Modified Test Kit for Detecting Polar Compounds and Evaluating Their Distribution in Reused Frying Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Kit Development
- In a 250 mL beaker, 100 mL of a methanol (1:1) solution was prepared.
- A total of 40 g of Silica Gel G Type 60 and 0.1 g of Bromocresol Green were added to the solution.
- The pH of the solution was adjusted to 7.3 using 0.1 N NaOH, resulting in a blue-colored solution.
- Clean microscope slides were immersed in the prepared solution and rotated to ensure an even coating.
- The coated slides were removed and placed horizontally on paper towels to dry for 20 min.
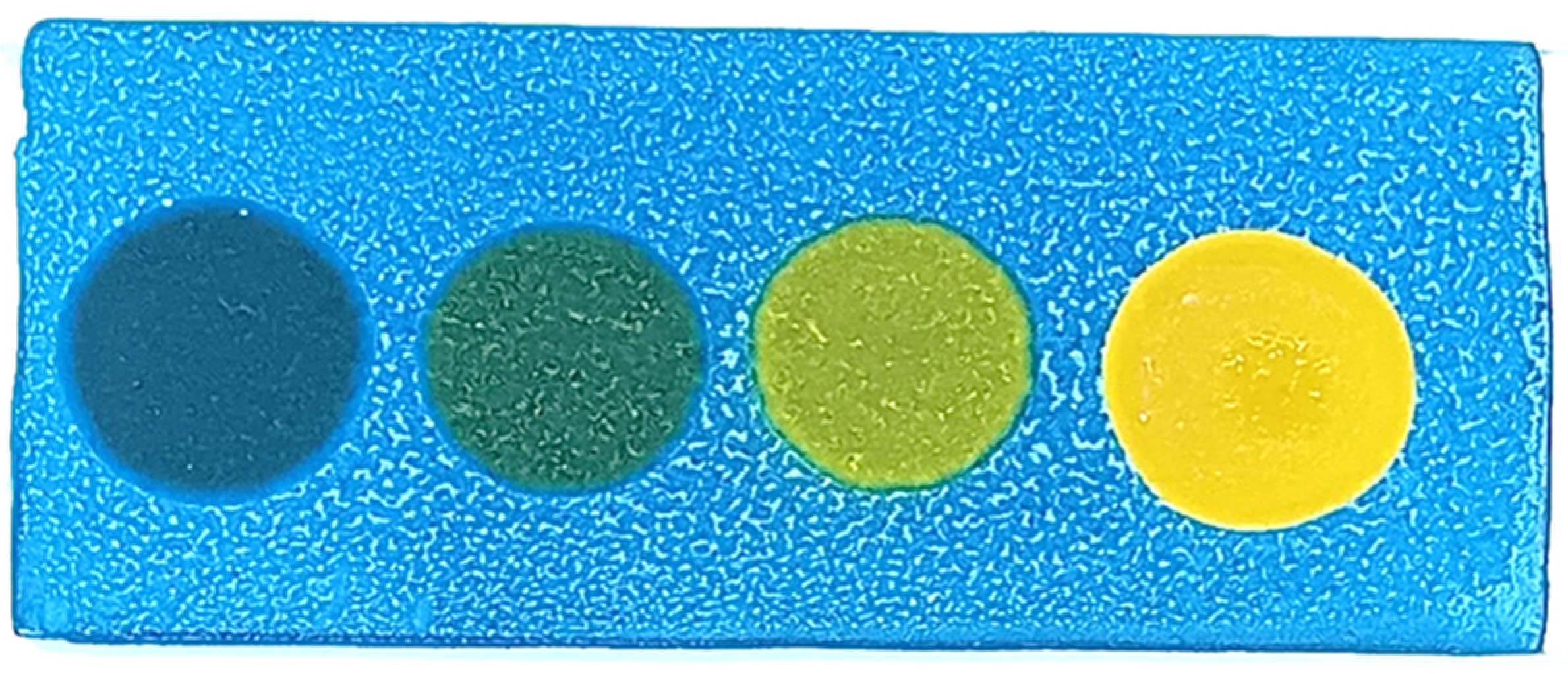
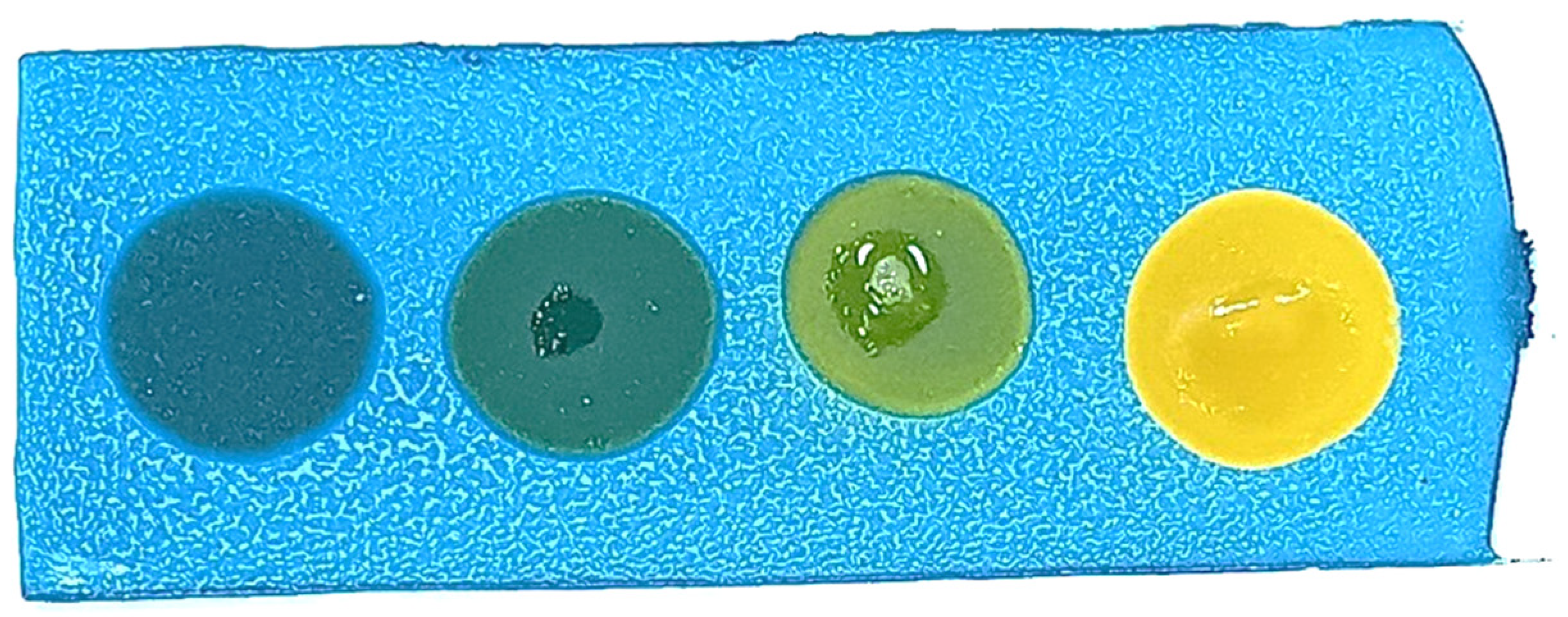
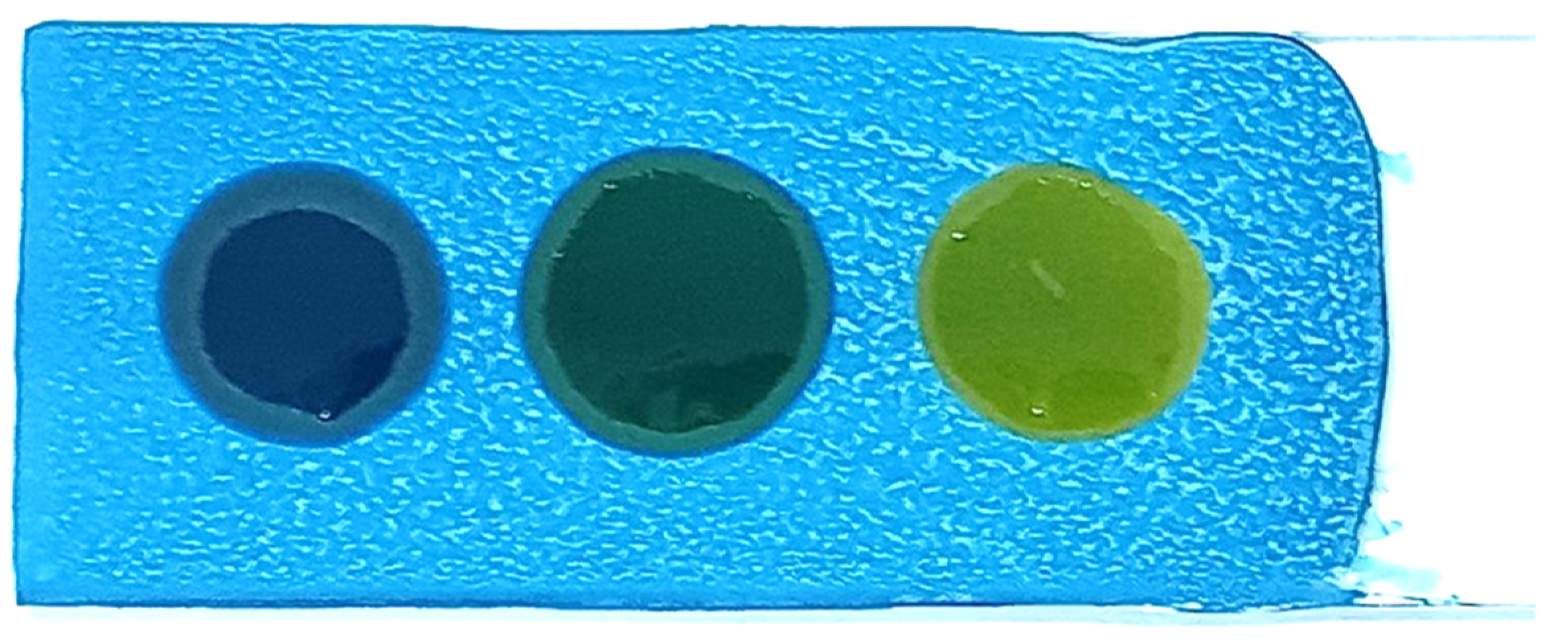
- The slides were baked in a hot-air oven at 100 °C for 1 h, producing blue-coated slides (Figure 1).
- The prepared slides were stored in an airtight, opaque container to prevent exposure to air, which could cause the blue coating to turn gray and render the slides unusable.
2.2. Evaluation of Test Kit Efficiency Compared to the Standard Method
- Sensitivity = A/(A + C) × 100%.
- Specificity = D/(B + D) × 100%.
- Positive predictive value = A/(A + B) × 100%.
- Negative predictive value = D/(C + D) × 100%.
- Accuracy = (A + D)/(A + B + C + D) × 100%.
3. Results
3.1. Efficiency of the Polar Compound Test Kit
- Blue: No polar compounds detected (<20% by weight).
- Green/Light green: Polar compounds detected (21–24% by weight).
- Yellow: High levels of polar compounds detected (≥25% by weight).
3.2. Distribution of Polar Compounds in Six Oil Types
3.2.1. Palm Oil
3.2.2. Soybean Oil
3.2.3. Canola Oil
3.2.4. Sunflower Oil
3.2.5. Rice Bran Oil
3.2.6. Coconut Oil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mL | Milliliter |
| GC | Gas Column Chromatography |
| NaOH | Sodium Hydroxide |
| IUPAC | International Union of Pure and Applied Chemistry |
References
- Ghobadi, S.; Akhlaghi, M.; Shams, S.; Mazloomi, S.M. Acid and Peroxide Values and Total Polar Compounds of Frying Oils in Fast Food Restaurants of Shiraz, Southern Iran. Int. J. Nutr. Sci. 2018, 3, 25–30. [Google Scholar]
- Xu, L.; Wu, G.; Yang, F.; Meng, L.; Huang, J.; Zhang, H.; Jin, Q.; Wang, X. Influence of Fried Food and Oil Type on the Distribution of Polar Compounds in Discarded Oil During Restaurant Deep Frying. Food Chem. 2019, 272, 12–17. [Google Scholar]
- Liu, Y.; Sun, L.; Bai, H.; Ran, Z. Detection for Frying Times of Various Edible Oils Based on Near-Infrared Spectroscopy. Appl. Sci. 2020, 10, 7789. [Google Scholar] [CrossRef]
- Warner, K.; Gupta, M. Frying Quality and Stability of Low-and Ultra-Low-Linolenic Acid Soybean Oils. J. Am. Oil Chem. Soc. 2003, 80, 275–280. [Google Scholar] [CrossRef]
- Susana, M.; Rodrigues, E.; Velasco, J.; Dobarganes, C. Quality of Used Frying Fats and Oils: Comparison of Rapid Tests Based on Chemical and Physical Oil Properties. Int. J. Food Sci. Technol. 2007, 42, 601–608. [Google Scholar]
- Joelle, N.; Afif, C.; Louka, N. Impact of a Novel Partial Defatting Technology on Oxidative Stability and Sensory Properties of Peanut Kernels. Food Chem. 2021, 334, 127581. [Google Scholar]
- Geeta, B.; Zhou, W.; Barlow, P.J.; Joshi, P.S.; Lo, H.L.; Chung, Y.K. Review of Rapid Tests Available for Measuring the Quality Changes in Frying Oils and Comparison with Standard Methods. Crit. Rev. Food Sci. Nutr. 2010, 50, 503–514. [Google Scholar]
- Martin, G.; Silwood, C.J.L.; Addis, P.; Claxson, A.; Serra, B.B.; Viana, M. Health Effects of Oxidized Heated Oils. Foodserv. Res. Int. 2001, 13, 41–55. [Google Scholar]
- Ministry of Public Health. Notification of the Ministry of Public Health (No. 283) B.E. 2547 (2004): Stipulation on the Amount of Polar Compounds in Oil Used for Frying or Cooking Food for Sale, Bangkok, Thailand, 2004. Available online: https://food.fda.moph.go.th/food-law/announ-moph-283 (accessed on 20 January 2025).
- Andras, S.; Bazar, G.; Locsmandi, L.; Romvari, R. Quality Alterations of Four Frying Fats During Long-Term Heating (Conventional Analysis and Nirs Calibration). J. Food Qual. 2010, 33, 42–58. [Google Scholar]
- Robern, H.; Leona, G. A Colorimetric Spot Test for Heated Oils. Can. Inst. Food Sci. Technol. J. 1981, 14, 150–152. [Google Scholar]
- International Union of Pure Applied Chemistry, Fats Applied Chemistry Division; Commission on Oils, Derivatives; Dieffenbacher, A.; Pocklington, W.D. Standard Methods for the Analysis of Oils, Fats, and Derivatives: 1st Supplement to the 7th Revised and Enlarged Edition; Blackwell Scientific Publications: Hoboken, NJ, USA, 1987. [Google Scholar]
- Xu, X.Q. A Modified Veri-Fry® Quick Test for Measuring Total Polar Compounds in Deep-Frying Oils. J. Am. Oil Chem. Soc. 1999, 76, 1087–1089. [Google Scholar] [CrossRef]
- Hassanien, M.F.R.; Sharoba, A.M. Rheological Characteristics of Vegetable Oils as Affected by Deep Frying of French Fries. J. Food Meas. Charact. 2014, 8, 171–179. [Google Scholar] [CrossRef]
- Chen, W.-A.; Chiu, C.P.; Cheng, W.-C.; Hsu, C.-K.; Kuo, M.-I. Total Polar Compounds and Acid Values of Repeatedly Used Frying Oils Measured by Standard and Rapid Methods. J. Food Drug Anal. 2013, 21, 3. [Google Scholar]
- Nataša, Š.; Žontar, T.P.; Ulrih, N.P. Dielectric Properties and Dipole Moment of Edible Oils Subjected to ‘Frying’thermal Treatment. Foods 2020, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.K.; Dash, U.M.A.; Rayaguru, K.; Krishnan, K.R. Physio-Chemical Changes During Repeated Frying of Cooked Oil: A Review. J. Food Biochem. 2016, 40, 371–390. [Google Scholar]
- Stier, R.F. Tests to Monitor Quality of Deep-Frying Fats and Oils. Eur. J. Lipid Sci. Technol. 2004, 106, 766–771. [Google Scholar] [CrossRef]
- Abrante-Pascual, S.; Nieva-Echevarría, B.; Goicoechea-Oses, E. Vegetable Oils and Their Use for Frying: A Review of Their Compositional Differences and Degradation. Foods 2024, 13, 4186. [Google Scholar] [CrossRef] [PubMed]




| Test Kit | Reference Method (GC) | |
|---|---|---|
| Positive | Negative | |
| Positive (≥ 25%) | True positive (A) | False positive (B) |
| Negative (< 25%) | False negative (C) | True negative (D) |
| Total | N+ = (A + C) | N− = (B + D) |
| Test kit | GC-column chromatography | Accuracy = 92.00% Sensitivity = 88.09% Specificity = 94.82% Positive predictive value = 92.50% Negative predictive value = 91.66% | |
| + | − | ||
| +(≥25%) * | 37 | 3 | |
| −(<25%) ** | 5 | 55 | |
| Time (h) | Test Kit Color | Polar Compounds (%) | Interpretation |
|---|---|---|---|
| 0–3 | Blue | ≤20 | Safe to use |
| 6–78 | Green/Light green | 21–24 | Approaching degradation |
| >81 | Yellow | ≥25 | “Do not use” |
| Time (h) | Test Kit Color | Polar Compounds (%) | Interpretation |
|---|---|---|---|
| 0–6 | Blue | ≤20 | Safe to use |
| 9–90 | Green/Light green | 21–24 | Approaching degradation |
| >90 | Yellow | ≥25 | “Do not use” |
| Time (h) | Test Kit Color | Polar Compounds (%) | Interpretation |
|---|---|---|---|
| 0–9 | Blue | ≤20 | Safe to use |
| 10–78 | Green/Light green | 21–24 | Approaching degradation |
| >81 | Yellow | ≥25 | “Do not use” |
| Time (h) | Test Kit Color | Polar Compounds (%) | Interpretation |
|---|---|---|---|
| 0–9 | Blue | <20 | Safe to use |
| 12–90 | Green/Light green | 20–24 | Approaching degradation |
| >90 | Not detectable | Not Detectable * | “Do not use” |
| Time (h) | Test Kit Color | Polar Compounds (%) | Interpretation |
|---|---|---|---|
| 0–6 | Blue | ≤20 | Safe to use |
| 7–90 | Green/Light green | 21–24 | Approaching degradation |
| >90 | Not detectable | Not Detectable | “Do not use” |
| Time (h) | Test Kit Color | Polar Compounds (%) | Interpretation |
|---|---|---|---|
| 0–3 | Blue | ≤20 | Safe to use |
| 4–48 | Green/Light green | 21–24 | Approaching degradation |
| >48 | Yellow | ≥25 | “Do not use” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yongyod, R.; Kerdsin, A. Modified Test Kit for Detecting Polar Compounds and Evaluating Their Distribution in Reused Frying Oil. Foods 2025, 14, 1572. https://doi.org/10.3390/foods14091572
Yongyod R, Kerdsin A. Modified Test Kit for Detecting Polar Compounds and Evaluating Their Distribution in Reused Frying Oil. Foods. 2025; 14(9):1572. https://doi.org/10.3390/foods14091572
Chicago/Turabian StyleYongyod, Rapeepan, and Anusak Kerdsin. 2025. "Modified Test Kit for Detecting Polar Compounds and Evaluating Their Distribution in Reused Frying Oil" Foods 14, no. 9: 1572. https://doi.org/10.3390/foods14091572
APA StyleYongyod, R., & Kerdsin, A. (2025). Modified Test Kit for Detecting Polar Compounds and Evaluating Their Distribution in Reused Frying Oil. Foods, 14(9), 1572. https://doi.org/10.3390/foods14091572






