The Effect of Muicle–Chitosan Edible Coatings on the Physical, Chemical, and Microbiological Quality of Cazon Fish (Mustelus lunulatus) Fillets Stored in Ice
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Extract Preparation
2.3. Evaluation of Antibacterial Activity
2.3.1. Bacterial Activation and Culture Preparation
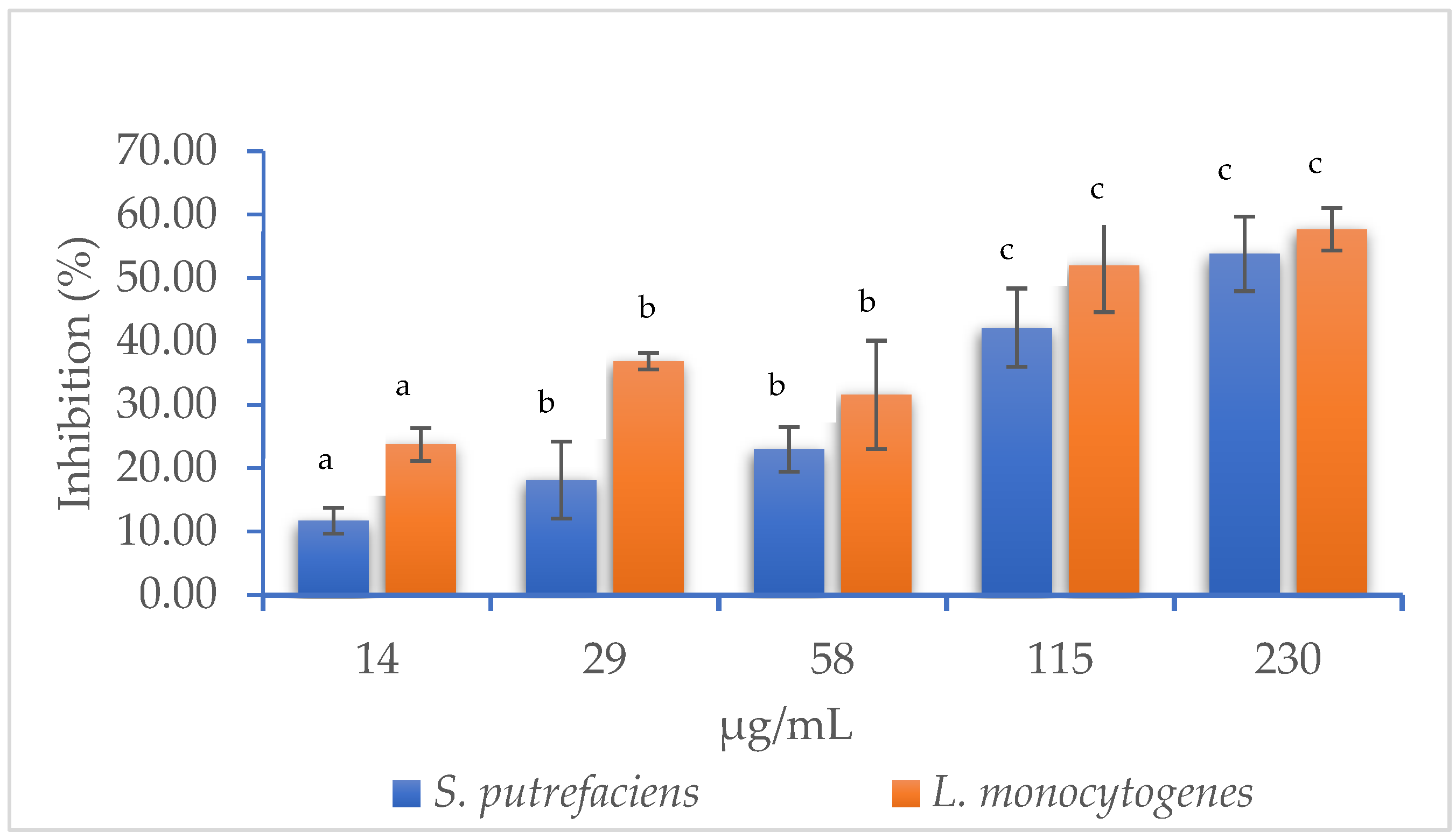
2.3.2. Determination of Minimum Inhibitory Concentration (MIC) and IC50
2.4. Determination of Phytochemical Compounds
2.4.1. Total Phenolic Content
2.4.2. Total Flavonoid Content
2.5. Preparation and Application of Treatments and Control
2.6. Ice Storage Study
2.7. Analytical Procedures
2.7.1. pH
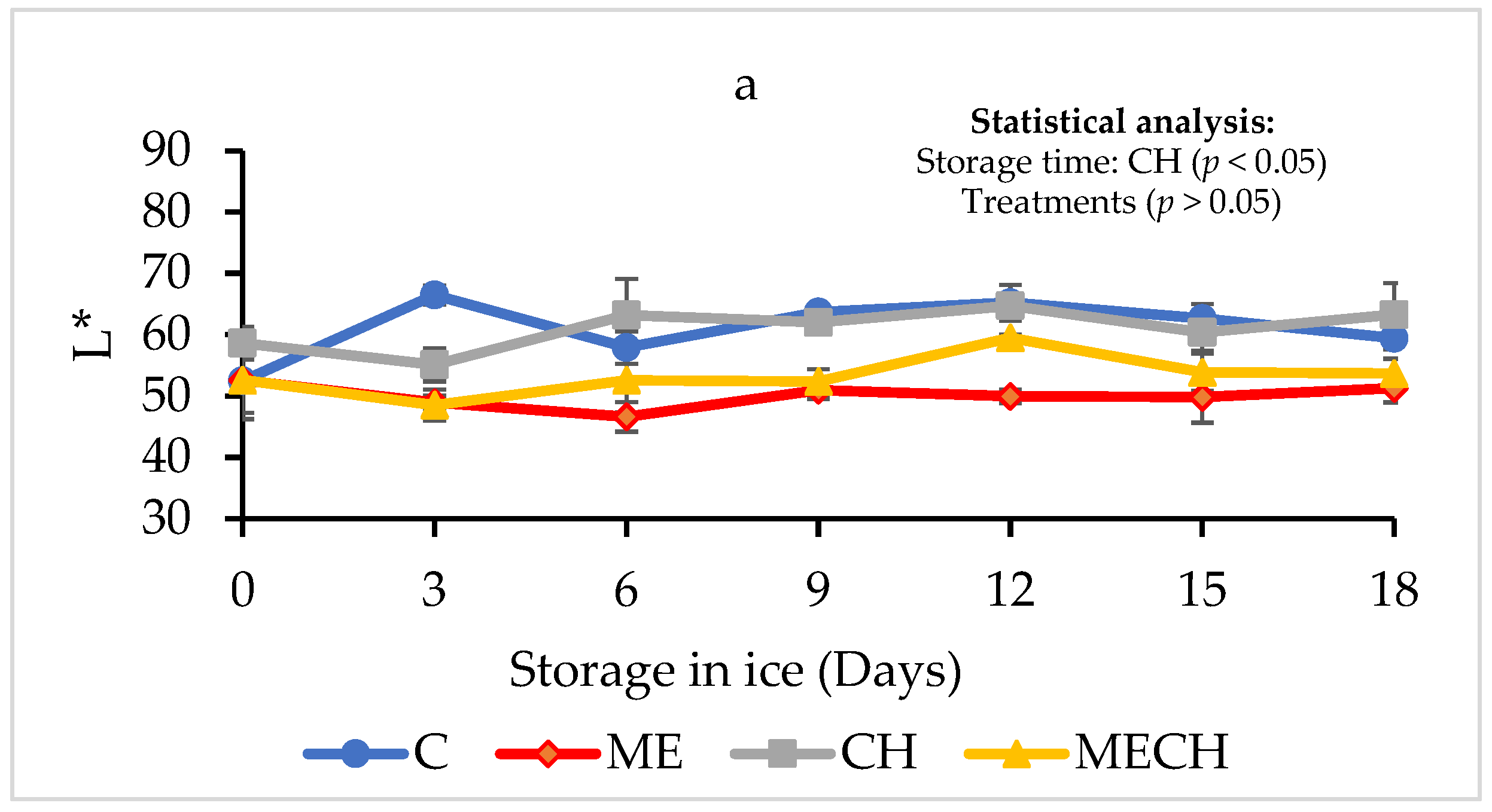
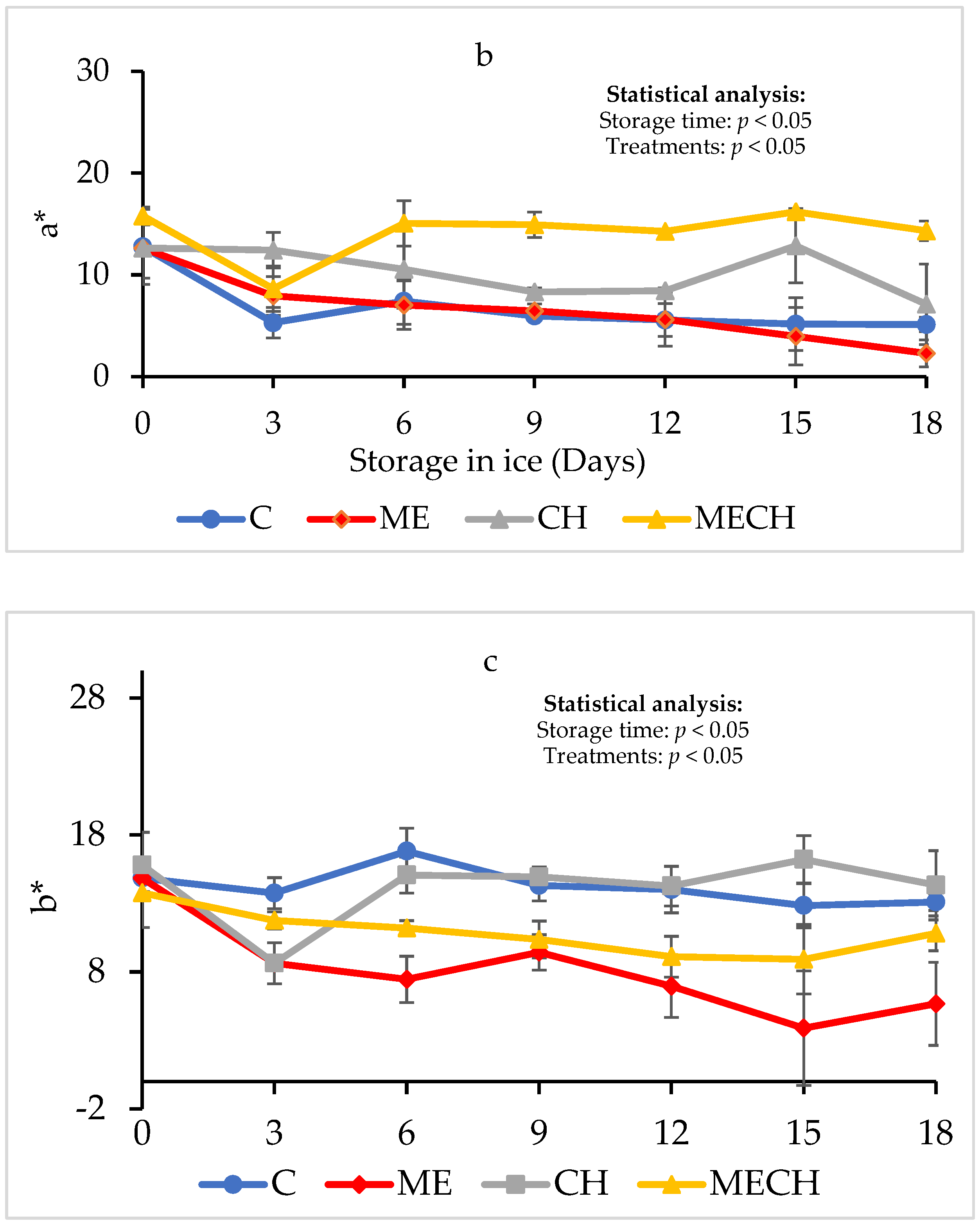
2.7.2. Colour
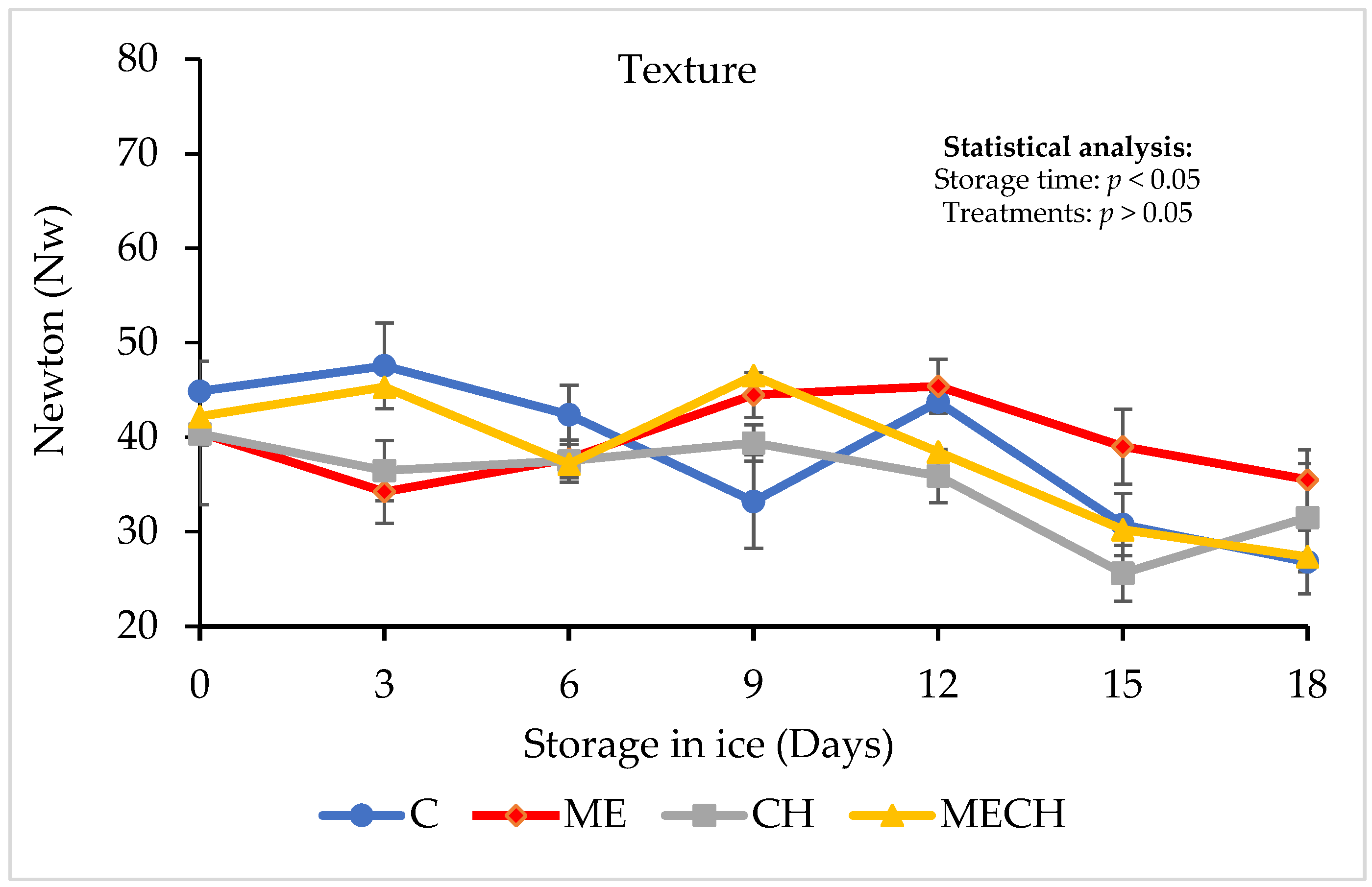
2.7.3. Texture
2.7.4. Water-Holding Capacity (WHC)
2.7.5. Total Volatile Basic Nitrogen (TVB-N)
2.7.6. Aerobic Mesophilic Count
2.8. Statistical Analysis
3. Results
3.1. Evaluation of Antibacterial Activity and Phytochemical Compounds with Antioxidant Potential in Muicle Extract
3.1.1. Minimum Inhibitory Concentration (MIC) and IC50 of Muicle Extract
3.1.2. Total Phenolic and Flavonoid Content in Muicle Extract
3.2. Evaluation of Physical Quality
3.2.1. pH Determination
3.2.2. Texture
3.2.3. Water-Holding Capacity (WHC)
3.2.4. Colour
3.3. Chemical Determinations
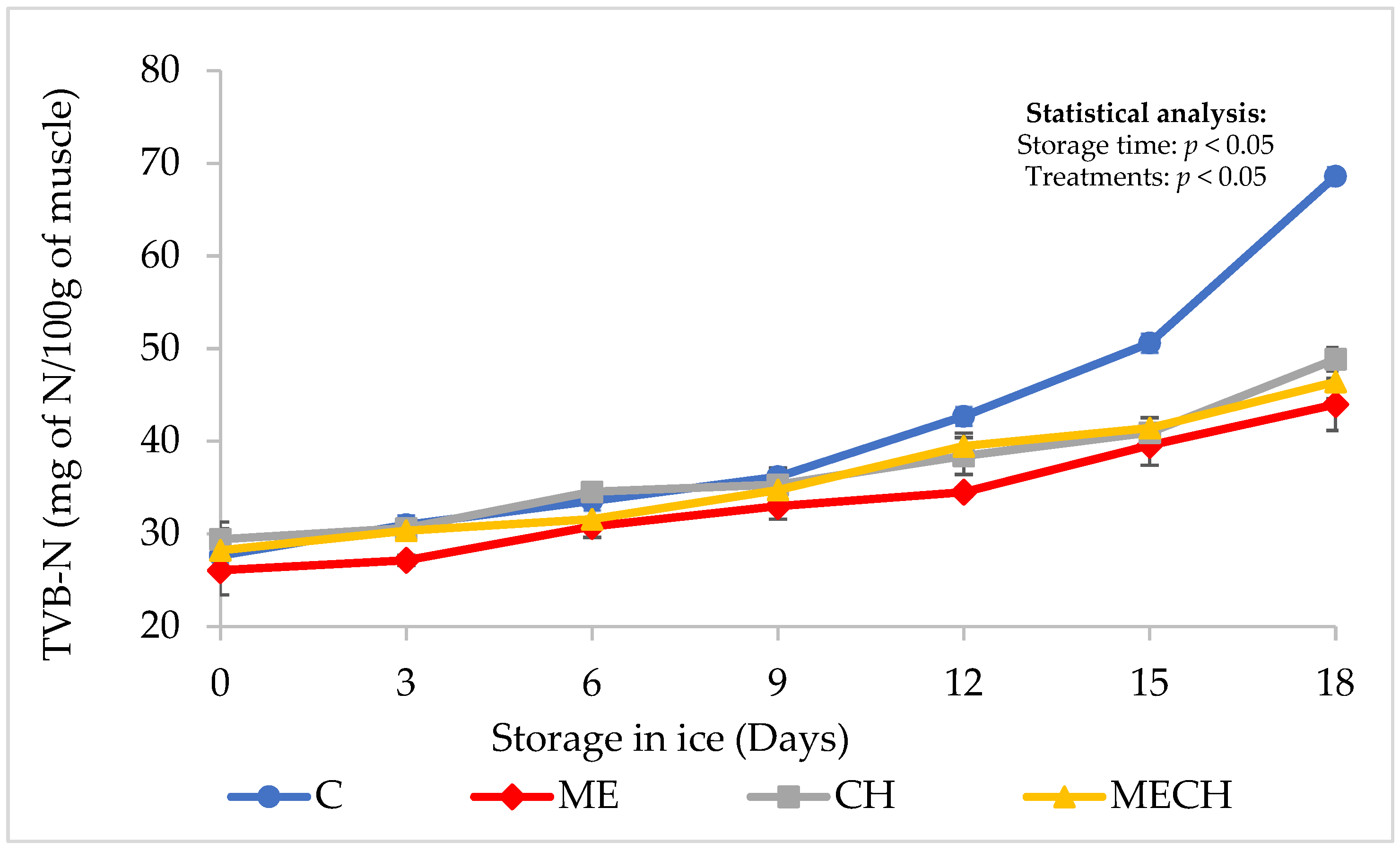
Total Volatile Basic Nitrogen (TVB-N)
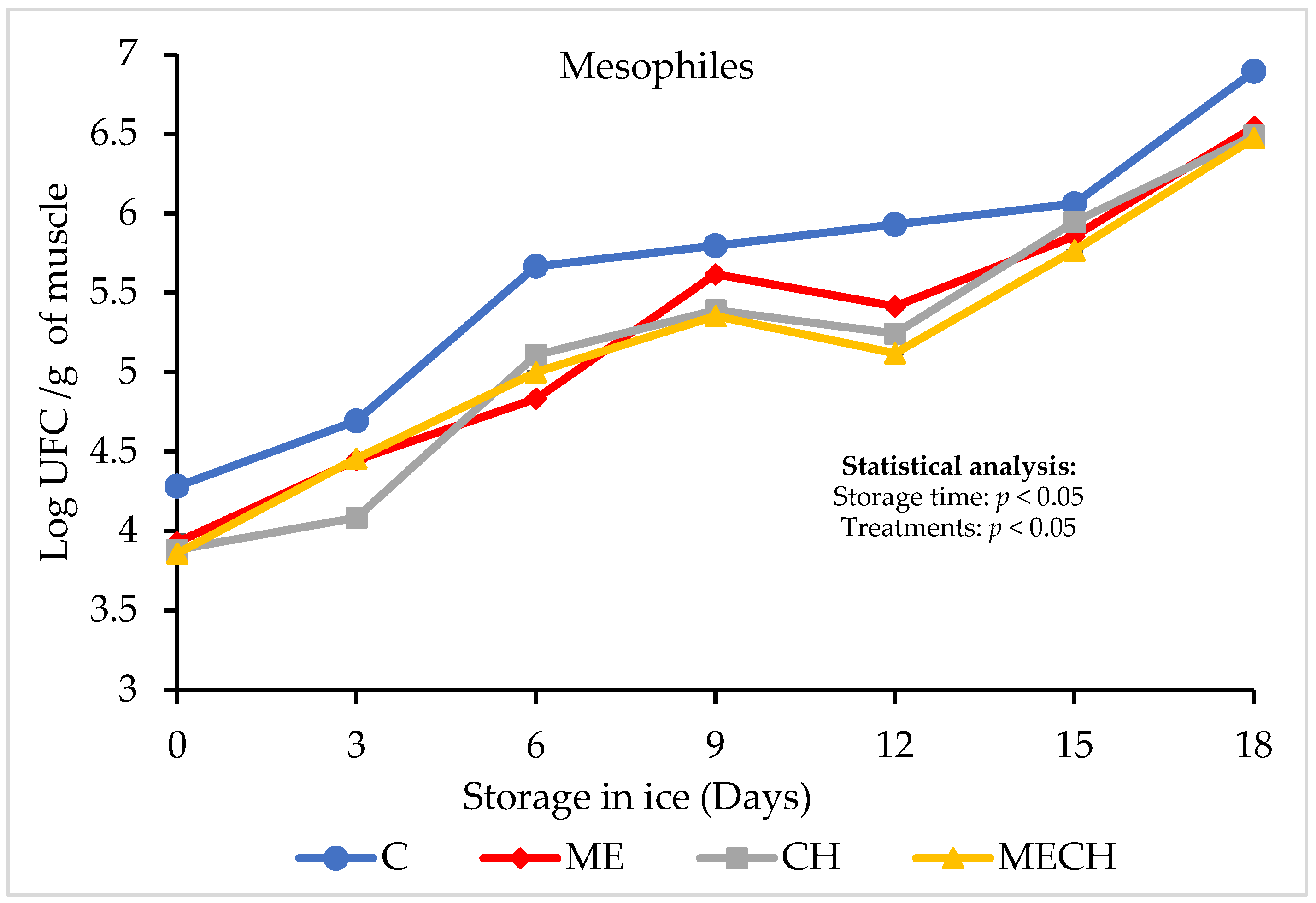
3.4. Microbiological Determinations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huss, H.H. Quality and Changes in Fresh Fish; FAO Fisheries Technical Paper No. 348; FAO: Rome, Italy, 1995; 195p, Available online: https://openknowledge.fao.org/handle/20.500.14283/v7180e (accessed on 15 February 2025).
- Koutsoumanis, K.; Nychas, G.J.E. Chemical and sensory changes associated with microbial flora of mediterranean boque (Boops boops) stored aerobically at 0, 3, 7, and 10 C. Appl. Environ. Microbiol. 1999, 65, 698–706. [Google Scholar] [CrossRef]
- Montoya-Camacho, N. Efecto de la Temperatura de Almacenamiento Sobre el Rigor Mortis y la Calidad del Músculo de Tilapia (Oreochromis niloticus). Master‘s Thesis, Universidad de Sonora, Hermosillo, Sonora, Mexico, 2013. [Google Scholar]
- Anuario Estadístico de Pesca. Comisión Nacional de Acuicultura y Pesca. 2021. Available online: https://nube.conapesca.gob.mx/sites/cona/dgppe/2021/ANUARIO_ESTADISTICO_DE_ACUACULTURA_Y_PESCA_2021.pdf (accessed on 15 February 2025).
- Badilla Flores, K.D. Análisis de las Capturas de Elasmobranquios Sujetos a Pesca Ribereña en Bahía de Kino, Sonora. Undergraduate Thesis, Bachelor’s Program in Biology, Departamento de Investigaciones Científicas y Tecnológicas, División de Ciencias Biológicas y de la Salud, Universidad de Sonora, Hermosillo, Sonora, Mexico, 2010; 97p. [Google Scholar]
- Salomón-Aguilar, C.A.; Villavicencio-Garayzar, C.J.; Reyes-Bonilla, H. Shark breeding grounds and seasons in the Gulf of California: Fishery management and conservation strategy. Cienc. Mar. 2009, 35, 369–388. [Google Scholar] [CrossRef]
- Crespo-Guerrero, J.M.; Rivera, M.G. Organización y transformaciones de la pesca comercial ribereña en el Parque Nacional Bahía de Loreto (Baja California Sur, México). J. Depopulation Rural Dev. Stud. 2017, 53, 59–96. [Google Scholar] [CrossRef]
- Marquez-Rios, E.; Castillo-Yanez, F.J.; Graciano-Verdugo, A.Z.; Jimenez-Ruiz, E.I.; Lugo-Sanchez, M.E.; Maeda-Martinez, A.N.; Ocano-Higuera, V.M. Impact of artesanal capture practices and post-capture handling on the quality of cazon fish muscle. Interciencia 2011, 36, 672–677. [Google Scholar]
- Sirohi, R.; Shahi, N.C.; Singh, A.; Richa, R. High-Pressure Processing of Seafoods. In Applied Agricultural Practices for Mitigating Climate Change; CRC Press: Boca Raton, FL, USA, 2019; Volume 2, pp. 229–240. [Google Scholar]
- Cropotova, J.; Tappi, S.; Genovese, J.; Rocculi, P.; Dalla Rosa, M.; Rustad, T. The combined effect of pulsed electric field treatment and brine salting on changes in the oxidative stability of lipids and proteins and color characteristics of sea bass (Dicentrarchus labrax). Heliyon 2021, 7, e05947. [Google Scholar] [CrossRef]
- Latief, R.; Dirpan, A.; Djalal, M.; Ramadhani, I.S.; Julyaningsih, A.H. Intelligent and Active Packaging System Application in Evaluating and Maintaining the Tuna (Thunnus Sp.) Fillets’ Quality at Cold Temperature. Curr. Res. Nutr. Food Sci. J. 2023, 11, 627–640. [Google Scholar] [CrossRef]
- Jiménez-Ruíz, E.I.; Ocaño-Higuera, V.M.; Valdez-Hurtado, S.; Cruz-Guzmán, J.A.; Otero-León, C.B.; Ruíz-Cruz, S.; Garzón-García, A.M.; Barrales-Cureño, H.J.; Canizales-Rodríguez, D.F.; Pérez-Martínez, C.J.; et al. Quality Improvement and Shelf-Life Extension of Iced Nile Tilapia Fillets Using Natural Garlic Extract. Fishes 2023, 8, 325. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Al-Gheethi, A.A.S.; Ghaleb, A.D.; Mahdi, A.A.; Al-Ansi, W.; Noman, A.E.; Al-Adeeb, A.; Olatoundé Odjo, A.K.; Du, Y.; Wei, M.; et al. Fabrication and characterization of chitosan/gelatin films loaded with microcapsules of Pulicaria jaubertii extract. Food Hydrocoll. 2022, 129, 107624. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Munekata, P.E.; Pateiro, M.; Domínguez, R.; Nieto, G.; Kumar, M.; Dhama, K.; Lorenzo, J.M. Bioactive compounds from fruits as preservatives. Foods 2023, 12, 343. [Google Scholar] [CrossRef]
- Valipour-Kootenaie, F.; Ariaii, P.; Khademi Shurmasti, D.; Nemati, M. Effect of chitosan edible coating enriched with eucalyptus essential oil and α-tocopherol on silver carp fillets quality during refrigerated storage. J. Food Saf. 2017, 37, e1229. [Google Scholar] [CrossRef]
- Yu, D.; Wu, L.; Regenstein, J.M.; Jiang, Q.; Yang, F.; Xu, Y.; Xia, W. Recent advances in quality retention of non-frozen fish and fishery products: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1747–1759. [Google Scholar] [CrossRef]
- Ramírez-Guerra, H.E.; Castillo-Yañez, F.J.; Montaño-Cota, E.A.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Canizales-Rodríguez, D.F.; Torres, W.; Montoya-Camacho, N.; Ocaño-Higuera, V.M. Protective effect of an edible tomato plant extract/chitosan coating on the quality and shelf life of sierra fish fillets. J. Chem. 2018, 2018, 2436045. [Google Scholar] [CrossRef]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z. Incorporation of salmon bone gelatine with chitosan, gallic acid and clove oil as edible coating for the cold storage of fresh salmon fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Lee, J.S.; Jahurul, M.H.A.; Pua, V.C.; Shapawi, R.; Chan, P.T. Effects of chitosan and ascorbic acid coating on the chilled tilapia fish (Oreochromis niloticus) fillet. J. Phys. Conf. Ser. 2019, 1358, 012009. [Google Scholar] [CrossRef]
- Vega-Avila, E.; Tapia-Aguilar, R.; Reyes-Chilpa, R.; Guzmán-Gutiérrez, S.L.; Pérez-Flores, J.; Velasco-Lezama, R. Actividad antibacteriana y antifúngica de Justicia spicigera. Rev. Latinoam. Química 2012, 40, 75–82. [Google Scholar]
- Sepulveda-Jimenez, G.; Reyna-Aquino, C.; Chaires-Martinez, L.; Bermudez-Torres, K.; Rodriguez-Monroy, M. Antioxidant Activity and Content of Phenolic Compounds and Flavonoids from Justicia spicigera. J. Biol. Sci. 2009, 9, 629–632. [Google Scholar] [CrossRef]
- Ortega-Cardona, C.E.; Aparicio Fernández, X. Quitosano: Una alternativa sustentable para el empaque de alimentos. Rev. Digit. Univ. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Velásquez, C.L. Quitina y quitosano: Materiales del pasado para el presente y el futuro. Av. Química 2006, 1, 15–21. [Google Scholar] [CrossRef]
- Burkhard, I.R.; Aon, M.A.; Bonavigna, R.A.; Kelly, C.L.; Villarreal, P. Relación entre el pH y el Nitrógeno Básico Volátil en filet de merluza como indicador de frescura. Jorn. Int. Vet. SNS 2023, 2, 1. [Google Scholar]
- Esparza-Espinoza, D.M.; del Carmen Santacruz-Ortega, H.; Plascencia-Jatomea, M.; Aubourg, S.P.; Salazar-Leyva, J.A.; Rodríguez-Felix, F.; Ezquerra-Brauer, J.M. Chemical-Structural Identification of Crude Gelatin from Jellyfish (Stomolophus meleagris) and Evaluation of Its Potential Biological Activity. Fishes 2023, 8, 246. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.D.J.; López-Mata, M.A.; Del Toro-Sanchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total phenolic, flavonoid, tomatine, and tomatidine contents and antioxidant and antimicrobial activities of extracts of tomato plant. Int. J. Anal. Chem. 2015, 2015, 284071. [Google Scholar] [CrossRef] [PubMed]
- Woyewoda, A.D.; Shaw, S.J.; Ke, P.J.; Burns, B.G. Recommended Laboratory Methods for Assessment of Fish Quality; Canadian Technical Report of Fisheries and Aquatic Sciences No. 1448; Canadian Institute of Fisheries Technologies: Halifax, NS, USA, 1986. [Google Scholar]
- Canizales-Rodríguez, D.F. Evaluación de la calidad y vida de anaquel del camarón Litopenaeus stylirostris Almacenado en Hielo y Caracterización de los Principales Compuestos Relacionados con su sabor y Deterioro. Master’s Thesis, Universidad de Sonora, Hermosillo, Sonora, Mexico, 2012. [Google Scholar]
- Cheng, C.S.; Hamann, D.D.; Webb, N.B.; Sidwell, V. Effects of species and storage time on minced fish gel texture. J. Food Sci. 1979, 44, 1087–1092. [Google Scholar] [CrossRef]
- NOM-092-SSA1-1994; NORMA Oficial Mexicana, Bienes y Servicios. Método para la Cuenta de Bacterias Aerobias en Placa. Diario Oficial de la Federación: Ciudad de México, Mexico, 1995. Available online: https://dof.gob.mx/nota_detalle.php?codigo=4886029&fecha=12/12/1995#gsc.tab=0 (accessed on 15 February 2025).
- Arunkumar, S.; Muthuselvam, M. Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J. Agric. Sci. 2009, 5, 572–576. [Google Scholar]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2019, 303, 75–89. [Google Scholar] [CrossRef]
- Cahuana-Tapia, L.M.; Cuello-Medina, G.E. Caracterización Fitoquímica, Cuantificación de Fenoles Totales, Flavonoides y Actividad Antioxidante del Extracto Etanólico de las Hojas Pos-Cosecha de Lycopersicum esculentum L. Bachelor’s Thesis, Universidad Nacional San Luis Gonzaga, Ica, Peru, 2019. [Google Scholar]
- Alvarez-Poblano, L.; Roman-Guerrero, A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Exogenous addition of muicle (Justicia spicigera Schechtendal) extract to white maize tortillas affects the antioxidant activity, texture, color, and in vitro starch digestibility. LWT 2020, 133, 110120. [Google Scholar] [CrossRef]
- Monroy, L.V.; Cauich, J.C.; Ortega, A.M.; Campos, M.S. Medicinal plants as potential functional foods or resources for obtaining anticancer activity metabolites. In Oncological Functional Nutrition; Academic Press: Cambridge, MA, USA, 2021; pp. 161–194. [Google Scholar]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Copes, J.A.; Pellicer, K.E.; Hoyo, G.D.; Brocardo, M.S.; Giannuzzi, L. Estudio del comportamiento del H en filetes de pejerrey de laguna (Odonthestes bonariensis), conservados con y sin vacío, utilizando distintos tratamientos y almacenados a temperaturas de 4, 0 y-1.5 º C. Analecta Vet. 2008, 28, 21–25. [Google Scholar]
- Ocaño-Higuera, V.M.; Marquez-Ríos, E.; Canizales-Dávila, M.; Castillo-Yáñez, F.J.; Pacheco-Aguilar, R.; Lugo-Sánchez, M.E.; Graciano-Verdugo, A.Z. Postmortem changes in cazon fish muscle stored on ice. Food Chem. 2009, 116, 933–938. [Google Scholar] [CrossRef]
- Tomé, E.; Iglesias, M.; Kodaira, M.; González, A. Efecto de la temperatura de almacenamiento en el rigor mortis y en la estabilidad de la tilapia (Oreochromis spp.) cultivada. Rev. Cient. FCV-LUZ 2000, 11, 373–378. [Google Scholar]
- Duran, A.; Erdemli, U.; Karakaya, M.; Tyilmaz, M. Effects of slaughter methods on physical, biochemical and microbiological quality of rainbow trout Oncorhynchus mykiss and mirror carp Cyprinus carpio filleted in pre-, in-or post-rigor periods. Fish. Sci. 2008, 74, 1146–1156. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Park, S.-W.; Shin, H.-S. Fish Freshness Indicator for Sensing Fish Quality during Storage. Foods 2023, 12, 1801. [Google Scholar] [CrossRef]
- Yan, Q.; Guo, M.; Chen, B.; Zhang, C.; Li, D.; Xie, J. Molecular characterization of spoilage microbiota in high CO2 refrigerated large yellow croaker (Larimichthys crocea) fillets using metagenomic and metabolomic approaches. Food Biosci. 2023, 56, 103227. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Berizi, E.; Hosseinzadeh, S.; Shekarforoush, S.S.; Barbieri, G. Microbial, chemical, textural and sensory properties of coated rainbow trout by chitosan combined with pomegranate peel extract during frozen storage. Int. J. Biol. Macromol. 2018, 106, 1004–1013. [Google Scholar] [CrossRef]
- Chang, L.; Li, Y.; Bai, X.; Xia, X.; Xu, W. Inhibition of Chitosan Ice Coating on the Quality Deterioration of Quick-Frozen Fish Balls during Repeated Freeze–Thaw Cycles. Foods 2023, 12, 717. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, F.; Zhang, W.B.; Parisi, G.; Du, Z.Y.; Zhang, M.L. The flesh texture of teleost fish: Characteristics and interventional strategies. Rev. Aquac. 2024, 16, 508–535. [Google Scholar] [CrossRef]
- Chaparro-Hernandez, S.; Ruiz-Cruz, S.; Marquez-Rios, E.; Ocano-Higuera, V.M.; Valenzuela-Lopez, C.C.; Ornelas-Paz, J.D.J.; Del-Toro-Sánchez, C.L. Effect of chitosan-carvacrol edible coatings on the quality and shelf life of tilapia (Oreochromis niloticus) fillets stored in ice. Food Sci. Technol. 2015, 35, 734–741. [Google Scholar] [CrossRef]
- Moreira López, J.J. Incidencia del Tiempo y Temperatura de Almacenamiento en la Calidad Microbiológica y Estabilidad de la Textura en Carne de Chame (Dormitatos latinfrons). Master’s Thesis, Escuela Superior Politécnica Agropecuaria de Manabí “Manuel Félix López” (ESPAM MFL), Calceta, Ecuador, 2022. Available online: http://repositorio.espam.edu.ec/handle/42000/1926 (accessed on 18 March 2022).
- Massa, A.E.; Agüeria, D.A.; Campos, C.A.; Czerner, M.; Fernández, L.; Miscoria, S.A.; Primost, M.A.; Volpedo, A. Relevamiento de aspectos técnicos de pH y otros parámetros de calidad establecidos por Brasil para el ingreso de productos pesqueros congelados: Valores de referencia para merluza común (Merluccius hubbsi); Red de Seguridad Alimentaria, Consejo Nacional de Investigaciones Científicas y Técnicas (RSA–CONICET): Buenos Aires, Argentina, 2020. [Google Scholar]
- Sánchez-Valencia, J.; Sánchez-Alonso, I.; Martinez, I.; Careche, M. Estimation of frozen storage time or temperature by kinetic modeling of the Kramer shear resistance and water holding capacity (WHC) of hake (Merluccius merluccius, L.) muscle. J. Food Eng. 2014, 120, 37–43. [Google Scholar] [CrossRef]
- Fennema, O.R. Comparative water holding properties of various muscle foods: A critical review relating to definitions, methods of measurement, governing factors, comparative data and mechanistic matters. J. Muscle Foods 1990, 1, 363–381. [Google Scholar] [CrossRef]
- Wijesundara, W.M.N.M.; Jayawardana, B.C.; Vidanarachchi, J.K. Changes in postmortem quality and shelf life determination of frigate mackerel (Auxis Thazard) and sting ray (Dasyatis Margarita) during ice storage. Annu. Académic Sess. 2012, 198–201. [Google Scholar]
- Chan, S.S.; Roth, B.; Jessen, F.; Jakobsen, A.N.; Lerfall, J. Water holding properties of Atlantic salmon. Compr. Rev. Food Sci. Food Saf. 2022, 21, 477–498. [Google Scholar] [CrossRef]
- Hassoun, A.; Karoui, R. Quality evaluation of fish and other seafood by traditional and nondestructive instrumental methods: Advantages and limitations. Crit. Rev. Food Sci. Nutr. 2017, 57, 1976–1998. [Google Scholar] [CrossRef] [PubMed]
- Montaño-Cota, E.A. Efecto de un Extracto Etanólico Obtenido a Partir de la Planta de Tomate (Solanum lycopersicum) Incorporado en un Recubrimiento Comestible a Base de Quitosano Sonre la Frescura, Calidad y vida de Anaquel del Filete de Sierra (Scomberomorus sierra) Almacenado en Hielo. Bachelor’s Thesis, Universidad de Sonora, Hermosillo, Sonora, Mexico, 2016; 62p. [Google Scholar]
- Da Silva Santos, F.M.; da Silva, A.I.M.; Vieira, C.B.; de Araújo, M.H.; da Silva, A.L.C.; Carneiro-da-Cunha, M.D.G.; Silva de Souza, B.W.; de Souza Bezerra, R. Use of chitosan coating in increasing the shelf life of liquid smoked Nile tilapia (Oreochromis niloticus) fillet. J. Food Sci. Technol. 2017, 54, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.O.; Ravishankar, C.N.; Lalitha, K.V.; Gopal, T.S. Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocoll. 2012, 26, 167–174. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C.; Moreno-Hernández, J.M.; Pacheco-Aguilar, R. Seafood proteins. In Proteins in Food Processing; Woodhead Publishing: Duxford, Cambridge, UK, 2018; pp. 445–475. [Google Scholar]
- Salueña, B.H.; Gamasa, C.S.; Rubial, J.M.D.; Odriozola, C.A. CIELAB color paths during meat shelf life. Meat Sci. 2019, 157, 107889. [Google Scholar] [CrossRef]
- Castillo-Yáñez, F.J.; Pacheco-Aguilar, R.; Márquez-Ríos, E.; Lugo-Sánchez, M.E.; Lozano-Taylor, J. Freshness loss in sierra fish (Scomberomorus sierra) muscle stored in ICE as affected by postcapture handling practices. J. Food Biochem. 2007, 31, 56–67. [Google Scholar] [CrossRef]
- Jinadasa, B.K.K.K. Determination of quality of marine fishes based on total volatile base nitrogen test (TVB-N). Nat. Sci. 2014, 5, 106–111. [Google Scholar]
- NOM-242-SSA1-2009; NORMA Oficial Mexicana Productos y servicios. Productos de la pesca frescos, refrigerados, congelados y procesados. Especificaciones sanitarias y métodos de prueba. Diario Oficial de la Federación, Gobierno de la República: Ciudad de México, Mexico, 2011.
- European Union. Council regulation (EC) N 2406/96 of 23 December 1996 laying down common marketing standards for certain fishery products. Off. J. L 1996, 334, 1–15. [Google Scholar]
- Ocaño-Higuera, V.M.; Maeda-Martínez, A.N.; Marquez-Ríos, E.; Canizales-Rodríguez, D.F.; Castillo-Yáñez, F.J.; Ruíz-Bustos, E.; Graciano-Verdugo, A.Z.; Plascencia-Jatomea, M. Freshness assessment of ray fish stored in ice by biochemical, chemical and physical methods. Food Chem. 2011, 125, 49–54. [Google Scholar] [CrossRef]
- Reátegui-Quispe, A.; Pariona-Velarde, D. Determinación de plomo, cadmio, mercurio y Bases Volátiles Nitrogenadas Totales (NBVT) en el músculo de tiburón azul (Prionace glauca) procedente de la zona sur del Perú. Rev. Biol. Mar. Oceanogr. 2019, 54, 336–342. [Google Scholar] [CrossRef]
- Banks, H.; Nickelson, R.; Finne, G. Shelf life studies on carbon dioxide packaged finfish from Gulf of Mexico. J. Food Sci. 1980, 45, 157–162. [Google Scholar] [CrossRef]
- Aref, S.; Habiba, R.; Morsy, N.; Abdel-Daim, M.M.; Zayet, F. Improvement of the shelf life of grey mullet (Mugil cephalus) fish steaks using edible coatings containing chitosan, nanochitosan, and clove oil during refrigerated storage. Food Prod. Process. Nutr. 2022, 4, 27. [Google Scholar] [CrossRef]
- International Commission on Microbiological Specifications for Foods (ICMSF). Microorganisms in Foods 7: Microbiological Testing in Food Safety Management; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002. [Google Scholar]


| S. putrefaciens | L. monocytogenes | |
|---|---|---|
| MIC (μg/mL ± SD) | 3.01 ± 0.73 a | 0.09 ± 0.07 b |
| IC50 (μg/mL ± SD) | 204.56 ± 20.22 a | 118.09 ± 14.51 b |
| 230 μg/mL of Extract | |
|---|---|
| Total phenols (mg GAE/100 g of sample) | 41.48 ± 3.28 |
| Flavonoids mg QE/100 g of sample | 27.52 ± 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Guzmán, J.A.; Garzón-García, A.M.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Valdez-Hurtado, S.; Paredes-Quijada, G.T.; Rodríguez-Figueroa, J.C.; Silvas-García, M.I.; Montoya-Camacho, N.; Ocaño-Higuera, V.M.; et al. The Effect of Muicle–Chitosan Edible Coatings on the Physical, Chemical, and Microbiological Quality of Cazon Fish (Mustelus lunulatus) Fillets Stored in Ice. Foods 2025, 14, 1619. https://doi.org/10.3390/foods14091619
Cruz-Guzmán JA, Garzón-García AM, Ruíz-Cruz S, Márquez-Ríos E, Valdez-Hurtado S, Paredes-Quijada GT, Rodríguez-Figueroa JC, Silvas-García MI, Montoya-Camacho N, Ocaño-Higuera VM, et al. The Effect of Muicle–Chitosan Edible Coatings on the Physical, Chemical, and Microbiological Quality of Cazon Fish (Mustelus lunulatus) Fillets Stored in Ice. Foods. 2025; 14(9):1619. https://doi.org/10.3390/foods14091619
Chicago/Turabian StyleCruz-Guzmán, José Alberto, Alba Mery Garzón-García, Saúl Ruíz-Cruz, Enrique Márquez-Ríos, Santiago Valdez-Hurtado, Gerardo Trinidad Paredes-Quijada, José Carlos Rodríguez-Figueroa, María Irene Silvas-García, Nathaly Montoya-Camacho, Victor Manuel Ocaño-Higuera, and et al. 2025. "The Effect of Muicle–Chitosan Edible Coatings on the Physical, Chemical, and Microbiological Quality of Cazon Fish (Mustelus lunulatus) Fillets Stored in Ice" Foods 14, no. 9: 1619. https://doi.org/10.3390/foods14091619
APA StyleCruz-Guzmán, J. A., Garzón-García, A. M., Ruíz-Cruz, S., Márquez-Ríos, E., Valdez-Hurtado, S., Paredes-Quijada, G. T., Rodríguez-Figueroa, J. C., Silvas-García, M. I., Montoya-Camacho, N., Ocaño-Higuera, V. M., Canizales-Rodríguez, D. F., & Jiménez-Ruíz, E. I. (2025). The Effect of Muicle–Chitosan Edible Coatings on the Physical, Chemical, and Microbiological Quality of Cazon Fish (Mustelus lunulatus) Fillets Stored in Ice. Foods, 14(9), 1619. https://doi.org/10.3390/foods14091619








