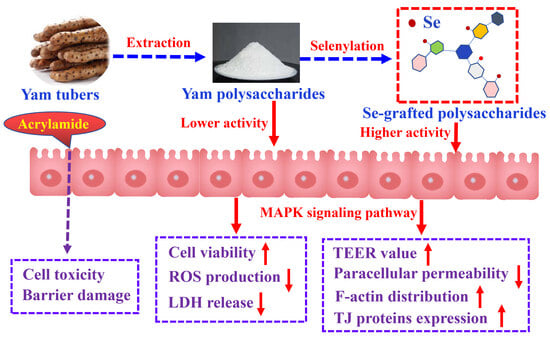
Covalent Grafting of Inorganic Selenium to the Water-Soluble and Nondigestive Chinese Yam Polysaccharides Causes Greater Protection of IEC-6 Cells with Acrylamide Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Line and Cell Culture
2.3. Preparation and Chemical Selenylation of YP
2.4. Assay of Cell Viability
2.5. Assay of Transepithelial Electrical Resistance (TEER) Value
2.6. Paracellular Permeability Assay
2.7. LDH Release Assay
2.8. Assay of ROS Production
2.9. Observation of the Intercellular Distribution of F-Actin
2.10. Quantitative Real-Time PCR and Western Blot Analyses
2.11. Statistical Analysis
3. Results
3.1. Cytotoxic Effect of Acrylamide on IEC-6 Cells
3.2. Effects of Three Polysaccharide Samples on LDH Release and ROS Level in Injured Cells
3.3. Effects of Three Polysaccharide Samples on Barrier Integrity of Injured Cells
3.4. Effects of Three Polysaccharide Samples on F-Actin Distribution Among IEC-6 Cells
3.5. Effects of Three Polysaccharide Samples on Expression of Three TJ Proteins in Injured Cells
3.6. Regulation of Three Polysaccharide Samples on MAPK Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthys, C.; Bilau, M.; Govaert, Y.; Moons, E.; De Henauw, S.; Willems, J.L. Risk assessment of dietary acrylamide intake in Flemish adolescents. Food Chem. Toxicol. 2005, 43, 271–278. [Google Scholar] [CrossRef]
- Chen, W.; Su, H.M.; Xu, Y.; Bao, T.; Zheng, X.D. Protective effect of wild raspberry (Rubus hirsutus Thunb.) extract against acrylamide-induced oxidative damage is potentiated after simulated gastrointestinal digestion. Food Chem. 2016, 196, 943–952. [Google Scholar] [CrossRef]
- Calleman, C.J. The metabolism and pharmacokinetics of acrylamide: Implications for mechanisms of toxicity and human risk estimation. Drug Metab. Rev. 1996, 28, 527–590. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, X.H. Chemical features of the oligochitosan-glycated caseinate digest and its enhanced protection on barrier function of the acrylamide-injured IEC-6 cells. Food Chem. 2019, 290, 246–254. [Google Scholar] [CrossRef]
- Kanu, A.N.; Ezeocha, C.V.; Ogunka, N.P. A review on bioactive compounds of yam varieties for human disease management. Asian Food Sci. J. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Huang, R.; Xie, J.H.; Yu, Y.; Shen, M.Y. Recent progress in the research of yam mucilage polysaccharides: Isolation, structure and bioactivities. Int. J. Biol. Macromol. 2020, 155, 1262–1269. [Google Scholar] [CrossRef]
- Pérez, S.; Mazeau, K.; du Penhoat, C.H. The three-dimensional structures of the pectic polysaccharides. Plant Physiol. Biochem. 2000, 38, 37–55. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.P.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Chen, J.; Zhang, D.Z.; Zhang, Y.F.; Wen, Y.H.; Li, L.H.; Zheng, L.H. Tumoricidal effects of a selenium (Se)-polysaccharide from Ziyang green tea on human osteosarcoma U-2 OS cells. Carbohydr. Polym. 2013, 98, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological activity of selenium and its impact on human health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Tang, Z.M.; Xiong, C.; Wu, F.F.; Zhao, J.R.; Zhao, X.-H. Enhanced Growth Inhibition and Apoptosis Induction in Human Colon Carcinoma HT-29 Cells of Soluble Longan Polysaccharides with a Covalent Chemical Selenylation. Nutrients 2022, 14, 1710. [Google Scholar] [CrossRef]
- Wang, Z.X.; Zhang, L.L.; Zhao, X.H. Antioxidant and Hypoglycemic Activities of the Soluble and Nondigestible Chinese Yam (Dioscorea opposita Thunb.) Polysaccharides with Covalent Se-Grafting. ACS Food Sci. Technol. 2025, 5, 734–742. [Google Scholar] [CrossRef]
- Hao, L.X.; Zhao, X.H. Immunomodulatory potentials of the water-soluble yam (Dioscorea opposita Thunb) polysaccharides for the normal and cyclophosphamide-suppressed mice. Food Agric. Immunol. 2016, 27, 667–677. [Google Scholar] [CrossRef]
- Surhio, M.M.; Wang, Y.; Xu, P.; Shah, F.; Li, J.; Ye, M. Antihyperlipidemic and hepatoprotective properties of selenium modified polysaccharide from Lachnum sp. Int. J. Biol. Macromol. 2017, 99, 88–95. [Google Scholar] [CrossRef]
- Sampath, C.; Chukkapalli, S.S.; Raju, A.; Alluri, L.S.C.; Srisai, D.; Gangula, P.R. Cinnamaldehyde protects against P. gingivalis induced intestinal epithelial barrier dysfunction in IEC-6 cells via the PI3K/Akt-Mediated NO/Nrf2 signaling pathway. Int. J. Mol. Sci. 2024, 25, 4734. [Google Scholar] [CrossRef]
- Yu, Y.H.; Wang, L.; Zhang, Q.; Zhang, X.N.; Zhao, X.H. Activities of the soluble and non-digestible longan (Dimocarpus longan Lour.) polysaccharides against HCT-116 cells as affected by a chemical selenylation. Curr. Res. Food Sci. 2022, 5, 1071–1083. [Google Scholar] [CrossRef]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef]
- Li, G.; Xiang, Y.; Zhao, J.; Chang, J.M. Saccharum alhagi polysaccharide-1 and -2 promote the immunocompetence of RAW264. 7 macrophages in vitro. Exp. Ther. Med. 2018, 15, e35563562. [Google Scholar] [CrossRef]
- Li, F.F.; Du, P.C.; Yang, W.Y.; Huang, D.F.; Nie, S.P.; Xie, M.Y. Polysaccharide from the seeds of Plantago asiatica L. alleviates nonylphenol induced intestinal barrier injury by regulating tight junctions in human Caco-2 cell line. Int. J. Biol. Macromol. 2020, 164, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhao, X.H.; Zhao, J.R.; Li, B.R. Galangin and kaempferol alleviate the indomethacin-caused cytotoxicity and barrier loss in rat intestinal epithelial (IEC-6) cells via mediating JNK/Src activation. ACS Omega 2021, 6, 15046–15056. [Google Scholar] [CrossRef]
- Canali, M.M.; Pedrotti, L.P.; Balsinde, J.; Ibarra, C.; Correa, S.G. Chitosan enhances transcellular permeability in human and rat intestine epithelium. Eur. J. Pharm. Biopharm. 2012, 80, e418425. [Google Scholar] [CrossRef]
- Drewe, J.; Beglinger, C.; Fricker, G. Effect of ischemia on intestinal permeability of lipopolysaccharides. Eur. J. Clin. Investig. 2001, 31, 138–144. [Google Scholar] [CrossRef]
- Li, Y.L.; Xu, B.; Xu, M.; Chen, D.P.; Xiong, Y.J.; Lian, M.Q.; Sun, Y.C.; Tang, Z.Y.; Wang, L.; Jiang, C.L.; et al. 6-Gingerol protects intestinal barrier from ischemia/reperfusion-induced damage via inhibition of p38 MAPK to NF-κB signalling. Pharmacol. Res. 2017, 119, 137–148. [Google Scholar] [CrossRef]
- Bouley, R.; Yui, N.; Terlouw, A.; Cheung, P.W.; Brown, D. Chlorpromazine induces basolateral aquaporin-2 accumulation via F-actin depolymerization and blockade of endocytosis in renal epithelial cells. Cell 2020, 9, 1057. [Google Scholar] [CrossRef]
- Heid, C.A.; Stevens, J.; Livak, K.J.; Williams, P.M. Real time quantitative PCR. Genome Res. 1996, 6, 986–994. [Google Scholar] [CrossRef]
- Yu, Y.H.; Zhao, X.H. Longan Polysaccharides with covalent selenylation combat the fumonisin B1-induced cell toxicity and barrier disruption in intestinal epithelial (IEC-6) cells. Nutrients 2023, 15, 4679. [Google Scholar] [CrossRef]
- Raldúa, D.; Casado, M.; Prats, E.; Faria, M.; Puig-Castellví, F.; Pérez, Y.; Alfonso, I.; Hsu, C.Y.; Arick II, M.A.; Garcia-Reyero, N.; et al. Targeting redox metabolism: The perfect storm induced by acrylamide poisoning in the brain. Sci. Rep. 2020, 10, e312. [Google Scholar] [CrossRef]
- Sahinturk, V.; Kacar, S.; Vejselova, D.; Kutlu, H.M. Acrylamide exerts its cytotoxicity in NIH/3T3 fibroblast cells by apoptosis. Toxicol. Ind. Health 2018, 34, 481–489. [Google Scholar] [CrossRef]
- Kacar, S.E.D.A.T.; Vejselova, D.; Kutlu, H.M.; Sahinturk, V. Acrylamide-derived cytotoxic, anti-proliferative, and apoptotic effects on A549 cells. Hum. Exp. Toxicol. 2018, 37, 468–474. [Google Scholar] [CrossRef]
- Nowak, A.; Zakłos-Szyda, M.; Żyżelewicz, D.; Koszucka, A.; Motyl, I. Acrylamide decreases cell viability, and provides oxidative stress, DNA damage, and apoptosis in human colon adenocarcinoma cell cine Caco-2. Molecules 2020, 25, 368. [Google Scholar] [CrossRef]
- Yan, F.F.; Zhao, L.; Chen, W.B.; Lu, Q.; Tang, C.; Wang, C.M.; Liu, R. Comparison of the inhibitory effects of procyanidins with different structures and their digestion products against acrylamide-induced cytotoxicity in IPEC-J2 cells. J. Funct. Foods 2020, 72, e104073. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Zhang, P.F.; Yang, D.X.; Xiao, J.H.; Hong, W.T.; Sun, H.M.; Xie, Q.Q.; Zeng, C.C. Artemisia argyi polysaccharide alleviates osmotic diarrhea by enhancing intestinal barrier protection and anti-inflammation. Int. J. Biol. Macromol. 2024, 282, e136779. [Google Scholar] [CrossRef]
- Li, Y.Y.; Sun, J.W.; Chen, L.; Lu, Y.M.; Wu, Q.X.; Yan, C.; Zhang, M.; Zhang, W.N. Structural characteristics of a polysaccharide from Armillariella tabescens and its protective effect on colitis mice via regulating gut microbiota and intestinal barrier function. Int. J. Biol. Macromol. 2024, 277, e133719. [Google Scholar] [CrossRef]
- Zhuang, S.; Ming, K.; Ma, N.; Sun, J.R.; Wang, D.H.; Ding, M.X.; Ding, Y. Portulaca oleracea L. polysaccharide ameliorates lipopolysaccharide-induced inflammatory responses and barrier dysfunction in porcine intestinal epithelial monolayers. J. Funct. Foods 2022, 91, e104997. [Google Scholar] [CrossRef]
- Shen, L. Tight junctions on the move: Molecular mechanisms for epithelial barrier regulation. Ann. N. Y. Acad. Sci. 2012, 1258, 9–18. [Google Scholar] [CrossRef]
- Moonwiriyakit, A.; Pathomthongtaweechai, N.; Steinhagen, P.R.; Chantawichitwong, P.; Satianrapapong, W.; Pongkorpsakol, P. Tight junctions: From molecules to gastrointestinal diseases. Tissue Barriers 2023, 11, 2077620. [Google Scholar] [CrossRef]
- Marcos-Ramiro, B.; García-Weber, D.; Millán, J. TNF-induced endothelial barrier disruption: Beyond actin and Rho. Thromb. Haemost. 2014, 112, 1088–1102. [Google Scholar] [CrossRef]
- Jin, M.Z.; Zhang, W.Q.; Zhang, X.M.; Huang, Q.L.; Chen, H.; Ye, M. Characterization, chemical modification and bioactivities of a polysaccharide from Stropharia rugosoannulata. Process Biochem. 2023, 128, 30–39. [Google Scholar] [CrossRef]
- Wang, H.F.; Yuan, M.Y.; Li, G.H.; Tao, Y.X.; Wang, X.Y.; Ke, S.; Zhang, M.; Wang, A.Q.; Zhou, Z. Chemical characterization, antioxidant and immunomodulatory activities of acetylated polysaccharides from Cyperus esculentus. Food Chem. 2023, 427, e136734. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Li, Q.Y.; Bao, A.J.; Liu, X.R.; Zeng, J.Y.; Yang, X.P.; Yang, J.; Zhang, J.; Lei, Z. Synthesis of selenium-containing Artemisia sphaerocephala polysaccharides: Solution conformation and anti-tumor activities in vitro. Carbohydr. Polym. 2016, 152, 70–78. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.K.; Bogoyevitch, M.A. The c-Jun N-terminal protein kinase family of mitogen-activated protein kinases (JNK MAPKs). Int. J. Biochem. Cell Biol. 2001, 33, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Kim, S.; Chung, H.T.; Pae, H.O. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013, 528, 27–48. [Google Scholar] [CrossRef]
- Cheng, X.X.; Zhu, Y.H.; Huang, J.H.; Li, Y.F.; Jiang, X.L.; Yang, Q. A neutral polysaccharide from Persicaria hydropiper (L.) Spach ameliorates lipopolysaccharide-induced intestinal barrier injury via regulating the gut microbiota and modulating AKT/PI3K/mTOR and MAPK signaling pathways. J. Ethnopharmacol. 2024, 320, e117403. [Google Scholar] [CrossRef]
- Peng, L.; Guo, F.H.; Pei, M.J.; Tsao, R.; Wang, X.Y.; Jiang, L.; Sun, Y.; Xiong, H. Anti-inflammatory effect of lentil hull (Lens culinaris) extract via MAPK/NF-κB signaling pathways and effects of digestive products on intestinal barrier and inflammation in Caco-2 and Raw264. 7 co-culture. J. Funct. Foods 2022, 92, e105044. [Google Scholar] [CrossRef]
- Hu, P.; Zong, Q.F.; Zhao, Y.H.; Gu, H.T.; Liu, Y.Y.; Gu, F.; Liu, H.Y.; Ahmed, A.A.; Bao, W.H.; Cai, D. Lactoferrin attenuates intestinal barrier dysfunction and inflammation by modulating the MAPK pathway and gut microbes in mice. J. Nutr. 2022, 152, 2451–2460. [Google Scholar] [CrossRef]









| Genes | Primer Sequences (5′–3′) |
|---|---|
| ZO-1 | Forward: CCACCTCGCACGTATCACAAGC |
| Reverse: GGCAATGACACTCCTTCGTCTCTG | |
| Occludin | Forward: CCTCCTTACAGGCCGGATGA |
| Reverse: AGCATTGGTCGAACGTGCAT | |
| Claudin-1 | Forward: GTTTCATCCTGGCTTCGCTG |
| Reverse: AGCAGTCACGATGTTGTCCC | |
| p-p38 | Forward: CCTCAGCTCAGCGAGAGAAT |
| Reverse: GGCACATTTAAGCTGGGCAC | |
| p-JNK | Forward: GCGACTGGAATGAGAACACAG |
| Reverse: CTGGAACTTACTGAAGCCACC | |
| GAPDH | Forward: CCCTCTGGAAAGCTGTGG |
| Reverse: GCTTCACCACCTTCTTGATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-X.; Zhang, L.-L.; Zhao, X.-H. Covalent Grafting of Inorganic Selenium to the Water-Soluble and Nondigestive Chinese Yam Polysaccharides Causes Greater Protection of IEC-6 Cells with Acrylamide Injury. Foods 2025, 14, 1560. https://doi.org/10.3390/foods14091560
Wang Z-X, Zhang L-L, Zhao X-H. Covalent Grafting of Inorganic Selenium to the Water-Soluble and Nondigestive Chinese Yam Polysaccharides Causes Greater Protection of IEC-6 Cells with Acrylamide Injury. Foods. 2025; 14(9):1560. https://doi.org/10.3390/foods14091560
Chicago/Turabian StyleWang, Zhen-Xing, Li-Li Zhang, and Xin-Huai Zhao. 2025. "Covalent Grafting of Inorganic Selenium to the Water-Soluble and Nondigestive Chinese Yam Polysaccharides Causes Greater Protection of IEC-6 Cells with Acrylamide Injury" Foods 14, no. 9: 1560. https://doi.org/10.3390/foods14091560
APA StyleWang, Z.-X., Zhang, L.-L., & Zhao, X.-H. (2025). Covalent Grafting of Inorganic Selenium to the Water-Soluble and Nondigestive Chinese Yam Polysaccharides Causes Greater Protection of IEC-6 Cells with Acrylamide Injury. Foods, 14(9), 1560. https://doi.org/10.3390/foods14091560