Occurrence of Pesticides, Mycotoxins, and Heavy Metals in Distilled Alcoholic Beverages: A Review of Contaminants and Health Risks
Abstract
1. Introduction
2. Regulation and Legislation
3. Climate Change and Emerging Environmental Risks
| Contaminant Type | Examples | Origin | Influencing Factors | Literature | |
|---|---|---|---|---|---|
| Pesticides | herbicides fungicides insecticides acaricide plant growth regulators | grain raw materials residues fruit raw materials residues | pesticide stability distillation efficiency | [13,27] | |
| Mycotoxins | AFT DON FUM ZEA T-2/HT-2 OTA PAT | fungal contamination of raw materials | climate conditions storage conditions | [28,29] | |
| Heavy Metals | Essential | Cu Zn Co Cr Mn Fe | environmental pollution equipment contamination | water source soil contamination distillation setup | [4] |
| Non-essential | Pb Cd Hg As | ||||
4. Occurrence of Mycotoxins in Cereal-Based Raw Materials
5. Occurrence of Pesticides in Fruit-Based Raw Materials
6. Metals in Alcoholic Beverages and Public Health Implications
7. Migration into Distillates
8. Risk Assessment
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 15-ADON | 15-acetyl-deoxynivalenol |
| 3-ADON | 3-acetyl-deoxynivalenol |
| AFB1 | aflatoxin B1 |
| AFB2 | aflatoxin B2 |
| AFG1 | aflatoxin G1 |
| AFG2 | aflatoxin G2 |
| AFT | aflatoxins |
| As | arsenic |
| Cd | cadmium |
| Co | cobalt |
| Cr | chromium |
| Cu | copper |
| D3G | deoxinyvalenol-3-glucoside |
| DON | deoxynivalenol |
| FB1 | fumonisin B1 |
| Fe | iron |
| FUM | fumonisins |
| Hg | mercury |
| IARC | International Agency for Research on Cancer |
| MCPA | 4-chloro-2-methylphenoxyacetic acid |
| ML | Maximum Level |
| Mn | manganese |
| MRL | Maximum Residue Level |
| Ni | nickel |
| NIV | nivalenol |
| OTA | ochratoxin A |
| PAT | patulin |
| Pb | lead |
| Rf | retention factor |
| SDHI | succinate dehydrogenase inhibitors |
| Sn | tin |
| THPI | tetrahydrophthalimide |
| TLC | thin-layer chromatography |
| ZEA | zearalenone |
| Zn | zinc |
References
- REGULATION (EU) 2019/787 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 17 April 2019 on the Definition, Description, Presentation and Labelling of Spirit Drinks, the Use of the Names of Spirit Drinks in the Presentation and Labelling of Other Foodstuffs, the Protection of Geographical Indications for Spirit Drinks, the Use of Ethyl Alcohol and Distillates of Agricultural Origin in Alcoholic Beverages, and Repealing Regulation (EC) No 110/2008. Available online: https://eur-lex.europa.eu/ (accessed on 23 February 2025).
- He, N.X.; Bayen, S. An Overview of Chemical Contaminants and Other Undesirable Chemicals in Alcoholic Beverages and Strategies for Analysis. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3916–3950. [Google Scholar] [CrossRef]
- Pang, X.N.; Li, Z.J.; Chen, J.Y.; Gao, L.J.; Han, B.Z. A Comprehensive Review of Spirit Drink Safety Standards and Regulations from an International Perspective. J. Food Prot. 2017, 80, 431–442. [Google Scholar] [CrossRef]
- Pál, L.; Muhollari, T.; Bujdosó, O.; Baranyai, E.; Nagy, A.; Árnyas, E.; Ádány, R.; Sándor, J.; McKee, M.; Szűcs, S. Heavy Metal Contamination in Recorded and Unrecorded Spirits. Should We Worry? Regul. Toxicol. Pharmacol. 2020, 116, 104723. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Sun, B. Chinese Baijiu and Whisky: Research Reservoirs for Flavor and Functional Food. Foods 2023, 12, 2841. [Google Scholar] [CrossRef]
- Stanzer, D.; Hanousek Čiča, K.; Blesić, M.; Smajić Murtić, M.; Mrvčić, J.; Spaho, N. Alcoholic Fermentation as a Source of Congeners in Fruit Spirits. Foods 2023, 12, 1951. [Google Scholar] [CrossRef]
- Carballo, D.; Fernández-Franzón, M.; Ferrer, E.; Pallarés, N.; Berrada, H. Dietary Exposure to Mycotoxins through Alcoholic and Non-Alcoholic Beverages in Valencia, Spain. Toxins 2021, 13, 438. [Google Scholar] [CrossRef]
- Rovetto, E.I.; Luz, C.; La Spada, F.; Meca, G.; Riolo, M.; Cacciola, S.O. Diversity of Mycotoxins and Other Secondary Metabolites Recovered from Blood Oranges Infected by Colletotrichum, Alternaria, and Penicillium Species. Toxins 2023, 15, 407. [Google Scholar] [CrossRef]
- Pleadin, J.; Zadravec, M.; Lešić, T.; Frece, J.; Markov, K.; Vasilj, V. Climate Change—A Potential Threat for Increasing Occurrences of Mycotoxins. Vet. Stanica 2020, 51, 659–671. [Google Scholar] [CrossRef]
- Petrović, E.; Ćosić, J.; Vrandečić, K.; Godena, S. Occurrence of Mycotoxins in Food and Beverages. J. Cent. Eur. Agric. 2023, 24, 137–150. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Fattore, M.; Albrizio, S.; Berrada, H.; Mañes, J. Occurrence of Fusarium Mycotoxins and Their Dietary Intake through Beer Consumption by the European Population. Food Chem. 2015, 178, 149–155. [Google Scholar] [CrossRef]
- Shin, J.; Luo, Y. Relationship between Exposure to Multiple Heavy Metals and Depressive Symptoms in the US: The Impact of Alcohol Consumption. Heliyon 2024, 10, e40221. [Google Scholar] [CrossRef]
- Shin, J.A.; Cho, H.; Seo, D.W.; Jeong, H.G.; Kim, S.C.; Lee, J.H.; Hong, S.T.; Lee, K.T. Approach Study for Mass Balance of Pesticide Residues in Distillers’ Stillage along with Distillate and Absence Verification of Pesticides in Distilled Spirits from Pilot-Scale of Distillation Column. Molecules 2019, 24, 2572. [Google Scholar] [CrossRef]
- Urkude, R.; Dhurvey, V.; Kochhar, S. Pesticide Residues in Beverages. In Quality Control in the Beverage Industry: Volume 17: The Science of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 529–560. ISBN 9780128166819. [Google Scholar]
- Wallace, D.R.; Buha Djordjevic, A. Heavy Metal and Pesticide Exposure: A Mixture of Potential Toxicity and Carcinogenicity. Curr. Opin. Toxicol. 2020, 19, 72–79. [Google Scholar] [CrossRef]
- REGULATION (EC) No 396/2005 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC (Text with EEA Relevance). 2006. Available online: https://eur-lex.europa.eu/ (accessed on 24 February 2025).
- COMMISSION REGULATION (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance). 2023. Available online: https://eur-lex.europa.eu/ (accessed on 25 February 2025).
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate Changes and Food Quality: The Potential of Microbial Activities as Mitigating Strategies in the Wine Sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef]
- Casu, A.; Camardo Leggieri, M.; Toscano, P.; Battilani, P. Changing Climate, Shifting Mycotoxins: A Comprehensive Review of Climate Change Impact on Mycotoxin Contamination. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13323. [Google Scholar] [CrossRef]
- Kovač, M.; Bulaić, M.; Jakovljević, J.; Nevistić, A.; Rot, T.; Kovač, T.; Šarkanj, I.D.; Šarkanj, B. Mycotoxins, Pesticide Residues, and Heavy Metals Analysis of Croatian Cereals. Microorganisms 2021, 9, 216. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Gelderblom, W.C.A.; Shephard, G.S.; Vismer, H.F. Mycotoxins: A Global Problem. In Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; CABI Publishing: Oxfordshire, UK, 2008; pp. 29–39. ISBN 9781845930820. [Google Scholar]
- Fernández-Cruz, M.L.; Mansilla, M.L.; Tadeo, J.L. Mycotoxins in Fruits and Their Processed Products: Analysis, Occurrence and Health Implications. J. Adv. Res. 2010, 1, 113–122. [Google Scholar] [CrossRef]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin Risks under a Climate Change Scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Post-Harvest Control Strategies: Minimizing Mycotoxins in the Food Chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 Contamination in Maize in Europe Increases Due to Climate Change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Inoue, T.; Nagatomi, Y.; Kinami, T.; Uyama, A.; Mochizuki, N. Fate of Pesticides in a Distilled Spirit of Barley Shochu during the Distillation Process. Biosci. Biotechnol. Biochem. 2010, 74, 2518–2522. [Google Scholar] [CrossRef]
- Shin, J.A.; Lee, K.T. Migration Degree of Selected Mycotoxins in the Distillation Process and Their Determination in Distilled Spirits from Pilot-Scale Continuous Distillation. Foods 2023, 12, 4189. [Google Scholar] [CrossRef]
- Nagatomi, Y.; Inoue, T.; Uyama, A.; Mochizuki, N. The Fate of Mycotoxins during the Distillation Process of Barley Shochu, a Distilled Alcoholic Beverage. Biosci. Biotechnol. Biochem. 2012, 76, 202–204. [Google Scholar] [CrossRef]
- Delcour, I.; Spanoghe, P.; Uyttendaele, M. Literature Review: Impact of Climate Change on Pesticide Use. Food Res. Int. 2015, 68, 7–15. [Google Scholar] [CrossRef]
- Martínez-Megías, C.; Mentzel, S.; Fuentes-Edfuf, Y.; Moe, S.J.; Rico, A. Influence of Climate Change and Pesticide Use Practices on the Ecological Risks of Pesticides in a Protected Mediterranean Wetland: A Bayesian Network Approach. Sci. Total Environ. 2023, 878, 163018. [Google Scholar] [CrossRef]
- Tsenang, M.; Pheko-ofitlhile, T.; Mokgadi, J.; Masamba, W.; Phokedi, G.N. A Validated ICP-MS Method for the Screening and Quantitative Analysis of Heavy Metal Contaminants in Home-Brewed Alcoholic Beverages of Botswana. Food Humanit. 2023, 1, 1125–1133. [Google Scholar] [CrossRef]
- Witkowska, D.; Słowik, J.; Chilicka, K. Review Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef]
- Jeričević, A.; Ilyin, I.; Vidič, S. Modelling of Heavy Metals: Study of Impacts Due to Climate Change. NATO Sci. Peace Secur. Ser. C Environ. Secur. 2012, 125, 175–189. [Google Scholar] [CrossRef]
- Udom, G.J.; Turyahabwe, B.; Aturamu, A.; Aziakpono, O.M.; Agbana, R.D.; Joseph, O.G.; Udom, N.W.G.; Mugide, N.; Odey, O.P.; Olot, H.; et al. Heavy Metal and Metalloid Pollution: A Systematic Review of Health Implications for Pregnant Women, Children, and Geriatrics in the East African Region. Environ. Adv. 2025, 19, 100620. [Google Scholar] [CrossRef]
- Ługowoj, S.; Balcerek, M. Traditional and New Raw Materials for Spirit Beverage Production. Acta Univ. Lodziensis. Folia Biol. Oecologica 2021, 17, 70–78. [Google Scholar] [CrossRef]
- Kovač, M.; Bulaić, M.; Nevistić, A.; Rot, T.; Babić, J.; Panjičko, M.; Kovač, T.; Šarkanj, B. Regulated Mycotoxin Occurrence and Co-Occurrence in Croatian Cereals. Toxins 2022, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as Human Carcinogens—The IARC Monographs Classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Ameye, M.; Phan, L.T.K.; Devlieghere, F.; De Saeger, S.; Eeckhout, M.; Audenaert, K. Post-Harvest Contamination of Maize by Fusarium Verticillioides and Fumonisins Linked to Traditional Harvest and Post-Harvest Practices: A Case Study of Small-Holder Farms in Vietnam. Int. J. Food Microbiol. 2021, 339, 109022. [Google Scholar] [CrossRef]
- Wan, J.; Chen, B.; Rao, J. Occurrence and Preventive Strategies to Control Mycotoxins in Cereal-Based Food. Compr. Rev. Food Sci. Food Saf. 2020, 19, 928–953. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Ruprich, J. Producers and Important Dietary Sources of Ochratoxin A and Citrinin. Toxins 2013, 5, 1574–1586. [Google Scholar] [CrossRef]
- Al-Anati, L.; Petzinger, E. Immunotoxic Activity of Ochratoxin A. J. Vet. Pharmacol. Ther. 2006, 29, 79–90. [Google Scholar] [CrossRef]
- Więckowska, M.; Cichon, N.; Szelenberger, R.; Gorniak, L.; Bijak, M. Ochratoxin A and Its Role in Cancer Development: A Comprehensive Review. Cancers 2024, 16, 3473. [Google Scholar] [CrossRef]
- Abbas, A.; Yli-Mattila, T. Biocontrol of Fusarium Graminearum, a Causal Agent of Fusarium Head Blight of Wheat, and Deoxynivalenol Accumulation: From In Vitro to In Planta. Toxins 2022, 14, 299. [Google Scholar] [CrossRef]
- Kovač, M.; Šubarić, D.; Bulaić, M.; Kovač, T.; Šarkanj, B. Yesterday Masked, Today Modified; What Do Mycotoxins Bring Next? Arch. Ind. Hyg. Toxicol. 2018, 69, 196–214. [Google Scholar] [CrossRef]
- Oluwakayode, A.; Greer, B.; Meneely, J.; Berthiller, F.; Krska, R.; Medina, A. Impact of Environmental Conditions on the Concentrations of Trichothecenes, Their Glucosides, and Emerging Fusarium Toxins in Naturally Contaminated, Irradiated, and Fusarium Langsethiae Inoculated Oats. Toxins 2024, 16, 166. [Google Scholar] [CrossRef] [PubMed]
- Balló, A.; Busznyákné Székvári, K.; Czétány, P.; Márk, L.; Török, A.; Szántó, Á.; Máté, G. Estrogenic and Non-Estrogenic Disruptor Effect of Zearalenone on Male Reproduction: A Review. Int. J. Mol. Sci. 2023, 24, 1578. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Gurikar, C.; Mary George, M.; Gurikar, C. Patulin: A Potentially Harmful Food Contaminant. Artic. Int. J. Chem. Stud. 2022, 10, 11–18. [Google Scholar]
- Zhao, Y.; Xu, W.; Liu, R.; Guo, L.; Liu, P. Determination and Analysis of Patulin in Apples, Hawthorns, and Their Products by High-Performance Liquid Chromatography. Mycotoxin Res. 2024, 40, 235–244. [Google Scholar] [CrossRef]
- Spaho, N. Distillation Techniques in the Fruit Spirits Production. In Distillation—Innovative Applications and Modeling; InTech: London, UK, 2017. [Google Scholar]
- Philippe, V.; Neveen, A.; Marwa, A.; Ahmad Basel, A.Y. Occurrence of Pesticide Residues in Fruits and Vegetables for the Eastern Mediterranean Region and Potential Impact on Public Health. Food Control 2021, 119, 107457. [Google Scholar] [CrossRef]
- Grewal, A.S.; Singla, A.; Kamboj, P.; Dua, J.S. Pesticide Residues in Food Grains, Vegetables and Fruits: A Hazard to Human Health. J. Med. Chem. Toxicol. 2017, 2, 40–46. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of Pesticides Use in Agriculture: Their Benefits and Hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Maksymiv, I. Pesticides: Benefits and Hazards. J. Vasyl Stefanyk Precarpathian Natl. Univ. 2015, 2, 70–76. [Google Scholar] [CrossRef]
- Kowalska, G.; Pankiewicz, U.; Kowalski, R. Assessment of Pesticide Content in Apples and Selected Citrus Fruits Subjected to Simple Culinary Processing. Appl. Sci. 2022, 12, 1417. [Google Scholar] [CrossRef]
- Izah, S.C.; Inyang, I.R.; Angaye, T.C.N.; Okowa, I.P. A Review of Heavy Metal Concentration and Potential Health Implications of Beverages Consumed in Nigeria. Toxics 2017, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Bansal, O.P. Health Impacts of the Potentially Toxic Metals Present in Milk, Dairy Products, Chocolates, Alcoholic and Non-Alcoholic Beverages: A Review. IOSR J. Environ. Sci. 2020, 14, 35–46. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The Essential Metals for Humans: A Brief Overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, L.; Gómez-Nieto, B.; Gismera, M.J.; Sevilla, M.T.; Procopio, J.R. Direct Determination of Copper and Zinc in Alcoholic and Non-Alcoholic Drinks Using High-Resolution Continuum Source Flame Atomic Absorption Spectrometry and Internal Standardization. Spectrochim. Acta Part B At. Spectrosc. 2018, 147, 21–27. [Google Scholar] [CrossRef]
- Ince, M.; Kaplan Ince, O.; Onal, A. Exposure to Copper and Risk Assessment for Human Health via Consumption of Alcoholic Beverages. J. Food Sci. Technol. 2021, 58, 510–519. [Google Scholar] [CrossRef]
- Iwegbue, C.M.A.; Ojelum, A.L.; Bassey, F.I. A Survey of Metal Profiles in Some Traditional Alcoholic Beverages in Nigeria. Food Sci. Nutr. 2014, 2, 724–733. [Google Scholar] [CrossRef]
- Siviero, G.; Cinosi, A.; Monticelli, D.; Seralessandri, L. Determination of Trace Metals in Spirits by Total Reflection X-Ray Fluorescence Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2018, 144, 15–19. [Google Scholar] [CrossRef]
- Muhollari, T.; Szűcs, S.; Sajtos, Z.; McKee, M.; Baranyai, E.; Ádány, R.; Pál, L. Heavy Metals in Unrecorded Albanian Rakia: A Pilot Study on a Potential Public Health Risk. Heliyon 2023, 9, e13717. [Google Scholar] [CrossRef]
- Pelegrín, C.J.; Flores, Y.; Jiménez, A.; Garrigós, M.C. Recent Trends in the Analysis of Chemical Contaminants in Beverages. Beverages 2020, 6, 32. [Google Scholar] [CrossRef]
- Chen, C.Y.; Aggarwal, S.K.; Chung, C.H.; You, C.F. Advanced Mass Spectrometry for Beverage Safety and Forensic. In Safety Issues in Beverage Production: Volume 18: The Science of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–269. ISBN 9780128166796. [Google Scholar]
- Yigit, N.; Velioglu, Y.S. Effects of Processing and Storage on Pesticide Residues in Foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 3622–3641. [Google Scholar] [CrossRef]
- Raters, M.; Matissek, R. Thermal Stability of Aflatoxin B1 and Ochratoxin A. Mycotoxin Res. 2008, 24, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Pietri, A.; Bertuzzi, T.; Agosti, B.; Donadini, G. Transfer of Aflatoxin B1 and Fumonisin B1 from Naturally Contaminated Raw Materials to Beer during an Industrial Brewing Process. Food Addit. Contam. —Part A 2010, 27, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nagatomi, Y.; Uyama, A.; Mochizuki, N. Fate of Mycotoxins during Beer Brewing and Fermentation. Biosci. Biotechnol. Biochem. 2013, 77, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Hall, C.E. Mold and Mycotoxin Problems Encountered during Malting and Brewing. Int. J. Food Microbiol. 2007, 119, 89–94. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Carreon-Alvarez, A.; Barcena-Soto, M.; Casillas, N. Metals in Alcoholic Beverages: A Review of Sources, Effects, Concentrations, Removal, Speciation, and Analysis. J. Food Compos. Anal. 2008, 21, 672–683. [Google Scholar] [CrossRef]
- Muntean, N.; Frențiu, T.; Rákos, G.; Covaci, E. The Influence of the Distillation Process on the Content of Metals in Home- And Industrially-Brewed Alcoholic Beverages—Risk Assessment to Human Health. Stud. Univ. Babes-Bolyai Chem. 2022, 67, 215–234. [Google Scholar] [CrossRef]
- Codex Alimentarius, General Standard for Food Additives Codex STAN 192-1995, 8 July 1995. 1995. Available online: https://www.fao.org/ (accessed on 26 February 2025).
- Chlebicz, A.; Śliżewska, K. In Vitro Detoxification of Aflatoxin B1, Deoxynivalenol, Fumonisins, T-2 Toxin and Zearalenone by Probiotic Bacteria from Genus Lactobacillus and Saccharomyces Cerevisiae Yeast. Probiotics Antimicrob. Proteins 2020, 12, 289–301. [Google Scholar] [CrossRef]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin Occurrence in Feed and Feed Raw Materials Worldwide: Long-Term Analysis with Special Focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Louppis, A.P.; Kontominas, M.G. Recent Developments (2020–2023) on the Use of LC in the Determination of Food Contaminants. Separations 2024, 11, 342. [Google Scholar] [CrossRef]
- Brouziotis, A.A.; Heise, S.; Saviano, L.; Zhang, K.; Giarra, A.; Bau, M.; Tommasi, F.; Guida, M.; Libralato, G.; Trifuoggi, M. Levels of Rare Earth Elements on Three Abandoned Mining Sites of Bauxite in Southern Italy: A Comparison between TXRF and ICP-MS. Talanta 2024, 275, 126093. [Google Scholar] [CrossRef] [PubMed]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Gorniak, L.; Stela, M.; Bijak, M. The Existing Methods and Novel Approaches in Mycotoxins’ Detection. Molecules 2021, 26, 3981. [Google Scholar] [CrossRef] [PubMed]
- Dearfield, K.L.; Hoelzer, K.; Kause, J.R. Review of Various Approaches for Assessing Public Health Risks in Regulatory Decision Making: Choosing the Right Approach for the Problem. J. Food Prot. 2014, 77, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
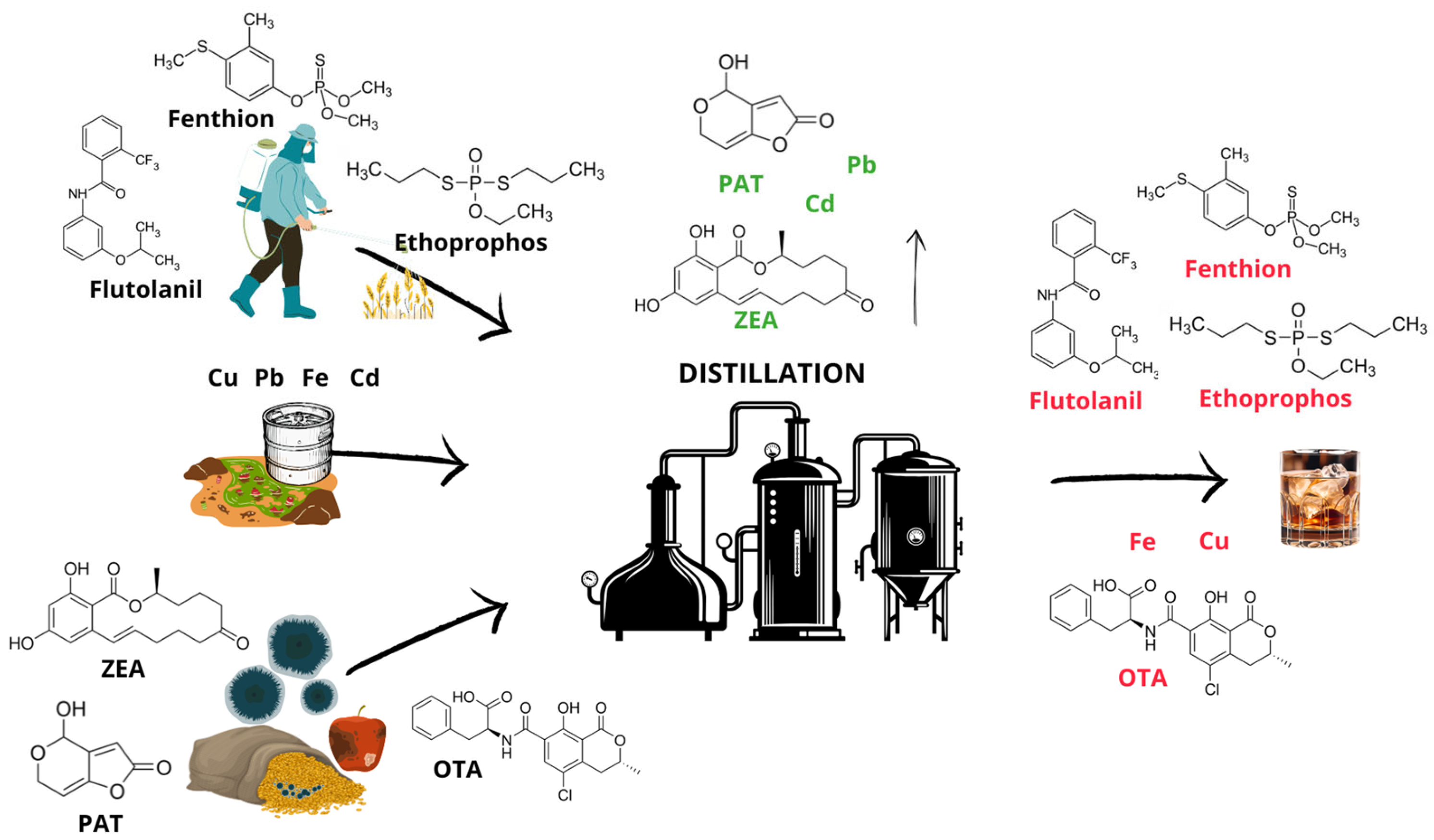
| Type of Spirit Beverage | Raw Material | Analyte | Spike (mg/L) | Conc. in Distillate (mg/L) | Literature |
|---|---|---|---|---|---|
| Sake | Fermented rice bran powder | Terbufos | 0.02 | ND 2 | [13] |
| 0.04 | 0.0018 | ||||
| Iprobenfon | 0.28 | ND | |||
| Fenthion | 0.14 | 0.0024 | |||
| 0.33 | 0.01 | ||||
| Flutolanil | 1.27 | 0.01 | |||
| Ethoprophos | 0.35 | 0.05 | |||
| Pb | 10 | ND | |||
| Cd | 10 | ND | |||
| AFB1 | 0.04 | ND | [28] | ||
| OTA | 0.02 | 0.00019 | |||
| DON | 4 | ND | |||
| ZEA | 0.8 | ND | |||
| Barley shochu | Fermented barley mash | PAT | 0.05 | ND | [29] |
| NIV | 0.05 | ND | |||
| DON | 0.05 | ND | |||
| AFG2 | 0.01 | ND | |||
| AFG1 | 0.01 | ND | |||
| AFB2 | 0.01 | ND | |||
| AFB1 | 0.01 | ND | |||
| HT-2 | 0.05 | ND | |||
| T-2 | 0.05 | ND | |||
| ZEA | 0.05 | ND | |||
| FB1 | 0.05 | ND | |||
| OTA | 0.05 | ND | |||
| FB2 | 0.05 | ND | |||
| Cycloate | 0.05 | 0.022 1 | [27] | ||
| Cadusafos | 0.05 | 0.009 1 | |||
| Diallate | 0.05 | 0.015 1 | |||
| Ethoprophos | 0.05 | 0.015 1 | |||
| Thiometon | 0.05 | 0.012 1 | |||
| Terbufos | 0.05 | 0.008 1 | |||
| Recorded spirits (n = 97): Palinka (n = 25), whiskey (n = 21), vodka (n = 16), brandy (n = 18), rum (n = 6), artificially flavored spirits (n = 5), gin (n = 3), tequila (n = 2), absinthe (n = 1) | - | Copper | - | 0.0–15.3 | [4] |
| Cobalt | - | 0.0–0.31 | |||
| Chromium | - | 0.0–0.71 | |||
| Iron | - | 0.0–34.63 | |||
| Manganese | - | 0.0–3.38 | |||
| Nickel | - | 0.0–77.16 | |||
| Zinc | - | 0.0–7.49 | |||
| Tin | - | 0.0–3.47 | |||
| Unrecorded spirits (n = 100): Palinka | - | Copper | - | 0.0–51.60 | |
| Iron | - | 0.0–11.91 | |||
| Manganese | - | 0.0–0.79 | |||
| Nickel | - | 0.0–6.86 | |||
| Zinc | - | 0.0–16.96 | |||
| Tin | - | 0.0–4.10 |
| Contaminants | Analysis Methods | Advantages | Disadvantages | Literature |
|---|---|---|---|---|
| Heavy metals | ICP/MS, ICP/OES | High sensitivity, ability to analyze multiple elements simultaneously | High operating costs, high argon consumption, complex sample prep, time-consuming | [65,79] |
| TXRF | Fast, low cost, minimal sample preparation, for high-concentration samples | High detection limits, spectral interferences, less precise for trace elements | ||
| GFAAS | Improved sensitivity, lower detection limits | Complex sample preparation, slower analysis | ||
| FAAS | Fast analysis, relatively low cost | Lower sensitivity compared to other techniques | ||
| Pesticides | GC/MS, GC/MS/MS | High sensitivity, good separation | Requires extensive sample preparation, expensive equipment | |
| HPLC/MS, HPLC/MS/MS | Excellent selectivity, suitable for complex matrices | High operational costs, potential matrix interferences | ||
| Mycotoxins | HPLC-FLD/UV/MS | High sensitivity, allows simultaneous detection of multiple mycotoxins, applicable for various matrices | Requires derivatization for some mycotoxins, expensive equipment, complex sample preparation | [80] |
| LC-MS/MS | High selectivity and sensitivity, reliable identification, applicable to complex matrices | High operational costs, requires highly trained personnel | ||
| ELISA | Fast and simple screening method, enables high-throughput testing, requires low sample volume and minimal clean-up | Matrix interferences may affect accuracy, requires thorough validation for different food matrices | ||
| LFIA | Simple, fast results, low cost, suitable for large-scale on-site screening, no need for sample clean-up | Potential interferences, limited sensitivity for trace analytes | ||
| Biosensors | High sensitivity, real-time detection, possibility of multi-toxin analysis, portable, suitable for on-site testing | Matrix interference, antibody cross-reactivity, necessity of matrices’ validation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rot, T.; Gavran, S.; Babić, J.; Lončarić, A. Occurrence of Pesticides, Mycotoxins, and Heavy Metals in Distilled Alcoholic Beverages: A Review of Contaminants and Health Risks. Foods 2025, 14, 1303. https://doi.org/10.3390/foods14081303
Rot T, Gavran S, Babić J, Lončarić A. Occurrence of Pesticides, Mycotoxins, and Heavy Metals in Distilled Alcoholic Beverages: A Review of Contaminants and Health Risks. Foods. 2025; 14(8):1303. https://doi.org/10.3390/foods14081303
Chicago/Turabian StyleRot, Tomislav, Sunčana Gavran, Jurislav Babić, and Ante Lončarić. 2025. "Occurrence of Pesticides, Mycotoxins, and Heavy Metals in Distilled Alcoholic Beverages: A Review of Contaminants and Health Risks" Foods 14, no. 8: 1303. https://doi.org/10.3390/foods14081303
APA StyleRot, T., Gavran, S., Babić, J., & Lončarić, A. (2025). Occurrence of Pesticides, Mycotoxins, and Heavy Metals in Distilled Alcoholic Beverages: A Review of Contaminants and Health Risks. Foods, 14(8), 1303. https://doi.org/10.3390/foods14081303






