Ripening-Associated Changes in Fatty Acid Composition and Nutritional Indices in Caciocavallo Silano PDO Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm Management and Sample Collection
2.2. Chemical Composition of Feed and Cheese
2.3. Salt Content in Cheese
2.4. Lactose Analysis in Cheese
2.5. Fatty Acids Analysis in Unifeed and Cheese
2.6. Atherogenicity Index (AI) and Thrombogenicity Index (TI)
2.7. Statistical Analysis
3. Results
3.1. Chemical Composition of Feed
3.2. Chemical Composition of Cheese
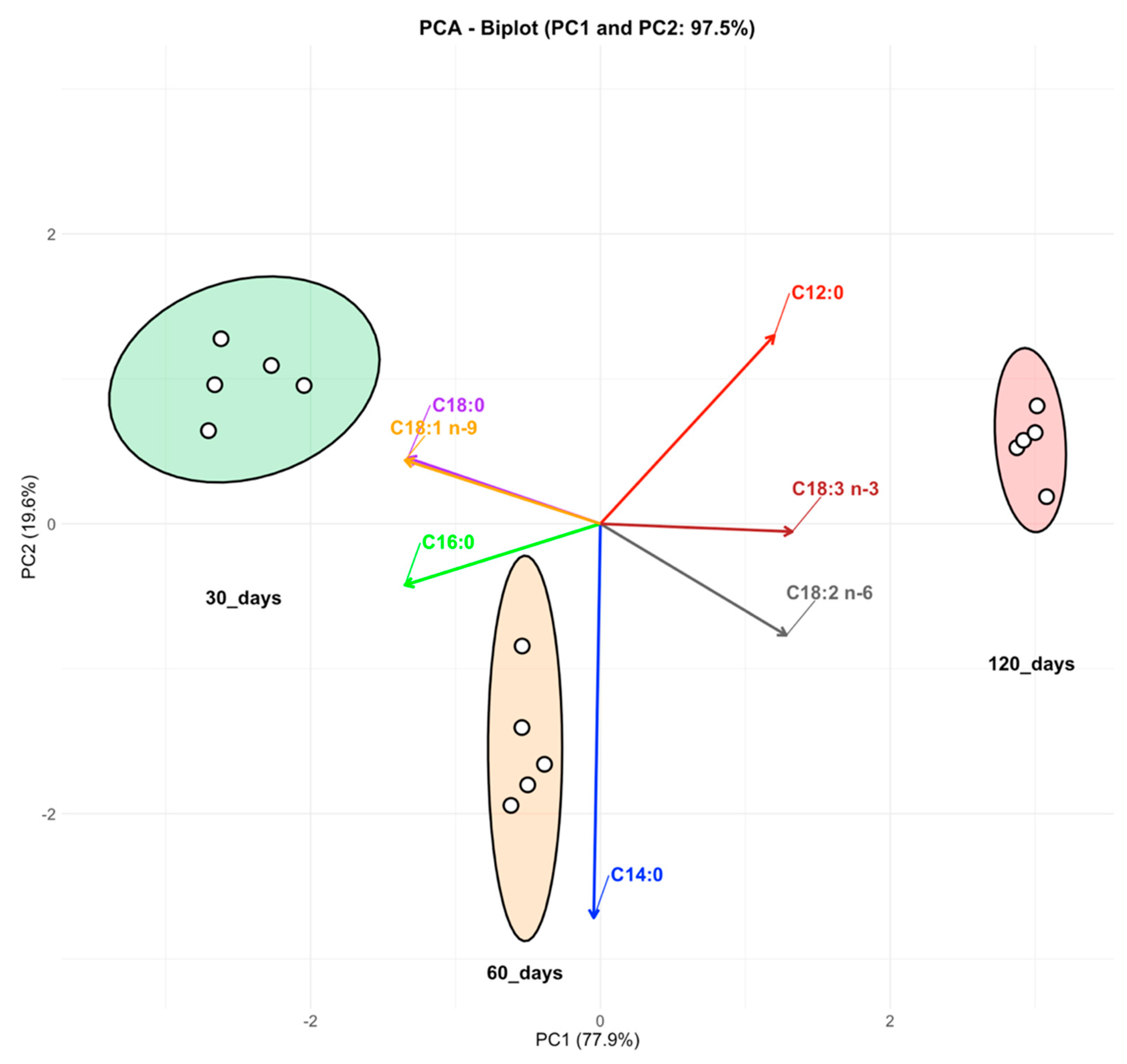
3.3. Fatty Acid Profile of Cheese
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Didonna, A.; Renna, M.; Santamaria, P. Traditional Italian Agri-Food Products: A Unique Tool with Untapped Potential. Agriculture 2023, 13, 1313. [Google Scholar] [CrossRef]
- Pilone, V.; De Lucia, C.; Del Nobile, M.A.; Contò, F. Policy Developments of Consumer’s Acceptance of Traditional Products Innovation: The Case of Environmental Sustainability and Shelf Life Extension of a PGI Italian Cheese. Trends Food Sci. Technol. 2015, 41, 83–94. [Google Scholar] [CrossRef]
- Wägeli, S.; Janssen, M.; Hamm, U. Organic Consumers’ Preferences and Willingness-to-Pay for Locally Produced Animal Products. Int. J. Consum. Stud. 2016, 40, 357–367. [Google Scholar] [CrossRef]
- Van Loo, E.J.; Grebitus, C.; Roosen, J. Explaining Attention and Choice for Origin Labeled Cheese by Means of Consumer Ethnocentrism. Food Qual. Prefer. 2019, 78, 103716. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale. Available online: https://www.gazzettaufficiale.it/eli/id/1999/10/12/099G0423/sg (accessed on 1 April 2025).
- Succi, M.; Aponte, M.; Tremonte, P.; Niro, S.; Sorrentino, E.; Iorizzo, M.; Tipaldi, L.; Pannella, G.; Panfili, G.; Fratianni, A.; et al. Variability in Chemical and Microbiological Profiles of Long-Ripened Caciocavallo Cheeses. J. Dairy Sci. 2016, 99, 9521–9533. [Google Scholar] [CrossRef] [PubMed]
- Muto, F.; Biondino, D.; Crisci, G.M.; Marabini, S.; Procopio, F.; Scarciglia, F.; Vai, G.B. Pages of Earth History in an Exceptional Uniqueness: The Geo-Heritage of the Sila National Park and Its Spheroidal Boulders Geosite (Northern Calabria, Italy). Geoheritage 2024, 16, 36. [Google Scholar] [CrossRef]
- Ercolini, D.; Frisso, G.; Mauriello, G.; Salvatore, F.; Coppola, S. Microbial Diversity in Natural Whey Cultures Used for the Production of Caciocavallo Silano PDO Cheese. Int. J. Food Microbiol. 2008, 124, 164–170. [Google Scholar] [CrossRef]
- Chilliard, Y.; Glasser, F.; Ferlay, A.; Bernard, L.; Rouel, J.; Doreau, M. Diet, Rumen Biohydrogenation and Nutritional Quality of Cow and Goat Milk Fat. Eur. J. Lipid Sci. Technol. 2007, 109, 828–855. [Google Scholar] [CrossRef]
- Martin, B.; Verdier-Metz, I.; Buchin, S.; Hurtaud, C.; Coulon, J.-B. How Do the Nature of Forages and Pasture Diversity Influence the Sensory Quality of Dairy Livestock Products? Anim. Sci. 2005, 81, 205–212. [Google Scholar] [CrossRef]
- Braghieri, A.; Piazzolla, N.; Romaniello, A.; Paladino, F.; Ricciardi, A.; Napolitano, F. Effect of Adjuncts on Sensory Properties and Consumer Liking of Scamorza Cheese. J. Dairy Sci. 2015, 98, 1479–1491. [Google Scholar] [CrossRef]
- Ablondi, M.; Sabbioni, A.; Stocco, G.; Cipolat-Gotet, C.; Dadousis, C.; van Kaam, J.-T.; Finocchiaro, R.; Summer, A. Genetic Diversity in the Italian Holstein Dairy Cattle Based on Pedigree and SNP Data Prior and After Genomic Selection. Front. Vet. Sci. 2022, 8, 773985. [Google Scholar] [CrossRef] [PubMed]
- Kotsampasi, B.; Karatzia, M.A.; Tsiokos, D.; Chadio, S. Nutritional Strategies to Alleviate Stress and Improve Welfare in Dairy Ruminants. Animals 2024, 14, 2573. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, O.; Samková, E.; Křížová, L.; Hasoňová, L.; Kala, R. Role of Fatty Acids in Milk Fat and the Influence of Selected Factors on Their Variability—A Review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef] [PubMed]
- Piraino, P.; Zotta, T.; Ricciardi, A.; Parente, E. Discrimination of Commercial Caciocavallo Cheeses on the Basis of the Diversity of Lactic Microflora and Primary Proteolysis. Int. Dairy J. 2005, 15, 1138–1149. [Google Scholar] [CrossRef]
- Morea, M.; Matarante, A.; Di Cagno, R.; Baruzzi, F.; Minervini, F. Contribution of Autochthonous Non-Starter Lactobacilli to Proteolysis in Caciocavallo Pugliese Cheese. Int. Dairy J. 2007, 17, 525–534. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Pasquale, I.; De Angelis, M.; Gobbetti, M. Accelerated Ripening of Caciocavallo Pugliese Cheese with Attenuated Adjuncts of Selected Nonstarter Lactobacilli. J. Dairy Sci. 2012, 95, 4784–4795. [Google Scholar] [CrossRef]
- AOAC 948.12; Moisture in Cheese. Method II (Rapid Screening). AOAC Official Method. Oxford University Press: Oxford, UK, 2002; 17th Ed. AOAC International, Gaithersburg, MD. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=1145 (accessed on 2 April 2025).
- AOAC 935.42-1935; Ash of Cheese. Gravimetric Method. AOAC International: Rockville, MD, USA, 2015. Available online: http://files.foodmate.com/2013/files_2824.html (accessed on 2 April 2025).
- AOAC 942.05; Ash of Animal Feed. AOAC Official Method. Oxford University Press: Oxford, UK, 1943. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&cPath=1&products_id=2464 (accessed on 2 April 2025).
- AOAC Official Method 920.39; Fat (Crude) or Ether Extract in Animal Feed|PDF. Official Methods of Analysis (2000) 17th Ed., AOAC INTERNATIONAL, Gaithersburg, MD, Official Method920.39. Available online: https://www.scribd.com/document/480055415/AOAC-920-39 (accessed on 2 April 2025).
- AOAC 933.05; Fat in Cheese. AOAC Official Method. Oxford University Press: Oxford, UK, 1996. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=366 (accessed on 2 April 2025).
- AOAC 920.39; Fat (Crude) or Ether Extract in Animal Feed. AOAC Official Method. Oxford University Press: Oxford, UK, 1920. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=1165 (accessed on 2 April 2025).
- AOAC 978.10; Fiber (Crude) in Animal Feed and Pet Food. AOAC Official Method. Oxford University Press: Oxford, UK, 1996. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R1169 (accessed on 2 April 2025).
- AOAC 973.18; Fiber (Acid Detergent) and Lignin (H2SO4) in Animal Feed. AOAC Official Method. Oxford University Press: Oxford, UK, 1977. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=1165 (accessed on 2 April 2025).
- European Parliament and Council. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 Text with EEA Relevance. Off. J. Eur. Union 2011, L 304, 18–63. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- ISO 5509:2000. Available online: https://www.iso.org/standard/11560.html (accessed on 2 April 2025).
- Galluzzo, F.G.; Cumbo, V.; Cammilleri, G.; Calabrese, V.; Pulvirenti, A.; Cicero, N.; Pantano, L.; Mascetti, A.; Lo Cascio, G.; Bacchi, E. Fatty Acids Composition of Stomach Oil of Scopoli’s Shearwater (Calonectris diomedea) from Linosa’s Colony. Animals 2022, 12, 1069. [Google Scholar] [CrossRef]
- Peycheva, K.; Panayotova, V.; Stancheva, R.; Makedonski, L.; Merdzhanova, A.; Cicero, N.; Parrino, V.; Fazio, F. Trace Elements and Omega-3 Fatty Acids of Wild and Farmed Mussels (Mytilus galloprovincialis) Consumed in Bulgaria: Human Health Risks. Int. J. Environ. Res. Public Health 2021, 18, 10023. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, A.; Cavallo, M.; Menchetti, L.; Angelucci, E.; Cartoni Mancinelli, A.; Vaudo, G.; Marconi, S.; Camilli, E.; Galli, F.; Castellini, C. The Healthy Fatty Index Allows for Deeper Insights into the Lipid Composition of Foods of Animal Origin When Compared with the Atherogenic and Thrombogenicity Indexes. Foods 2024, 13, 1568. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.; Theobald, H.E. The Health Effects of Dietary Unsaturated Fatty Acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Pantano, L.; Cascio, G.L.; Alongi, A.; Cammilleri, G.; Vella, A.; Macaluso, A.; Cicero, N.; Migliazzo, A.; Ferrantelli, V. Fatty Acids Determination in Bronte Pistachios by Gas Chromatographic Method. Nat. Prod. Res. 2016, 30, 2378–2382. [Google Scholar] [CrossRef]
- Moreira, R.V.; Costa, M.P.; Frasao, B.S.; Sobral, V.S.; Cabral, C.C.; Rodrigues, B.L.; Mano, S.B.; Conte-Junior, C.A. Effect of Ripening Time on Bacteriological and Physicochemical Goat Milk Cheese Characteristics. Food Sci. Biotechnol. 2020, 29, 459–467. [Google Scholar] [CrossRef]
- Maniaci, G.; Di Grigoli, A.; Bonanno, A.; Giosuè, C.; Ilardi, V.; Alabiso, M. Fatty Acids as Biomarkers of the Production Season of Caciocavallo Palermitano Cheese. Animals 2021, 11, 2675. [Google Scholar] [CrossRef]
- Bonanno, A.; Tornambè, G.; Bellina, V.; De Pasquale, C.; Mazza, F.; Maniaci, G.; Di Grigoli, A. Effect of Farming System and Cheesemaking Technology on the Physicochemical Characteristics, Fatty Acid Profile, and Sensory Properties of Caciocavallo Palermitano Cheese. J. Dairy Sci. 2013, 96, 710–724. [Google Scholar] [CrossRef]
- Scerra, M.; Chies, L.; Caparra, P.; Cilione, C.; Foti, F. Effect of Only Pasture on Fatty Acid Composition of Cow Milk and Ciminà Caciocavallo Cheese. J. Food Res. 2016, 5, 20. [Google Scholar] [CrossRef]
- Niro, S.; Succi, M.; Tremonte, P.; Sorrentino, E.; Coppola, R.; Panfili, G.; Fratianni, A. Evolution of Free Amino Acids during Ripening of Caciocavallo Cheeses Made with Different Milks. J. Dairy Sci. 2017, 100, 9521–9531. [Google Scholar] [CrossRef] [PubMed]
- Ianni, A.; Martino, C.; Innosa, D.; Bennato, F.; Grotta, L.; Martino, G. Zinc Supplementation of Lactating Dairy Cows: Effects on Chemical-Nutritional Quality and Volatile Profile of Caciocavallo Cheese. Asian-Australas. J. Anim. Sci. 2019, 33, 825. [Google Scholar] [CrossRef]
- Fallico, V.; McSweeney, P.L.H.; Siebert, K.J.; Horne, J.; Carpino, S.; Licitra, G. Chemometric Analysis of Proteolysis During Ripening of Ragusano Cheese*. J. Dairy Sci. 2004, 87, 3138–3152. [Google Scholar] [CrossRef]
- Panari, G.; Mariani, P.; Summer, A.; Guidetti, R.; Pecorari, M. Change of the Composition and Evolution of the Proteolysis of Parmigiano-Reggiano Cheese during Ripening as Regards the Shape (Outer and Inner Part) of the Wheel. Sci. E Tec. Latt.-Casearia 2003, 54, 199–212. [Google Scholar]
- Di Trana, A.; Sabia, E.; Di Rosa, A.R.; Addis, M.; Bellati, M.; Russo, V.; Dedola, A.S.; Chiofalo, V.; Claps, S.; Di Gregorio, P.; et al. Caciocavallo Podolico Cheese, a Traditional Agri-Food Product of the Region of Basilicata, Italy: Comparison of the Cheese’s Nutritional, Health and Organoleptic Properties at 6 and 12 Months of Ripening, and Its Digital Communication. Foods 2023, 12, 4339. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Masucci, F.; Napolitano, F.; Braghieri, A.; Romano, R.; Manzo, N.; Di Francia, A. Fatty Acid and Sensory Profiles of Caciocavallo Cheese as Affected by Management System. J. Dairy Sci. 2014, 97, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Tardiolo, G.; La Fauci, D.; Riggio, V.; Daghio, M.; Di Salvo, E.; Zumbo, A.; Sutera, A.M. Gut Microbiota of Ruminants and Monogastric Livestock: An Overview. Animals 2025, 15, 758. [Google Scholar] [CrossRef] [PubMed]
- Selner, D.R.; Schultz, L.H. Effects of Feeding Oleic Acid or Hydrogenated Vegetable Oils to Lactating Cows1. J. Dairy Sci. 1980, 63, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Steele, W.; Moore, J.H. The Effects of Mono-Unsaturated and Saturated Fatty Acids in the Diet on Milk-Fat Secretion in the Cow. J. Dairy Res. 1968, 35, 353–360. [Google Scholar] [CrossRef]
- Ros, E. Dietary Cis-Monounsaturated Fatty Acids and Metabolic Control in Type 2 Diabetes23. Am. J. Clin. Nutr. 2003, 78, 617S–625S. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M. Monounsaturated Fatty Acids and Risk of Cardiovascular Disease. Circulation 1999, 100, 1253–1258. [Google Scholar] [CrossRef]
- Naghshi, S.; Aune, D.; Beyene, J.; Mobarak, S.; Asadi, M.; Sadeghi, O. Dietary Intake and Biomarkers of Alpha Linolenic Acid and Risk of All Cause, Cardiovascular, and Cancer Mortality: Systematic Review and Dose-Response Meta-Analysis of Cohort Studies. BMJ 2021, 375, n2213. [Google Scholar] [CrossRef]
- Rodriguez, D.; Lavie, C.J.; Elagizi, A.; Milani, R.V. Update on Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Health. Nutrients 2022, 14, 5146. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Bishehkolaei, M.; Pathak, Y. Influence of Omega N-6/n-3 Ratio on Cardiovascular Disease and Nutritional Interventions. Hum. Nutr. Metab. 2024, 37, 200275. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Yu, Q.; Song, S.; Brenna, J.T.; Shen, Y.; Ye, K. Higher Ratio of Plasma Omega-6/Omega-3 Fatty Acids Is Associated with Greater Risk of All-Cause, Cancer, and Cardiovascular Mortality: A Population-Based Cohort Study in UK Biobank. eLife 2024, 12, RP90132. [Google Scholar] [CrossRef]
- Feeney, E.L.; Lamichhane, P.; Sheehan, J.J. The Cheese Matrix: Understanding the Impact of Cheese Structure on Aspects of Cardiovascular Health—A Food Science and a Human Nutrition Perspective. Int. J. Dairy Technol. 2021, 74, 656–670. [Google Scholar] [CrossRef]
- Mureşan, C.C.; Marc, R.A.; Anamaria Semeniuc, C.; Ancuţa Socaci, S.; Fărcaş, A.; Fracisc, D.; Rodica Pop, C.; Rotar, A.; Dodan, A.; Mureşan, V.; et al. Changes in Physicochemical and Microbiological Properties, Fatty Acid and Volatile Compound Profiles of Apuseni Cheese during Ripening. Foods 2021, 10, 258. [Google Scholar] [CrossRef]
- Katz, M.; Medina, R.; Gonzalez, S.; Oliver, G. Esterolytic and Lipolytic Activities of Lactic Acid Bacteria Isolated from Ewe’s Milk and Cheese. J. Food Prot. 2002, 65, 1997–2001. [Google Scholar] [CrossRef]
- Medina, R.B.; Katz, M.B.; González, S.; Oliver, G. Determination of Esterolytic and Lipolytic Activities of Lactic Acid Bacteria. In Public Health Microbiology: Methods and Protocols; Spencer, J.F.T., Ragout de Spencer, A.L., Eds.; Humana Press: Totowa, NJ, USA, 2004; pp. 465–470. ISBN 978-1-59259-766-6. [Google Scholar]
- Paszczyk, B.; Polak-Śliwińska, M.; Zielak-Steciwko, A.E. Chemical Composition, Fatty Acid Profile, and Lipid Quality Indices in Commercial Ripening of Cow Cheeses from Different Seasons. Animals 2022, 12, 198. [Google Scholar] [CrossRef]
- Paszczyk, B.; Łuczyńska, J. The Comparison of Fatty Acid Composition and Lipid Quality Indices in Hard Cow, Sheep, and Goat Cheeses. Foods 2020, 9, 1667. [Google Scholar] [CrossRef]
| Unifeed | % |
|---|---|
| Moisture | 8.8 |
| Ash | 5.3 |
| Protein | 12.4 |
| Fat | 4.0 |
| Fiber | 6.22 |
| NFE | 63.28 |
| Fatty Acid | Unifeed (%) |
|---|---|
| C8:0 | 1.19 |
| C10:0 | 2.99 |
| C11:0 | 0.05 |
| C12:0 | 0.06 |
| C13:0 | 0.03 |
| C14:0 | 0.11 |
| C15:0 | 0.02 |
| C15:1 | 0.01 |
| C16:0 | 12.54 |
| C16:1 n9 | 0.04 |
| C16:1 n7 | 0.08 |
| C17:0 | 0.09 |
| C17:1 | 0.03 |
| C18:0 | 2.95 |
| C18:1 n9 | 31.41 |
| C18:1 n7 | 0.82 |
| C18:2 | 47.67 |
| C18:3 n6 | 0.03 |
| C18:3 n3 | 1.74 |
| C20:0 | 0.64 |
| C20:1 n11 | 0.07 |
| C20:1 n9 | 0.43 |
| C20:2 n6 | 0.04 |
| C21:0 | 0.03 |
| C20:4 n3 | 0.02 |
| C22:0 | 0.40 |
| C22:1 n11 | 0.01 |
| C22:1 n9 | 0.07 |
| C23:0 | 0.08 |
| C24:0 | 0.38 |
| C24:1 | 0.06 |
| Caciocavallo Silano | Moisture | Ash | Protein | Fat | Salt | Lactose |
|---|---|---|---|---|---|---|
| 30 days | 46.55 ± 0.19 | 2.82 ± 0.01 | 24.52 ± 0.81 | 24.94 ± 0.04 | 1.17 ± 0.14 | <LOQ |
| 60 days | 37.20 ± 0.17 | 3.53 ± 0.04 | 29.08 ± 0.74 | 28.6 ± 0.13 | 1.59 ± 0.25 | <LOQ |
| 120 days | 32.0 ± 0.09 | 4.54 ± 0.18 | 31.5 ± 0.86 | 30.0 ± 0.07 | 1.96 ± 0.20 | <LOQ |
| Ripening | ||||
|---|---|---|---|---|
| Fatty Acid | 30 Days | 60 Days | 120 Days | Significance |
| C4:0 | 2.94 ± 0.25 | 2.97 ± 0.21 | 3.05 ± 0.16 | ns |
| C6:0 | 2.29 ± 0.09 | 2.60 ± 0.06 | 2.55 ± 0.53 | ns |
| C8:0 | 1.29 ± 0.05 c | 1.61 ± 0.06 b | 1.98 ± 0.05 a | *** |
| C10:0 | 3.18 ± 0.04 a | 2.45 ± 0.02 c | 2.77 ± 0.05 b | *** |
| C12:0 | 3.30 ± 0.04 b | 3.19 ± 0.04 c | 3.93 ± 0.02 a | *** |
| C13:0 | 0.06 ± 0.03 | 0.08 ± 0.04 | 0.10 ± 0.04 | ns |
| C14:0 | 10.90 ± 0.02 b | 11.10 ± 0.05 a | 10.93 ± 0.02 b | *** |
| C15:0 | 1.09 ± 0.11 ab | 1.15 ± 0.13 a | 0.93 ± 0.13 b | * |
| C16:0 | 32.72 ± 0.02 a | 32.50 ± 0.02 b | 31.69 ± 0.02 c | *** |
| C16:1 n9 | 0.05 ± 0.02 | 0.04 ± 0.01 | 0.06 ± 0.02 | ns |
| C16:1 n7 | 0.06 ± 0.03 b | 0.13 ± 0.02 a | 0.15 ± 0.03 a | *** |
| C17:0 | 0.66 ± 0.03 b | 1.01 ± 0.02 b | 1.10 ± 0.02 c | *** |
| C17:1 | 0.25 ± 0.02 c | 0.28 ± 0.01 b | 0.31 ± 0.02 a | *** |
| C18:0 | 11.74 ± 0.08 a | 11.45 ± 0.02 b | 11.19 ± 0.03 c | *** |
| C18:1 n9 | 22.60 ± 0.02 a | 21.95 ± 0.01 b | 21.33 ± 0.02 c | *** |
| C18:1 n7 | 0.03 ± 0.02 | 0.04 ± 0.02 | 0.03 ± 0.02 | ns |
| C18:2 n6 (LA) | 2.17 ± 0.03 c | 2.35 ± 0.03 b | 2.47 ± 0.03 a | *** |
| C18:3 n6 | 0.09 ± 0.01 c | 0.18 ± 0.02 b | 0.25 ± 0.01 a | *** |
| C18:3 n3 (ALA) | 0.44 ± 0.01 c | 0.49 ± 0.03 b | 0.59 ± 0.01 a | *** |
| C20:0 | 0.25 ± 0.01 b | 0.27 ± 0.02 b | 0.32 ± 0.02 a | *** |
| C20:1 n9 | 2.38 ± 0.04 b | 2.47 ± 0.03 a | 2.49 ± 0.02 a | *** |
| C20:2 n6 | 0.12 ± 0.02 a | 0.09 ± 0.02 b | 0.10 ± 0.01 ab | * |
| C20:3 n6 | 0.12 ± 0.02 a | 0.17 ± 0.02 a | 0.19 ± 0.02 a | *** |
| C20:4 n6 | 0.16 ± 0.05 | 0.15 ± 0.02 | 0.12 ± 0.02 | ns |
| C20:3 n3 | 0.01 ± 0.00 b | 0.03 ± 0.01 a | 0.02 ± 0.00 b | * |
| C20:5 n3 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | ns |
| C22:0 | 0.03 ± 0.02 ab | 0.02 ± 0.01 b | 0.04 ± 0.01 a | * |
| C22:1 n9 | 0.03 ± 0.02 | 0.04 ± 0.02 | 0.03 ± 0.01 | ns |
| C22:2 | 0.10 ± 0.02 | 0.10 ± 0.03 | 0.14 ± 0.01 | ns |
| C22:5 n6 | 0.05 ± 0.01 | 0.04 ± 0.02 | 0.04 ± 0.01 | ns |
| C22:5 n3 | 0.07 ± 0.03 a | 0.06 ± 0.02 a | 0.02 ± 0.01 b | * |
| C24:0 | 0.09 ± 0.02 c | 0.13 ± 0.02 b | 0.16 ± 0.01 a | *** |
| C22:6 n3 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | ns |
| C24:1 n9 | 0.13 ± 0.01 | 0.13 ± 0.02 | 0.10 ± 0.01 | ns |
| ∑SFA | 70.54 ± 0.45 | 70.61 ± 0.26 | 70.79 ± 0.46 | ns |
| ∑MUFA | 26.03 ± 0.12 a | 25.69 ± 0.08 b | 25.26 ± 0.06 c | *** |
| ∑PUFA | 3.42 ± 0.14 c | 3.74 ± 0.13 b | 4.00 ± 0.01 a | *** |
| ∑ n6/n3 | 4.60 ± 0.09 a | 4.70 ± 0.05 b | 4.60 ± 0.03 a | *** |
| AI | 3.19 ± 0.01 a | 3.27 ± 0.01 b | 3.32 ± 0.01 c | *** |
| TI | 4.31 ± 0.06 a | 4.36 ± 0.02 b | 4.38 ± 0.02 b | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tardiolo, G.; Di Salvo, E.; Tringali, S.; Bartolomeo, G.; Genovese, C.; Furfaro, M.E.; Sutera, A.M.; Virga, A.N.; Cicero, N.; Zumbo, A. Ripening-Associated Changes in Fatty Acid Composition and Nutritional Indices in Caciocavallo Silano PDO Cheese. Foods 2025, 14, 1566. https://doi.org/10.3390/foods14091566
Tardiolo G, Di Salvo E, Tringali S, Bartolomeo G, Genovese C, Furfaro ME, Sutera AM, Virga AN, Cicero N, Zumbo A. Ripening-Associated Changes in Fatty Acid Composition and Nutritional Indices in Caciocavallo Silano PDO Cheese. Foods. 2025; 14(9):1566. https://doi.org/10.3390/foods14091566
Chicago/Turabian StyleTardiolo, Giuseppe, Eleonora Di Salvo, Simona Tringali, Giovanni Bartolomeo, Claudia Genovese, Maria Elena Furfaro, Anna Maria Sutera, Antonino Nazareno Virga, Nicola Cicero, and Alessandro Zumbo. 2025. "Ripening-Associated Changes in Fatty Acid Composition and Nutritional Indices in Caciocavallo Silano PDO Cheese" Foods 14, no. 9: 1566. https://doi.org/10.3390/foods14091566
APA StyleTardiolo, G., Di Salvo, E., Tringali, S., Bartolomeo, G., Genovese, C., Furfaro, M. E., Sutera, A. M., Virga, A. N., Cicero, N., & Zumbo, A. (2025). Ripening-Associated Changes in Fatty Acid Composition and Nutritional Indices in Caciocavallo Silano PDO Cheese. Foods, 14(9), 1566. https://doi.org/10.3390/foods14091566










