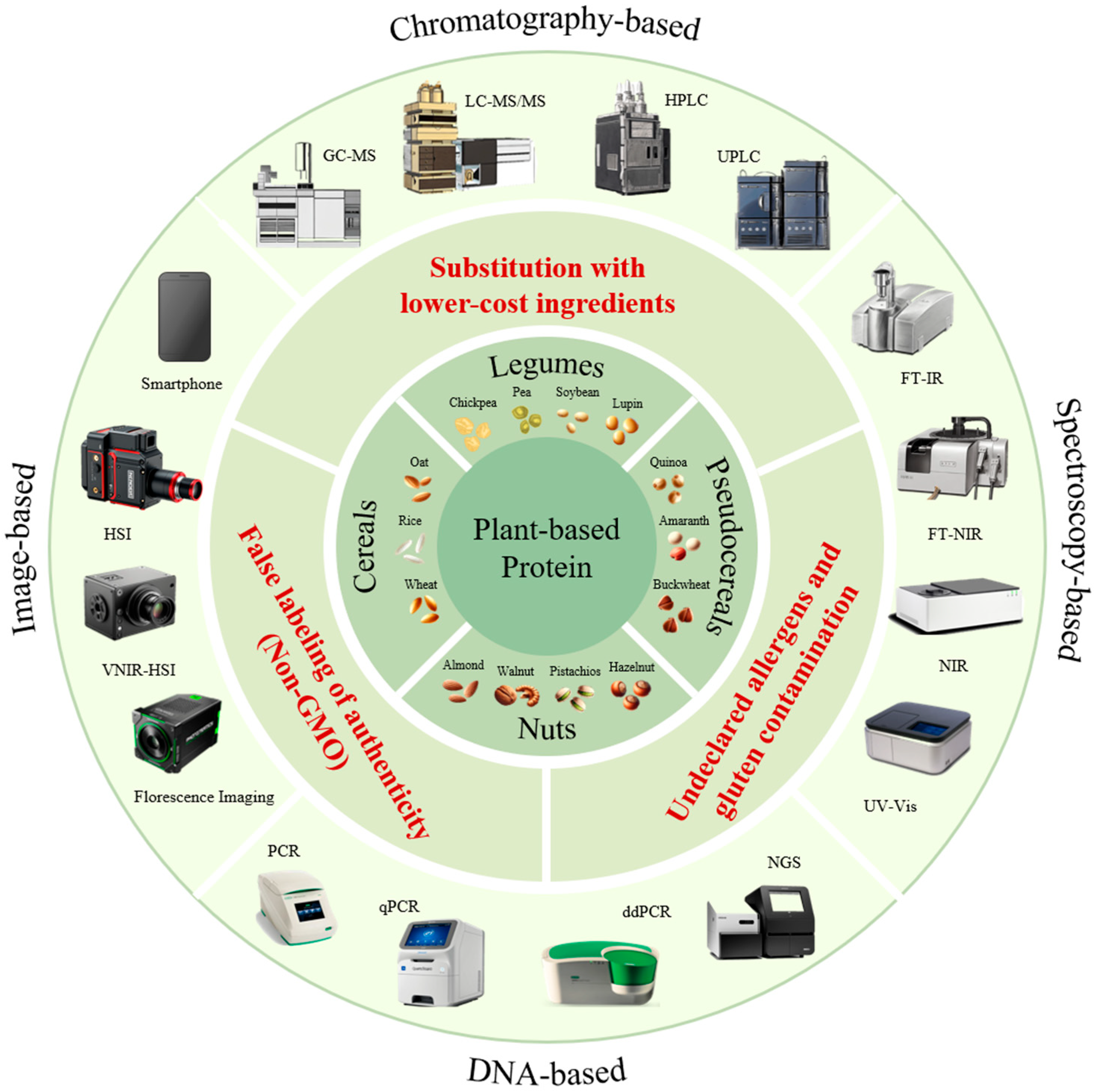
Food Fraud in Plant-Based Proteins: Analytical Strategies and Regulatory Perspectives
Abstract
1. Introduction
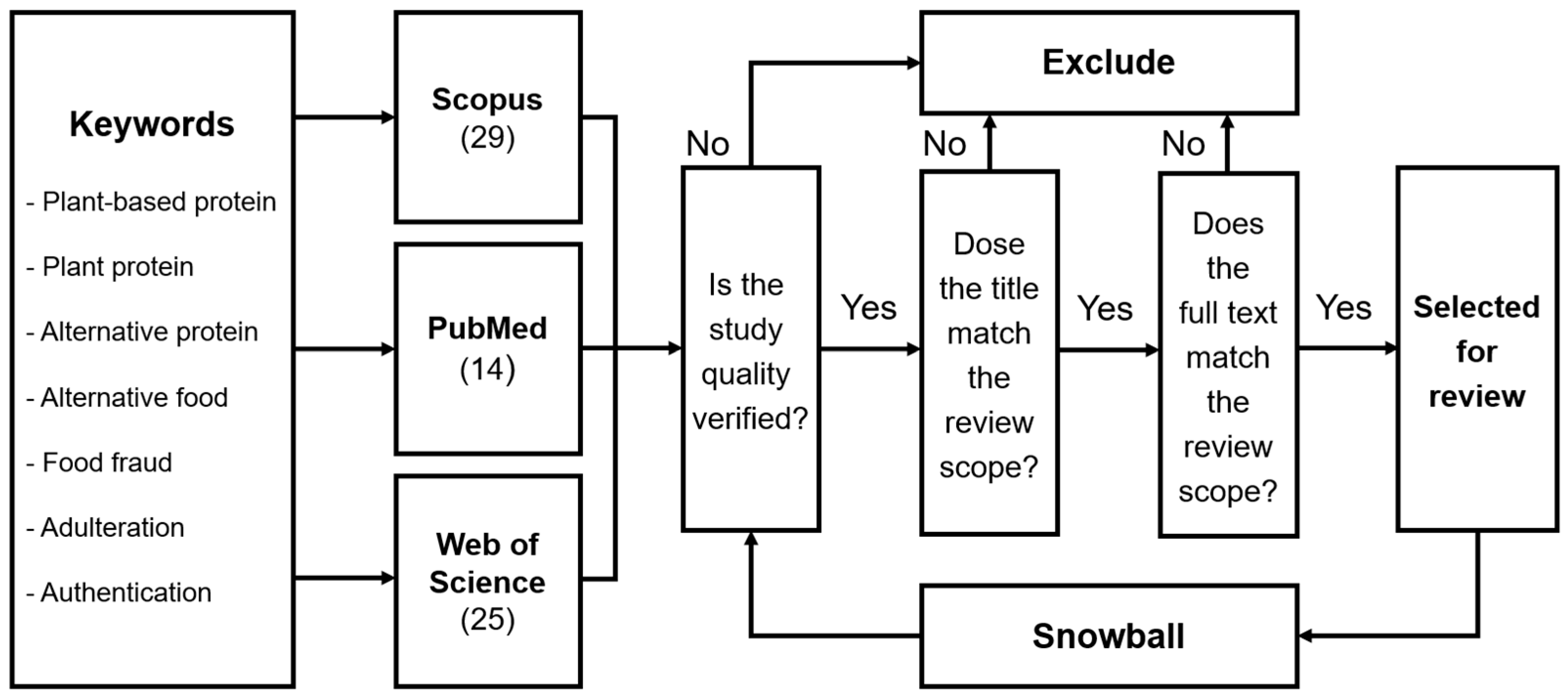
2. Methodological Framework for Literature Review
3. Comprehensive Review of Major Plant Protein Sources
3.1. Soybean
3.2. Pea
3.3. Lupin
3.4. Chickpea
3.5. Wheat
3.6. Oat
3.7. Rice
3.8. Nuts
| Category | Plant Source | Nutritional Properties | Functional Properties | References |
|---|---|---|---|---|
| Legumes | Soybean | High in essential amino acids (lysine and threonine); low in fat; rich in isoflavones | Strong emulsification, gelation, and water-holding capacity; commonly used in meat analogues | [30,31,32,33,34] |
| Pea | Rich in lysine, threonine, valine, and leucine; non-GMO, cholesterol-free, and cost-effective protein source | Gel formation, foam stabilization, and emulsification; high-moisture extrusion enables fibrous meat-like textures | [7,39,40,41] | |
| Lupin | Good lysine content but low in cysteine and methionine | Strong emulsification and gelling properties; enhances nutritional value when combined with cereals | [42,43,45,46] | |
| Chickpea | Rich in isoleucine, lysine, and tryptophan; lower allergenicity compared to soybean; contains bioactive peptides | Hypolipidemic and antihypertensive effects; good protein bioavailability | [48,49,50,51,52,53] | |
| Cereals | Wheat | High in gluten-forming proteins (gliadin and glutenin); rich in essential amino acids, except lysine | Strong viscoelastic properties due to gluten network formation; ideal for bakery and texturized protein products | [33,54] |
| Oat | Moderate protein content (12–20%); low gluten; limited levels of sulfur-containing amino acids | High thermal stability; good gel formation | [58,59,60] | |
| Rice | Gluten-free; rich in glutamine and proline; low in lysine and methionine; high digestibility | Poor solubility; improved via enzymatic hydrolysis and high-pressure processing | [61,62,65] | |
| Nuts | Almond, walnut, pistachio, hazelnut | High protein content with balanced amino acid profile; rich in tocopherols, B vitamins, and beneficial fatty acids | Strong emulsifying and foaming properties; used in plant-based beverages and dairy alternatives | [67,68,69] |
4. Food Fraud Risks Associated with the Plant-Based Protein Industry
| Category | Characteristics | Examples | References |
|---|---|---|---|
| Economically motivated adulteration (EMA) | Deliberate substitution, dilution, or misrepresentation of plant protein sources for economic gain. This includes the addition of lower-cost ingredients or inflation of protein content using non-protein nitrogen compounds. | Adulteration of high-value plant proteins (e.g., chickpea flour) with lower-cost alternatives (e.g., pea and grass pea). Fraudulent substitution of premium durum wheat with common wheat. Melamine contamination to falsely increase protein content in plant-based protein powders. | [77,78,79,82,83,84,85,86] |
| Mislabeling and supply chain integrity violations | False claims regarding the origin, processing method, or composition of plant-based protein products. This includes the misrepresentation of genetically modified status, organic labeling fraud, and false species declaration. | Fraudulent labeling of GMO soy as non-GMO to exploit premium pricing. False country-of-origin claims, such as selling common rice as premium basmati rice to increase market value. | [15,80,87,88] |
| Health and safety risks | Unintentional or intentional contamination of plant-based protein sources with allergens, gluten, or toxic compounds, posing risks to consumers. | Adulteration of gluten-free oat products with wheat, rye, or barley. Cross-contamination of quinoa flour with undeclared soy, maize, or wheat proteins. Undisclosed presence of allergens in plant-based protein formulations, leading to severe allergic reactions. | [89,90,91,92] |
5. Detection Methods for Food Fraud in Plant-Based Proteins
5.1. Chromatography-Based Detection
5.2. DNA-Based Detection
5.3. Spectroscopy-Based Detection
| Analytical Methods | Food | Adulterant and Fraud | References | |
|---|---|---|---|---|
| Chromatography-based | LC-MS/MS | Meat products | Adulteration with 23 plant-based proteins (LOD < 200 µg/g) | [95] |
| LC-MS/MS | Plant-based products | Detection of grains (buckwheat, wheat, rye, barley, oats); LOD between 0.028 and 0.056 g/L | [96] | |
| LC-MS/MS | Grain products | Nitrogen-rich adulterants to artificially inflate protein content | [100] | |
| UPLC-MS | Lupin and lentil seeds | Eight types of lupin and lentil seeds | [99] | |
| UPLC-MS/MS | Plant protein beverages | Almond, peanut, walnut, and soybean adulteration (LOQs between 0.01 and 0.5 g/L) | [97] | |
| UPLC-MS/MS | Durum wheat | Common wheat (LOD < 100 µg/g) | [98] | |
| GC-MS | Basmati rice | Seven different rice varieties | [103] | |
| GC-MS | Cereal grains and oilseed plants | Differentiation among plant species | [104] | |
| DNA-based | PCR | Pea flour | Wheat or soy adulteration (LOD 0.1%) | [105] |
| qPCR | Chickpea, quinoa, coix seed, and rice | Detection of unique gene markers (LOD < 0.01%) | [106] | |
| qPCR | Plant-based products | Vertebrate contamination (threshold 0.1%) | [107] | |
| qPCR | Durum wheat | Common wheat (LOD < 0.15%) | [108] | |
| ddPCR | Soybean | Quantification of GMO adulterants (LOD 0.01%) | [110] | |
| ddPCR | Soybean | Quantification of five genetically modified soybean events (LOQ 0.1%) | [109] | |
| ddPCR | Durum wheat | Common wheat, rye, and barley (LOD < 1%) | [111] | |
| DNA Barcoding | Black-gram-based products | Detection of wheat and white pea flour adulteration (5% contamination) | [84] | |
| NGS | Plant protein powder supplements | Diverse species contamination (soybean, chia seeds, quinoa, etc.) | [112] | |
| Spectroscopy-based | FT-IR | Wheat flour | Adulteration with barley flour (0.30% detection) | [113] |
| FT-MIR | Quinoa flour | Adulteration with soybean, maize, and wheat flours (1–10%) | [90] | |
| NIR | Chickpea flour | Maize flour adulteration (1–90%) | [115] | |
| NIR | Hazelnut | Almond or chickpea adulteration (3%) | [116] | |
| NIR | Cashew | Adulteration with peanut, Brazil nut, macadamia, and pecan (0.1–10%) | [117] | |
| FT-NIR | Pistachio powder | Adulteration with green pea and peanut (5–40%) | [118] | |
| FT-NIR | Plant protein powders | Authentication of whey, soy, and wheat adulteration (10–40%) | [119] | |
| Imaging-based | HSI | Quinoa flour | Detection of wheat, rice, soybean, and corn contamination with chemometric analysis (R2 = 0.99) | [120] |
| HSI | Wheat flour | Peanut and walnut adulteration detection (LOD 0.03%) | [121] | |
| SWIR-HSI | Almond powder | Peanut adulteration detection (100% specificity) | [122] | |
| VNIR-HSI | Ground beef | Soy protein adulteration (LOD 0.74%) | [123] | |
| HSI | Wheat flour | Peanut and walnut powder adulteration detection (LOD 0.5%) | [124] | |
| Visible Imaging with AI | Rice varieties | Authentication and fraud detection (93–99% accuracy) | [125] | |
| Multiple Imaging Sensor | Skimmed milk powder | Detection of plant protein adulterants (10–50%) | [126] | |
5.4. Imaging-Based Detection
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| qPCR | Quantitative polymerase chain reaction |
| dPCR | Digital polymerase chain reaction |
| ddPCR | Droplet digital polymerase chain reaction |
| PCR | Polymerase chain reaction |
| NGS | Next-generation sequencing |
| DNA | Deoxyribonucleic acid |
| GMO | Genetically modified organism |
| EMA | Economically motivated adulteration |
| FT-IR | Fourier-transform infrared spectroscopy |
| FT-MIR | Fourier-transform mid-infrared spectroscopy |
| NIR | Near-infrared spectroscopy |
| FT-NIR | Fourier-transform near-infrared spectroscopy |
| HSI | Hyperspectral imaging |
| SWIR-HSI | Shortwave infrared hyperspectral imaging |
| VNIR-HSI | Visible and near-infrared hyperspectral imaging |
| AI | Artificial intelligence |
| LC-MS/MS | Liquid chromatography tandem mass spectrometry |
| UPLC-MS | Ultra-performance liquid chromatography–mass spectrometry |
| UPLC-MS/MS | Ultra-performance liquid chromatography–tandem mass spectrometry |
| GC-MS | Gas chromatography–mass spectrometry |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| VOC | Volatile organic compound |
References
- Lee, S.Y.; Lee, D.Y.; Jeong, J.W.; Kim, J.H.; Yun, S.H.; Joo, S.-T.; Choi, I.; Choi, J.S.; Kim, G.-D.; Hur, S.J. Studies on meat alternatives with a focus on structuring technologies. Food Bioprocess Technol. 2023, 16, 1389–1412. [Google Scholar] [CrossRef]
- Wood, P.; Tavan, M. A review of the alternative protein industry. Curr. Opin. Food Sci. 2022, 47, 100869. [Google Scholar] [CrossRef]
- Khanashyam, A.C.; Mundanat, A.S.; Sajith Babu, K.; Thorakkattu, P.; Krishnan, R.; Abdullah, S.; Bekhit, A.E.A.; McClements, D.J.; Santivarangkna, C.; Nirmal, N.P. Emerging alternative food protein sources: Production process, quality parameters, and safety point of view. Crit. Rev. Biotechnol. 2025, 45, 1–22. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A. Food in the anthropocene: The EAT–lancet commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. A brief review of the science behind the design of healthy and sustainable plant-based foods. NPJ Sci. Food 2021, 5, 17. [Google Scholar] [CrossRef]
- Ahmad, M.; Qureshi, S.; Akbar, M.H.; Siddiqui, S.A.; Gani, A.; Mushtaq, M.; Hassan, I.; Dhull, S.B. Plant-based meat alternatives: Compositional analysis, current development and challenges. Appl. Food Res. 2022, 2, 100154. [Google Scholar] [CrossRef]
- Pastrana-Pastrana, Á.J.; Rodríguez-Herrera, R.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C. Plant proteins, insects, edible mushrooms and algae: More sustainable alternatives to conventional animal protein. J. Future Foods 2025, 5, 248–256. [Google Scholar] [CrossRef]
- Possidónio, C.; Prada, M.; Graça, J.; Piazza, J. Consumer perceptions of conventional and alternative protein sources: A mixed-methods approach with meal and product framing. Appetite 2021, 156, 104860. [Google Scholar] [CrossRef]
- Grossmann, L.; Weiss, J. Alternative protein sources as technofunctional food ingredients. Annu. Rev. Food Sci. Technol. 2021, 12, 93–117. [Google Scholar] [CrossRef]
- Hong, E.; Lee, S.Y.; Jeong, J.Y.; Park, J.M.; Kim, B.H.; Kwon, K.; Chun, H.S. Modern analytical methods for the detection of food fraud and adulteration by food category. J. Sci. Food Agric. 2017, 97, 3877–3896. [Google Scholar] [CrossRef]
- Aslam, N.; Fatima, R.; Altemimi, A.B.; Ahmad, T.; Khalid, S.; Hassan, S.A.; Aadil, R.M. Overview of industrial food fraud and authentication through chromatography technique and its impact on public health. Food Chem. 2024, 460, 140542. [Google Scholar] [CrossRef]
- Spink, J.; Bedard, B.; Keogh, J.; Moyer, D.C.; Scimeca, J.; Vasan, A. International survey of food fraud and related terminology: Preliminary results and discussion. J. Food Sci. 2019, 84, 2705–2718. [Google Scholar] [CrossRef] [PubMed]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant proteins: Assessing their nutritional quality and effects on health and physical function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef] [PubMed]
- Faller, A.C.; Kesanakurti, P.; Arunachalam, T. Chapter 14—Fraud in grains and cereals. In Food Fraud; Hellberg, R.S., Everstine, K., Sklare, S.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 281–308. [Google Scholar] [CrossRef]
- Fuso, A.; Leni, G.; Prandi, B.; Lolli, V.; Caligiani, A. Novel foods/feeds and novel frauds: The case of edible insects. Trends Food Sci. Technol. 2024, 147, 104457. [Google Scholar] [CrossRef]
- Vinothkanna, A.; Dar, O.I.; Liu, Z.; Jia, A.-Q. Advanced detection tools in food fraud: A systematic review for holistic and rational detection method based on research and patents. Food Chem. 2024, 446, 138893. [Google Scholar] [CrossRef] [PubMed]
- Kitchenham, B. Procedures for Performing Systematic Reviews. Keele Univ. 2004, 33, 1–26. [Google Scholar]
- Arrizubieta, J.I.; Ukar, O.; Ostolaza, M.; Mugica, A. Study of the Environmental Implications of Using Metal Powder in Additive Manufacturing and Its Handling. Metals 2020, 10, 261. [Google Scholar] [CrossRef]
- Wohlin, C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. In Proceedings of the 18th International Conference on Evaluation Assessment in Software Engineering, Berlin, Germany, 30 March 2014; p. 38. [Google Scholar]
- Kennedy, S.P. Chapter 2—History of food fraud and development of mitigation requirements and standards. In Food Fraud; Hellberg, R.S., Everstine, K., Sklare, S.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 9–22. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Delcour, J.A.; Joye, I.J.; Pareyt, B.; Wilderjans, E.; Brijs, K.; Lagrain, B. Wheat gluten functionality as a quality determinant in cereal-based food products. Annu. Rev. Food Sci. Technol. 2012, 3, 469–492. [Google Scholar] [CrossRef]
- Campbell, L.; Rempel, C.B.; Wanasundara, J.P.D. Canola/rapeseed protein: Future opportunities and directions—Workshop proceedings of IRC 2015. Plants 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Munialo, C.D. A review of alternative plant protein sources, their extraction, functional characterisation, application, nutritional value and pinch points to being the solution to sustainable food production. Int. J. Food Sci. 2024, 59, 462–472. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Zhao, Y.; Jiang, L.; Sui, X. Structural, extraction and safety aspects of novel alternative proteins from different sources. Food Chem. 2024, 436, 137712. [Google Scholar] [CrossRef]
- Meenakshi Sundaram, G.S.; Das, D.; Emiola-Sadiq, T.; Sajeeb Khan, A.; Zhang, L.; Meda, V. Developments in the Dry Fractionation of Plant Components: A Review. Separations 2024, 11, 332. [Google Scholar] [CrossRef]
- Pulivarthi, M.K.; Buenavista, R.M.; Bangar, S.P.; Li, Y.; Pordesimo, L.O.; Bean, S.R.; Siliveru, K. Dry Fractionation Process Operations in the Production of Protein Concentrates: A Review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4670–4697. [Google Scholar] [CrossRef]
- Hewage, A.; Olatunde, O.O.; Nimalaratne, C.; Malalgoda, M.; Aluko, R.E.; Bandara, N. Novel Extraction technologies for developing plant protein ingredients with improved functionality. Trends Food Sci. Technol. 2022, 129, 492–511. [Google Scholar] [CrossRef]
- Zhang, T.; Dou, W.; Zhang, X.; Zhao, Y.; Zhang, Y.; Jiang, L.; Sui, X. The development history and recent updates on soy protein-based meat alternatives. Trends Food Sci. Technol. 2021, 109, 702–710. [Google Scholar] [CrossRef]
- Matsumiya, K.; Murray, B.S. Soybean protein isolate gel particles as foaming and emulsifying agents. Food Hydrocoll. 2016, 60, 206–215. [Google Scholar] [CrossRef]
- Sun, C.; Ge, J.; He, J.; Gan, R.; Fang, Y. Processing, quality, safety, and acceptance of meat analogue products. Engineering 2021, 7, 674–678. [Google Scholar] [CrossRef]
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel protein sources for applications in meat-alternative products—Insight and challenges. Foods 2022, 11, 957. [Google Scholar] [CrossRef]
- Thrane, M.; Paulsen, P.V.; Orcutt, M.W.; Krieger, T.M. Chapter 2—Soy Protein: Impacts, production, and applications. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 23–45. [Google Scholar] [CrossRef]
- Katz, Y.; Gutierrez-Castrellon, P.; González, M.G.; Rivas, R.; Lee, B.W.; Alarcon, P. A Comprehensive review of sensitization and allergy to soy-based products. Clin. Rev. Allergy Immunol. 2014, 46, 272–281. [Google Scholar] [CrossRef]
- Wang, J.; He, Z.; Raghavan, V. Soybean allergy: Characteristics, mechanisms, detection and its reduction through novel food processing techniques. Crit. Rev. Food Sci. Nutr. 2023, 63, 6182–6195. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Baumgartner, S.; Lauter, K. Soybean (Glycine max) allergens—A review on an outstanding plant food with allergenic potential. ACS Food Sci. Technol. 2023, 3, 363–378. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 2593–2605. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, X.; Cheng, Y.; Cui, B.; Abd El-Aty, A.M. Impact of high moisture contents on the structure and functional properties of pea protein isolate during extrusion. Food Hydrocoll. 2022, 127, 107508. [Google Scholar] [CrossRef]
- Maningat, C.C.; Jeradechachai, T.; Buttshaw, M.R. Textured wheat and pea proteins for meat alternative applications. Cereal Chem. 2022, 99, 37–66. [Google Scholar] [CrossRef]
- Fontanari, G.G.; Batistuti, J.P.; Cruz, R.J.d.; Saldiva, P.H.N.; Arêas, J.A.G. Cholesterol-lowering effect of whole lupin (Lupinus albus) seed and its protein isolate. Food Chem. 2012, 132, 1521–1526. [Google Scholar] [CrossRef]
- Shrestha, S.; Hag, L.v.t.; Haritos, V.S.; Dhital, S. Lupin proteins: Structure, isolation and application. Trends Food Sci. Technol. 2021, 116, 928–939. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Torres, A.; Frias, J.; Vidal-Valverde, C. Semolina supplementation with processed lupin and pigeon pea flours improve protein quality of pasta. LWT-Food Sci. Technol. 2010, 43, 617–622. [Google Scholar] [CrossRef]
- Mennini, M.; Dahdah, L.; Mazzina, O.; Fiocchi, A. Lupin and other potentially cross-reactive allergens in peanut allergy. Curr. Allergy Asthma Rep. 2016, 16, 84. [Google Scholar] [CrossRef]
- Peeters, K.A.B.M.; Nordlee, J.A.; Penninks, A.H.; Chen, L.; Goodman, R.E.; Bruijnzeel-Koomen, C.A.F.M.; Hefle, S.L.; Taylor, S.L.; Knulst, A.C. Lupine allergy: Not simply cross-reactivity with peanut or soy. J. Allergy Clin. Immunol. 2007, 120, 647–653. [Google Scholar] [CrossRef]
- van de Noort, M. Chapter 11—Lupin: An important protein and nutrient source. In Sustainable Protein Sources, 2nd ed.; Nadathur, S., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 219–239. [Google Scholar] [CrossRef]
- Boukid, F. Chickpea (Cicer arietinum L.) protein as a prospective plant-based ingredient: A review. Int. J. Food Sci. 2021, 56, 5435–5444. [Google Scholar] [CrossRef]
- Xing, Q.; Dekker, S.; Kyriakopoulou, K.; Boom, R.M.; Smid, E.J.; Schutyser, M.A. Enhanced nutritional value of chickpea protein concentrate by dry separation and solid state fermentation. Innov. Food Sci. Emerg. Technol. 2020, 59, 102269. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, K.; Bai, Y.; Li, B.; Xu, W. Effect of high intensity ultrasound on physicochemical, interfacial and gel properties of chickpea protein isolate. LWT 2020, 129, 109563. [Google Scholar] [CrossRef]
- Shi, W.; Hou, T.; Guo, D.; He, H. Evaluation of hypolipidemic peptide (Val-Phe-Val-Arg-Asn) virtual screened from chickpea peptides by pharmacophore model in high-fat diet-induced obese rat. J. Funct. Foods 2019, 54, 136–145. [Google Scholar] [CrossRef]
- Gupta, N.; Bhagyawant, S.S. Enzymatic treatment improves ACE-I inhibiton and antiproliferative potential of chickpea. Vegetos 2019, 32, 363–369. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; del Mar Contreras, M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef]
- Flambeau, M.; Le Bourgot, C.; Van der Mijnsbrugge, A.; Respondek, F.; Redl, A. Chapter 4—Proteins from wheat: Sustainable production and new developments in nutrition-based and functional applications. In Sustainable Protein Sources, 2nd ed.; Nadathur, S., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 77–91. [Google Scholar] [CrossRef]
- Sharma, P.; Kotari, S. Barley: Impact of processing on physicochemical and thermal properties—A review. Food Rev. Int. 2017, 33, 359–381. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chemistry of wheat gluten proteins: Qualitative composition. Cereal Chem. 2023, 100, 23–35. [Google Scholar] [CrossRef]
- Anzani, C.; Boukid, F.; Drummond, L.; Mullen, A.M.; Álvarez, C. Optimising the use of proteins from rich meat co-products and non-meat alternatives: Nutritional, technological and allergenicity challenges. Food Res. Int. 2020, 137, 109575. [Google Scholar] [CrossRef] [PubMed]
- Mel, R.; Malalgoda, M. Oat protein as a novel protein ingredient: Structure, functionality, and factors impacting utilization. Cereal Chem. 2022, 99, 21–36. [Google Scholar] [CrossRef]
- Paudel, D.; Dhungana, B.; Caffe, M.; Krishnan, P. A review of health-beneficial properties of oats. Foods 2021, 10, 2591. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, C.; Bao, Y.; Liu, F.; Wang, H.; Wang, Y. Oat: Current state and challenges in plant-based food applications. Trends Food Sci. Technol. 2023, 134, 56–71. [Google Scholar] [CrossRef]
- Quiñones, R.S.; Macachor, C.P.; Quiñones, H.G. Development of gluten-free composite flour blends. Trop. Technol. J. 2015, 19, 3. [Google Scholar] [CrossRef]
- Sousa, R.; Portmann, R.; Dubois, S.; Recio, I.; Egger, L. Protein digestion of different protein sources using the INFOGEST static digestion model. Food Res. Int. 2020, 130, 108996. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Suen, P.K.; Zhang, C.-Q.; Mak, W.S.; Gu, M.-H.; Liu, Q.-Q.; Sun, S.S.-M. Improved growth performance, food efficiency, and lysine availability in growing rats fed with lysine-biofortified rice. Sci. Rep. 2017, 7, 1389. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Choi, I.; Han, J. Construction of rice protein-based meat analogues by extruding process: Effect of substitution of soy protein with rice protein on dynamic energy, appearance, physicochemical, and textural properties of meat analogues. Food Res. Int. 2022, 161, 111840. [Google Scholar] [CrossRef]
- Roy, T.; Singh, A.; Sari, T.P.; Homroy, S. Rice protein: Emerging insights of extraction, structural characteristics, functionality, and application in the food industry. J. Food Compost. Anal. 2023, 123, 105581. [Google Scholar] [CrossRef]
- Gonçalves, B.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Cosme, F. Composition of nuts and their potential health benefits-an overview. Foods 2023, 12, 942. [Google Scholar] [CrossRef]
- Qamar, S.; Manrique, Y.J.; Parekh, H.; Falconer, J.R. Nuts, cereals, seeds and legumes proteins derived emulsifiers as a source of plant protein beverages: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2742–2762. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; McKay, D.L.; Blumberg, J.B. The phytochemical composition and antioxidant actions of tree nuts. Asia Pac. J. Clin. Nutr. 2010, 19, 117. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC5012104/ (accessed on 25 April 2025).
- Tian, L.; You, X.; Zhang, S.; Zhu, Z.; Yi, J.; Jin, G. Enhancing functional properties and protein structure of almond protein isolate using high-power ultrasound treatment. Molecules 2024, 29, 3590. [Google Scholar] [CrossRef] [PubMed]
- Luparelli, A.; Losito, I.; De Angelis, E.; Pilolli, R.; Lambertini, F.; Monaci, L. Tree nuts and peanuts as a source of beneficial compounds and a threat for allergic consumers: Overview on methods for their detection in complex food products. Foods 2022, 11, 728. [Google Scholar] [CrossRef]
- Cuadrado, C.; Sanchiz, Á.; Linacero, R. Nut allergenicity: Effect of food processing. Allergies 2021, 1, 150–162. [Google Scholar] [CrossRef]
- Manning, L. Food fraud: Policy and food chain. Curr. Opin. Food Sci. 2016, 10, 16–21. [Google Scholar] [CrossRef]
- Moore, J.C.; DeVries, J.W.; Lipp, M.; Griffiths, J.C.; Abernethy, D.R. Total protein methods and their potential utility to reduce the risk of food protein adulteration. Compr. Rev. Food Sci. Food Saf. 2010, 9, 330–357. [Google Scholar] [CrossRef]
- Chan, E.Y.Y.; Griffiths, S.M.; Chan, C.W. Public-health risks of melamine in milk products. Lancet 2008, 372, 1444–1445. [Google Scholar] [CrossRef]
- Gimonkar, S.; Van Fleet, E.E.; Boys, K.A. Chapter 13—Dairy product fraud. In Food Fraud; Hellberg, R.S., Everstine, K., Sklare, S.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 249–279. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting nitrogen into protein—Beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Gossner, C.M.-E.; Schlundt, J.; Ben Embarek, P.; Hird, S.; Lo-Fo-Wong, D.; Beltran, J.J.O.; Teoh, K.N.; Tritscher, A. The melamine incident: Implications for international food and feed safety. Environ. Health Perspect. 2009, 117, 1803–1808. [Google Scholar] [CrossRef]
- Dobson, R.L.; Motlagh, S.; Quijano, M.; Cambron, R.T.; Baker, T.R.; Pullen, A.M.; Regg, B.T.; Bigalow-Kern, A.S.; Vennard, T.; Fix, A. Identification and characterization of toxicity of contaminants in pet food leading to an outbreak of renal toxicity in cats and dogs. Toxicol. Sci. 2008, 106, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Vega-Castellote, M.; Sánchez, M.-T.; Torres-Rodríguez, I.; Entrenas, J.-A.; Pérez-Marín, D. NIR sensing technologies for the detection of fraud in nuts and nut products: A review. Foods 2024, 13, 1612. [Google Scholar] [CrossRef] [PubMed]
- Everstine, K.; Spink, J.; Kennedy, S. Economically motivated adulteration (EMA) of food: Common characteristics of EMA incidents. J. Food Prot. 2013, 76, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Bosmali, I.; Ganopoulos, I.; Madesis, P.; Tsaftaris, A. Microsatellite and DNA-barcode regions typing combined with High Resolution Melting (HRM) analysis for food forensic uses: A case study on lentils (Lens culinaris). Food Res. Int. 2012, 46, 141–147. [Google Scholar] [CrossRef]
- Bala, M.; Sethi, S.; Sharma, S.; Mridula, D.; Kaur, G. Non-destructive determination of grass pea and pea flour adulteration in chickpea flour using near-infrared reflectance spectroscopy and chemometrics. J. Sci. Food Agric. 2023, 103, 1294–1302. [Google Scholar] [CrossRef]
- Scarafoni, A.; Ronchi, A.; Duranti, M. A real-time PCR method for the detection and quantification of lupin flour in wheat flour-based matrices. Food Chem. 2009, 115, 1088–1093. [Google Scholar] [CrossRef]
- Amane, D.; Ananthanarayan, L. Detection of adulteration in black gram-based food products using DNA barcoding. Food Control 2019, 104, 193–200. [Google Scholar] [CrossRef]
- Campmajó, G.; Saez-Vigo, R.; Saurina, J.; Núñez, O. High-performance liquid chromatography with fluorescence detection fingerprinting combined with chemometrics for nut classification and the detection and quantitation of almond-based product adulterations. Food Control 2020, 114, 107265. [Google Scholar] [CrossRef]
- Taylan, O.; Cebi, N.; Yilmaz, M.T.; Sagdic, O.; Ozdemir, D.; Balubaid, M. Rapid detection of green-pea adulteration in pistachio nuts using raman spectroscopy and chemometrics. J. Sci. Food Agric. 2021, 101, 1699–1708. [Google Scholar] [CrossRef]
- Desquilbet, M.; Bullock, D.S. Who pays the costs of Non-GMO segregation and identity preservation? Am. J. Agric. Econ. 2009, 91, 656–672. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Abu-Hassiba, A.E.-H.G.; ElRouby, M.T.; Abou-Hashim, F.; Omar, H.S. Food adulteration with genetically modified soybeans and maize, meat of animal species and ractopamine residues in different food products. Electron. J. Biotechnol. 2022, 55, 65–77. [Google Scholar] [CrossRef]
- Protudjer, J.L.P.; Roth-Walter, F.; Meyer, R. Nutritional considerations of plant-based diets for people with food allergy. Clin. Exp. Allergy 2024, 54, 895–908. [Google Scholar] [CrossRef]
- Rodríguez, S.D.; Rolandelli, G.; Buera, M.P. Detection of quinoa flour adulteration by means of FT-MIR spectroscopy combined with chemometric methods. Food Chem. 2019, 274, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.-S.; Tan, J.; Xie, J.-Y.; Li, M.-F. Rapid, simultaneous and non-destructive determination of maize flour and soybean flour adulterated in quinoa flour by front-face synchronous fluorescence spectroscopy. Food Control 2021, 130, 108329. [Google Scholar] [CrossRef]
- Worosz, M.R.; Wilson, N.L. A cautionary tale of purity, labeling and product literacy in the gluten-free market. J. Consum. Aff. 2012, 46, 288–318. [Google Scholar] [CrossRef]
- Fernando, I.; Jiangang, F.; Stephen, C.; and Close, D.C. A review of the emerging technologies and systems to mitigate food fraud in supply chains. Crit. Rev. Food Sci. Nutr. 2024, 1–28. [Google Scholar] [CrossRef]
- Williams, M.L.; Olomukoro, A.A.; Emmons, R.V.; Godage, N.H.; Gionfriddo, E. Matrix effects demystified: Strategies for resolving challenges in analytical separations of complex samples. J. Sep. Sci. 2023, 46, e2300571. [Google Scholar] [CrossRef]
- Spörl, J.; Speer, K.; Jira, W. A rapid LC-MS/MS multi-method for the detection of 23 foreign protein sources from legumes, oilseeds, grains, egg and milk in meat products. J. Food Compost. Anal. 2023, 124, 105628. [Google Scholar] [CrossRef]
- Seki, Y.; Nakamura, K.; Arimoto, C.; Kikuchi, H.; Yamakawa, H.; Nagai, H.; Ito, T.; Akiyama, H. Development of a simple and reliable high-performance liquid chromatography–tandem mass spectrometry approach to simultaneously detect grains specified in food allergen labeling regulation on processed food commodities. J. Chromatogr. A 2021, 1639, 461877. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Liu, Z.; Yang, Z.; Ma, J.; Zhang, Y.; Li, Q. Adulteration detection of plant protein beverages by UPLC-MS/MS based on signature peptide of allergen. Food Sci. Hum. Wellness 2024, 13, 3371–3380. [Google Scholar] [CrossRef]
- Russo, R.; Cusano, E.; Perissi, A.; Ferron, F.; Severino, V.; Parente, A.; Chambery, A. Ultra-high performance liquid chromatography tandem mass spectrometry for the detection of durum wheat contamination or adulteration. J. Mass Spectrom. 2014, 49, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Khattab, A.R.; Maamoun, A.A.; Kropf, M.; Heiss, A.G. UPLC-MS metabolome based classification of Lupinus and Lens seeds: A prospect for phyto-equivalency of its different accessions. Food Res. Int. 2019, 115, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.; Bessaire, T.; Tarres, A.; Goyon, A.; Delatour, T. Development of a quantitative multi-compound method for the detection of 14 nitrogen-rich adulterants by LC-MS/MS in food materials. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Bönick, J.; Huschek, G.; Rawel, H.M. Determination of wheat, rye and spelt authenticity in bread by targeted peptide biomarkers. J. Food Compost. Anal. 2017, 58, 82–91. [Google Scholar] [CrossRef]
- Nichani, K.; Uhlig, S.; Colson, B.; Hettwer, K.; Simon, K.; Bönick, J.; Uhlig, C.; Kemmlein, S.; Stoyke, M.; Gowik, P.; et al. Development of non-targeted mass spectrometry method for distinguishing spelt and wheat. Foods 2023, 12, 141. [Google Scholar] [CrossRef]
- Shannon, M.; Ratnasekhar, C.H.; McGrath, T.F.; Kapil, A.P.; Elliott, C.T. A two-tiered system of analysis to tackle rice fraud: The Indian Basmati study. Talanta 2021, 225, 122038. [Google Scholar] [CrossRef]
- Pastor, K.; Ilić, M.; Vujić, D.; Jovanović, D.; Ačanski, M. Characterization of fatty acids in cereals and oilseeds from the Republic of Serbia by gas chromatography—Mass spectrometry (GC/MS) with chemometrics. Anal. Lett. 2020, 53, 1177–1189. [Google Scholar] [CrossRef]
- Shin, J.; Kim, M.J.; Kim, H.Y. Development of triplex PCR for simultaneous detection of soybean and wheat. Food Sci. Biotechnol. 2021, 30, 801–806. [Google Scholar] [CrossRef]
- Zheng, Q.; Yin, X.; Yang, A.; Yu, N.; Xing, R.; Chen, Y.; Deng, R.; Cao, J. Precise authenticity of quinoa, coix Seed, wild rice and chickpea components using optimized TaqMan real-time PCR. Foods 2023, 12, 852. [Google Scholar] [CrossRef]
- Cottenet, G.; Blancpain, C. A real-time PCR method to assess the presence of vertebrate material in plant-based products. Food Control 2021, 125, 108001. [Google Scholar] [CrossRef]
- Carloni, E.; Amagliani, G.; Omiccioli, E.; Ceppetelli, V.; Del Mastro, M.; Rotundo, L.; Brandi, G.; Magnani, M. Validation and application of a quantitative real-time PCR assay to detect common wheat adulteration of durum wheat for pasta production. Food Chem. 2017, 224, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Yan, W.; He, Y.; Dong, L.; Xing, Z.; Li, C.; Xia, W.; Li, F. Development of a duplex digital PCR method to quantify five genetically modified soybean events. Food Anal. Methods 2022, 15, 294–306. [Google Scholar] [CrossRef]
- Demeke, T.; Eng, M. Detection of soybean GMO events using two multiplex droplet digital PCR assays. J AOAC Int. 2024, 108, 23–28. [Google Scholar] [CrossRef]
- Schulze, C.; Geuthner, A.C.; Mäde, D. Development and validation of a method for quantification of common wheat, durum wheat, rye and barley by droplet digital PCR. Eur. Food Res. Technol. 2021, 247, 2267–2283. [Google Scholar] [CrossRef]
- Faller, A.C.; Arunachalam, T.; Shanmughanandhan, D.; Kesanakurti, P.; Shehata, H.R.; Ragupathy, S.; Newmaster, S.G. Investigating appropriate molecular and chemical methods for ingredient identity testing of plant-based protein powder dietary supplements. Sci. Rep. 2019, 9, 12130. [Google Scholar] [CrossRef]
- Arslan, F.N.; Akin, G.; Karuk Elmas, Ş.N.; Üner, B.; Yilmaz, I.; Janssen, H.-G.; Kenar, A. FT-IR spectroscopy with chemometrics for rapid detection of wheat flour adulteration with barley flour. J. Verbrauch. Lebensm. 2020, 15, 245–261. [Google Scholar] [CrossRef]
- Dayananda, B.; Chahwala, P.; Cozzolino, D. The ability of near-infrared spectroscopy to discriminate plant protein mixtures: A preliminary study. AppliedChem 2023, 3, 428–436. [Google Scholar] [CrossRef]
- Bala, M.; Sethi, S.; Sharma, S.; Mridula, D.; Kaur, G. Prediction of maize flour adulteration in chickpea flour (besan) using near infrared spectroscopy. J. Food Sci. Technol. 2022, 59, 3130–3138. [Google Scholar] [CrossRef]
- López, M.I.; Trullols, E.; Callao, M.P.; Ruisánchez, I. Multivariate screening in food adulteration: Untargeted versus targeted modelling. Food Chem. 2014, 147, 177–181. [Google Scholar] [CrossRef]
- Miaw, C.S.W.; Martins, M.L.C.; Sena, M.M.; de Souza, S.V.C. Screening Method for the Detection of Other Allergenic Nuts in Cashew Nuts Using Chemometrics and a Portable Near-Infrared Spectrophotometer. Food Anal. Methods 2022, 15, 1074–1084. [Google Scholar] [CrossRef]
- Aykas, D.P.; Menevseoglu, A. A rapid method to detect green pea and peanut adulteration in pistachio by using portable FT-MIR and FT-NIR spectroscopy combined with chemometrics. Food Control 2021, 121, 107670. [Google Scholar] [CrossRef]
- De Géa Neves, M.; Poppi, R.J.; Breitkreitz, M.C. Authentication of plant-based protein powders and classification of adulterants as whey, soy protein, and wheat using FT-NIR in tandem with OC-PLS and PLS-DA models. Food Control 2022, 132, 108489. [Google Scholar] [CrossRef]
- Wu, Q.; Mousa, M.A.A.; Al-Qurashi, A.D.; Ibrahim, O.H.M.; Abo-Elyousr, K.A.M.; Rausch, K.; Abdel Aal, A.M.K.; Kamruzzaman, M. Global calibration for non-targeted fraud detection in quinoa flour using portable hyperspectral imaging and chemometrics. Curr. Res. Food Sci. 2023, 6, 100483. [Google Scholar] [CrossRef]
- Zheng, L.; Bao, Q.; Weng, S.; Tao, J.; Zhang, D.; Huang, L.; Zhao, J. Determination of adulteration in wheat flour using multi-grained cascade forest-related models coupled with the fusion information of hyperspectral imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 270, 120813. [Google Scholar] [CrossRef]
- Faqeerzada, M.A.; Lohumi, S.; Kim, G.; Joshi, R.; Lee, H.; Kim, M.S.; Cho, B.-K. Hyperspectral shortwave infrared image analysis for detection of adulterants in almond powder with one-class classification method. Sensors 2020, 20, 5855. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, X.; Ru, Y.; Chen, Q.; Wang, J.; Xu, L.; Zhou, H. Detection and visualization of soybean protein powder in ground beef using visible and near-infrared hyperspectral imaging. Infrared Phys. Technol. 2022, 127, 104401. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Ni, X.; Chu, X.; Li, Y.-F.; Sun, C. Evaluation of near-infrared hyperspectral imaging for detection of peanut and walnut powders in whole wheat flour. Appl. Sci. 2018, 8, 1076. [Google Scholar] [CrossRef]
- Izquierdo, M.; Lastra-Mejías, M.; González-Flores, E.; Pradana-López, S.; Cancilla, J.C.; Torrecilla, J.S. Visible imaging to convolutionally discern and authenticate varieties of rice and their derived flours. Food Control 2020, 110, 106971. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Alewijn, M.; Wijtten, M.; Weesepoel, Y. Detecting fraudulent additions in skimmed milk powder using a portable, hyphenated, optical multi-sensor approach in combination with one-class classification. Food Control 2021, 121, 107744. [Google Scholar] [CrossRef]
- Hassoun, A.; Måge, I.; Schmidt, W.F.; Temiz, H.T.; Li, L.; Kim, H.-Y.; Nilsen, H.; Biancolillo, A.; Aït-Kaddour, A.; Sikorski, M.; et al. Fraud in animal origin food products: Advances in emerging spectroscopic detection methods over the past five years. Foods 2020, 9, 1069. [Google Scholar] [CrossRef]
| Category | Chromatography-Based | DNA-Based | Spectroscopy-Based | Imaging-Based |
|---|---|---|---|---|
| Methods | LC-MS/MS, GC-MS, HPLC, UPLC-MS/MS | PCR, qPCR, ddPCR, NGS | FT-IR, NIR, Raman Spectroscopy | Hyperspectral Imaging, AI-assisted Visible Imaging |
| Accuracy | High to very high | Very high | Moderate to high | Moderate to high |
| Analysis speed | Moderate (30 min–2 h) | Fast to slow (30 min–48 h) | Very fast (few seconds–minutes) | Fast (real-time processing) |
| Cost | High | Moderate to high | Low to moderate | Moderate to high |
| Major applications | Detection of protein adulteration, nitrogen-rich fraud, species authentication | Species authentication, GMO detection, allergen identification | Rapid screening for ingredient substitution, non-destructive authentication | Real-time food fraud detection, authentication of powdered products |
| Advantages | High sensitivity and specificity, capable of detecting small molecular adulterants | Highly specific, capable of detecting gene even in processed foods | Non-destructive, rapid analysis, portable instruments available | Provides spatial and spectral data, enables AI-assisted real-time detection |
| Limitations | High cost, requires specialized training and equipment | Cannot directly detect protein adulteration, requires intact DNA | Limited sensitivity for minor adulterants, may require extensive calibration | High cost, requires AI-driven analysis, lower sensitivity for chemical adulteration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ham, J.-H.; Lee, Y.-J.; Lee, S.-S.; Kim, H.-Y. Food Fraud in Plant-Based Proteins: Analytical Strategies and Regulatory Perspectives. Foods 2025, 14, 1548. https://doi.org/10.3390/foods14091548
Ham J-H, Lee Y-J, Lee S-S, Kim H-Y. Food Fraud in Plant-Based Proteins: Analytical Strategies and Regulatory Perspectives. Foods. 2025; 14(9):1548. https://doi.org/10.3390/foods14091548
Chicago/Turabian StyleHam, Jun-Hyeok, Yeon-Jung Lee, Seung-Su Lee, and Hae-Yeong Kim. 2025. "Food Fraud in Plant-Based Proteins: Analytical Strategies and Regulatory Perspectives" Foods 14, no. 9: 1548. https://doi.org/10.3390/foods14091548
APA StyleHam, J.-H., Lee, Y.-J., Lee, S.-S., & Kim, H.-Y. (2025). Food Fraud in Plant-Based Proteins: Analytical Strategies and Regulatory Perspectives. Foods, 14(9), 1548. https://doi.org/10.3390/foods14091548








