sci. 2022, 12, 7854. [Google Scholar] [CrossRef]
- Pater, A.; Satora, P.; Januszek, M. Effect of Coriander Seed Addition at Different Stages of Brewing on Selected Parameters of Low-Alcohol Wheat Beers. Molecules 2024, 29, 844. [Google Scholar] [CrossRef]
- Shen, X.-J.; Chen, I.-H.; Chen, W.-H.; Hsu, Y.-C.; Tsai, C.-H. Field-isolated brewing yeast produces wheat beer flavor without wheat ingredients. LWT - Food Sci. Technol. 2024, 199, 116088. [Google Scholar] [CrossRef]
- Baiano, A.; Fiore, A.; la Gatta, B.; Tufariello, M.; Gerardi, C.; Savino, M.; Grieco, F. Single and Interactive Effects of Unmalted Cereals, Hops, and Yeasts on Quality of White-Inspired Craft Beers. Beverages 2023, 9, 9. [Google Scholar] [CrossRef]
- Palombi, L.; Tufariello, M.; Durante, M.; Fiore, A.; Baiano, A.; Grieco, F. Assessment of the Impact of Unmalted Cereals, Hops, and Yeast Strains on Volatolomic and Olfactory Profiles of Blanche Craft Beers: A Chemometric Approach. Food Chem. 2023, 416, 135783. [Google Scholar] [CrossRef]
- Kabelová, I.; Dvořáková, M.; Čížková, H.; Dostálek, P.; Melzoch, K. Determination of Free Amino Acids in Beers: A Comparison of Czech and Foreign Brands. J. Food Compos. Anal. 2008, 21, 736–741. [Google Scholar] [CrossRef]
- Xu, K.; Guo, M.; Du, J.; Zhang, Z. Cloudy Wheat Beer Enriched with Okra [Abelmoschus esculentus (L.) Moench]: Effects on Volatile Compound and Sensorial Attributes. Int. J. Food Prop. 2018, 21, 289–300. [Google Scholar] [CrossRef]
- Palmioli, A.; Alberici, D.; Ciaramelli, C.; Airoldi, C. Metabolomic Profiling of Beers: Combining ¹H NMR Spectroscopy and Chemometric Approaches to Discriminate Craft and Industrial Products. Food Chem. 2020, 327, 127025. [Google Scholar] [CrossRef]
- Li, X.; Jiang, K.; Jin, Y.; Liu, J. Comparative Study on Protein Composition and Foam Characteristics of Barley and Wheat Beer. Foods 2024, 13, 3400. [Google Scholar] [CrossRef]
- Pferdmenges, L.E.; Schröter, A.; Lohmayer, R.; Striegel, L.; Rychlik, M.; Müller, A.; Meinhardt, A.-K.; Trierweiler, B.; Hartmann, B.M.; Frommherz, L. Characterization of the Nutrient Composition of German Beer Styles for the German Nutrient Database. J. Food Compos. Anal. 2022, 105, 104181. [Google Scholar] [CrossRef]
- Song, Z.; Li, M.; Du, J.; Zhang, K. Effects of Non-Starch Polysaccharides from Pure Wheat Malt Beer on Beer Quality, In Vitro Antioxidant, Prebiotics, Hypoglycemic and Hypolipidemic Properties. Food Biosci. 2022, 47, 101780. [Google Scholar] [CrossRef]
- Li, M.; Du, J.; Zheng, Y. Non-Starch Polysaccharides in Wheat Beers and Barley Malt Beers: A Comparative Study. Foods 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Frank, W.; Humpfer, E.; Schäfer, H.; Keller, S.; Mörtter, M.; Spraul, M. Quality Control of Beer Using High-Resolution Nuclear Magnetic Resonance Spectroscopy and Multivariate Analysis. Eur. Food Res. Technol. 2005, 220, 215–221. [Google Scholar] [CrossRef]
- Mascia, I.; Fadda, C.; Karabín, M.; Dostálek, P.; Del Caro, A. Aging of Craft Durum Wheat Beer Fermented with Sourdough Yeasts. LWT - Food Sci. Technol. 2016, 65, 487–494. [Google Scholar] [CrossRef]
- Lee, B.; Shahin, F.; Selim, A.; Berjanskii, M.; Torres-Calzada, C.; Kovur, P.; Mandal, R.; Wishart, D. Automated Beer Analysis by NMR Spectroscopy. ACS Food Sci. Technol. 2025, 5, 378–388. [Google Scholar] [CrossRef]
- Vasas, M.; Tang, F.; Hatzakis, E. Application of NMR and Chemometrics for the Profiling and Classification of Ale and Lager American Craft Beer. Foods 2021, 10, 807. [Google Scholar] [CrossRef]
- Kaufmann, M.; Schwarz, K.J.; Dallmann, A.; Kuballa, T.; Bergmann, M. ¹H NMR Spectroscopic Discrimination of Different Beer Styles Combined with a Chemical Shift-Based Quantification Approach. Eur. Food Res. Technol. 2022, 248, 635–645. [Google Scholar] [CrossRef]
- Gerginova, D.; Chorbadzhiev, P.; Simova, S. Metabolomic Nuclear Magnetic Resonance Insights into Wine and Grape Ale Maturation. Beverages 2025, 11, 29. [Google Scholar] [CrossRef]
- Almeida, C.; Duarte, I.F.; Barros, A.; Rodrigues, J.; Spraul, M.; Gil, A.M. Composition of Beer by ¹H NMR Spectroscopy: Effects of Brewing Site and Date of Production. J. Agric. Food Chem. 2006, 54, 700–706. [Google Scholar] [CrossRef] [PubMed]
- OIV (International Organisation of Vine and Wine). Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2024. [Google Scholar]
- Grieco, F.; Fiore, A.; Gerardi, C.; Tufariello, M.; Romano, G.; Baiano, A. Optimisation of Quality Features of New Wheat Beers Fermented Through Sequential Inoculation of Non-Saccharomyces and Saccharomyces Yeasts. Heliyon 2024, 10, e37598. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, Y.-S. Analysis of Methanol, Fusel Alcohols, and Other Volatile Compounds of Local Specialty Alcoholic Beverages (Wine, Beer, and Soju) in Korea. Appl. Biol. Chem. 2025, 68, 11. [Google Scholar] [CrossRef]
- Zamani, N.; Rafizadeh, A.; Hassanian-Moghaddam, H.; Akhavan-Tavakoli, A.; Ghorbani-Samin, M.; Akhgari, M.; Shariati, S. Evaluation of Methanol Content of Illegal Beverages Using GC and an Easier Modified Chromotropic Acid Method: A Cross Sectional Study. Subst. Abuse Treat. Prev. Policy 2020, 15, 1. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Alcohol Drinking; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 44; IARC: Lyon, France, 1988. [Google Scholar]
- Annemüller, G.; Manger, H.-J. Gärung und Reifung des Bieres: Grundlagen, Technologie, Anlagentechnik, 3rd ed.; VLB Berlin: Berlin, Germany, 2020. [Google Scholar]
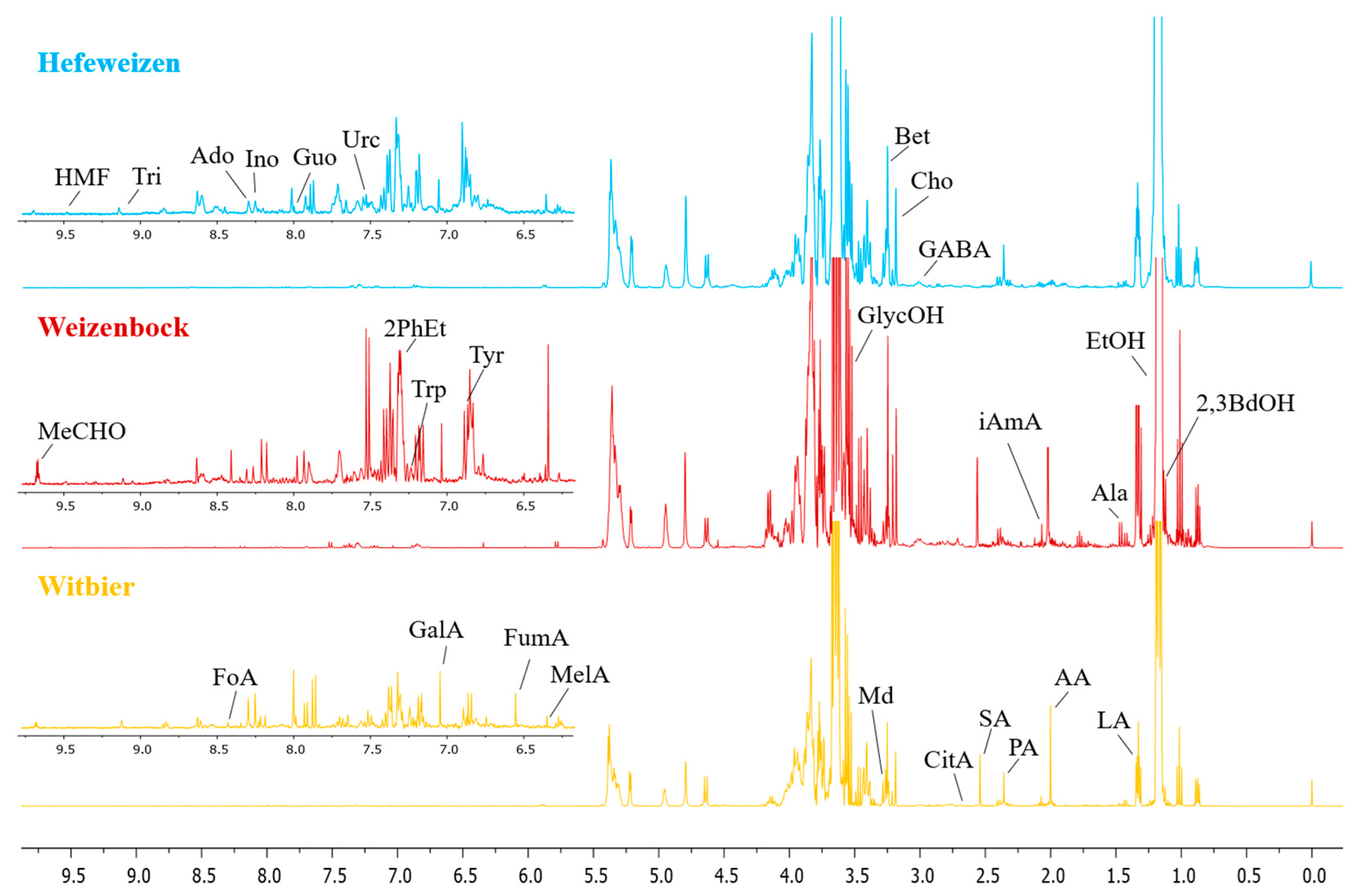
| Weissbier (Hefeweizen) | Weizenbock | Witbier (Blanche) | 1H δ in ppm | |
|---|---|---|---|---|
| min–max average | min–max average | min–max average | (multiplicities) | |
| Alcohols | ||||
| 1-propanol (1PrOH) | 9.3–50.1 24.8 | 22.7–38.2 32.9 | 9.6–22.2 15.6 | 1.54 (m) |
| 2-phenylethanol (2PhEt) | 18.8–64.7 41.6 | 36.9–134.1 73.0 | 21.4–57.2 30.4 | 7.31 (m) |
| 2,3-butanediol (2,3BdOH) | 56.2–256.5 132.0 | 120.0–237.7 187.7 | 74.4–138.4 101.0 | 1.13 (d) |
| Ethanol (EtOH) | 16,473.6–60,133.4 33,840.4 | 62,325.3–75,715.9 67,943.6 | 22,692.9–40,044.5 30,184.2 | 1.17 (t) |
| Glycerol (GlycOH) | 1172.2–3600.7 1915.7 | 3167.8–3963.8 3489.1 | 1233.6–2745.4 1663.8 | 3.55 (dd) |
| Isobutanol (iBuOH) | 27.7–103.2 58.9 | 58.3–235.0 134.9 | 9.8–59.2 31.3 | 1.74 (m) |
| Isopentanol (iPentOH) | 26.4–123.7 62.4 | 88.9–159.2 120.8 | 34.4–88.4 51.5 | 1.43 (q) |
| Methanol (MeOH) | 1.7–18.3 4.8 | 4.9–11.3 7.5 | 0.5–15.9 3.5 | 3.35 (s) |
| Saccharides | ||||
| αα-Trehalose (ααTr) | 2.2–101.9 21.3 | 12.4–56.4 29.9 | 6.1–95.3 21.4 | 5.18 (d) |
| Arabinose (Ar) | 6.4–61.6 31.8 | 25.5–68.8 46.8 | 16.6–50.2 32.7 | 4.49 (d) |
| Fructose (Fr) | - | - | 0.0–1724.0 114.9 | 4.03 (d) |
| Kojibiose (Kb) | 42.6–274.1 164.4 | 117.9–619.1 370.7 | 27.3–306.0 136.3 | 5.09 (d) |
| Maltodextrin (Md) | 2287.5–16,466.7 8592.7 | 11,073.9–30,777.6 21,468.3 | 4948.8–21,812.3 10,275.7 | 3.26 (dd) |
| Mannose (Mn) | 5.9–55.9 23.3 | 23.0–59.1 46.2 | 10.6–36.2 25.2 | 5.17 (d) |
| Sucrose (Su) | 12.0–253.3 96.5 | 49.3–157.5 105.7 | 22.1–238.7 113.0 | 4.20 (d) |
| Raffinose (Rf) | 21.5–149.1 65.4 | 75.5–144.0 115.2 | 12.8–105.8 34.7 | 5.02 (d) |
| Xylose (Xy) | 5.9–216.1 50.4 | 29.7–170.1 89.7 | 5.2–205.1 51.5 | 5.17 (d) |
| Organic acids | ||||
| Acetic acid (AA) | 29.5–715.5 207.6 | 423.7–922.5 653.9 | 18.6–286.6 106.8 | 2.00 (s) |
| Citric acid (CitA) | 0.0–201.8 108.6 | 73.8–196.0 123.8 | 0.0–144.8 82.5 | 2.65 (d) |
| Formic acid (FoA) | 0.0–17.5 3.5 | 4.0–13.2 7.6 | 0.6–4.9 2.3 | 8.42 (s) |
| Fumaric acid (FumA) | 0.5–6.6 2.8 | 0.5–6.4 4.2 | 0.5–8.6 3.4 | 6.54 (s) |
| GABA | 15.7–161.6 65.6 | 76.7–205.7 143.2 | 7.6–104.3 37.6 | 3.02 (t) |
| Gallic acid (GalA) | 6.4–13.2 9.7 | 10.5–20.0 14.4 | 6.2–15.4 10.0 | 7.04 (s) |
| Lactic acid (LA) | 44.8–1229.9 334.6 | 108.8–963.9 476.7 | 66.2–568.6 285.3 | 1.33 (d) |
| Maleic acid (MelA) | 0.8–9.2 2.8 | 4.3–19.9 9.7 | 1.2–5.0 2.2 | 6.35 (s) |
| Malic acid (MalA) | 0.0–150.8 66.6 | 41.6–106.7 74.4 | 0.0–201.5 78.4 | 2.76 (dd) |
| Pyruvic acid (PA) | 11.4–170.7 59.9 | 10.0–107.4 44.4 | 1.4–114.3 54.2 | 2.36 (s) |
| Succinic acid (SA) | 0.0–418.7 136.7 | 141.2–360.9 231.3 | 43.8–196.3 113.2 | 2.54 (s) |
| Tartaric acid (TA) | 1.2–17.8 5.4 | 7.6–45.1 22.4 | 1.9–13.4 5.0 | 4.56 (s) |
| Amino acids | ||||
| Alanine (Ala) | 2.4–85.6 31.9 | 115.2–198.3 167.4 | 4.4–148.1 33.3 | 1.47 (d) |
| Histidine (His) | 0.0–4.9 2.4 | 1.2–6.9 3.7 | 0.8–3.5 1.7 | 7.84 (s) |
| Isoleucine (Ile) | 1.0–21.3 6.9 | 19.0–68.9 49.4 | 1.0–20.5 8.4 | 1.00 (d) |
| Leucine (Leu) | 1.0–36.8 15.5 | 56.4–115.5 89.0 | 1.5–42.6 10.8 | 0.95 (d) |
| Phenylalanine (Phe) | 0.7–65.4 21.7 | 74.8–141.0 108.7 | 0.0–70.0 16.2 | 7.42 (m) |
| Pyroglutamic acid (Glp) | 56.9–311.2 139.8 | 214.4–313.7 277.3 | 24.0–130.8 83.5 | 2.49 (m) |
| Tryptophan (Trp) | 6.6–40.9 21.2 | 5.7–63.8 36.0 | 6.7–82.2 24.7 | 7.25 (t) |
| Tyrosine (Tyr) | 12.2–43.7 22.6 | 41.1–104.1 81.6 | 6.4–52.0 26.3 | 6.88 (d) |
| Valine (Val) | 0.7–48.7 11.3 | 97.5–152.4 116.3 | 1.0–79.4 16.3 | 0.98 (d) |
| Nucleosides and nucleobases | ||||
| Adenosine (Ado) | 0.0–29.5 14.9 | 0.5–20.0 8.0 | 0.0–56.7 18.4 | 8.36 (s) |
| Guanosine (Guo) | 7.4–62.6 24.6 | 35.7–87.6 63.3 | 6.1–30.7 15.2 | 7.98 (s) |
| Inosine (Ino) | 0.0–17.7 8.3 | 5.1–15.4 10.1 | 0.0–14.2 4.9 | 8.34 (s) |
| Thymidine (Thm) | 3.1–34.7 12.6 | 7.3–32.9 22.5 | 2.4–8.1 4.6 | 7.65 (s) |
| Uracil (Urc) | 8.0–57.9 21.8 | 36.2–107.6 63.2 | 2.6–37.1 13.3 | 7.53 (d) |
| Uridine (Urd) | 0.0–79.6 45.3 | 18.3–123.7 75.2 | 0.0–83.9 42.6 | 7.87 (d) |
| Other | ||||
| Acetaldehyde (MeCHO) | 0.2–20.5 2.7 | 0.5–7.4 2.9 | 0.0–4.8 1.2 | 9.67 (q) |
| Betaine (Bet) | 75.8–244.3 143.6 | 220.6–290.5 259.0 | 92.0–210.7 134.1 | 3.25 (s) |
| Choline (Cho) | 29.6–151.0 92.2 | 159.8–252.3 196.3 | 31.4–117.5 66.2 | 3.19 (s) |
| Hydroxymethylfurfural (HMF) | 0.0–2.4 0.7 | 0.3–1.6 0.9 | 0.5–7.3 1.4 | 9.45 (s) |
| Isoamyl acetate (iAmA) | 10.7–91.4 39.3 | 57.8–86.2 75.1 | 0.0–65.6 25.2 | 2.07 (s) |
| Trigonelline (Tri) | 1.0–7.0 3.2 | 4.3–8.8 6.4 | 1.3–9.1 4.0 | 9.11 (s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chorbadzhiev, P.; Gerginova, D.; Simova, S. Weiss or Wit: Chemical Profiling of Wheat Beers via NMR-Based Metabolomics. Foods 2025, 14, 1621. https://doi.org/10.3390/foods14091621
Chorbadzhiev P, Gerginova D, Simova S. Weiss or Wit: Chemical Profiling of Wheat Beers via NMR-Based Metabolomics. Foods. 2025; 14(9):1621. https://doi.org/10.3390/foods14091621
Chicago/Turabian StyleChorbadzhiev, Plamen, Dessislava Gerginova, and Svetlana Simova. 2025. "Weiss or Wit: Chemical Profiling of Wheat Beers via NMR-Based Metabolomics" Foods 14, no. 9: 1621. https://doi.org/10.3390/foods14091621
APA StyleChorbadzhiev, P., Gerginova, D., & Simova, S. (2025). Weiss or Wit: Chemical Profiling of Wheat Beers via NMR-Based Metabolomics. Foods, 14(9), 1621. https://doi.org/10.3390/foods14091621







