Goat Milk-Derived Extracellular Vesicles Alleviate Colitis Potentially Through Improved Gut Microbiota in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of GMEVs
2.2. Characterization of GMEVs
2.3. In Vitro Digestion Assay of GMEVs
2.4. Animal Experiments
2.5. Measurement of Serum Cytokines
2.6. Western Bolt Analysis
2.7. qRT-PCR Analysis
2.8. Histology and Immunohistochemistry Analysis
2.9. Microbiota 16S rRNA Gene Sequencing
2.10. Statistical Analysis
3. Results
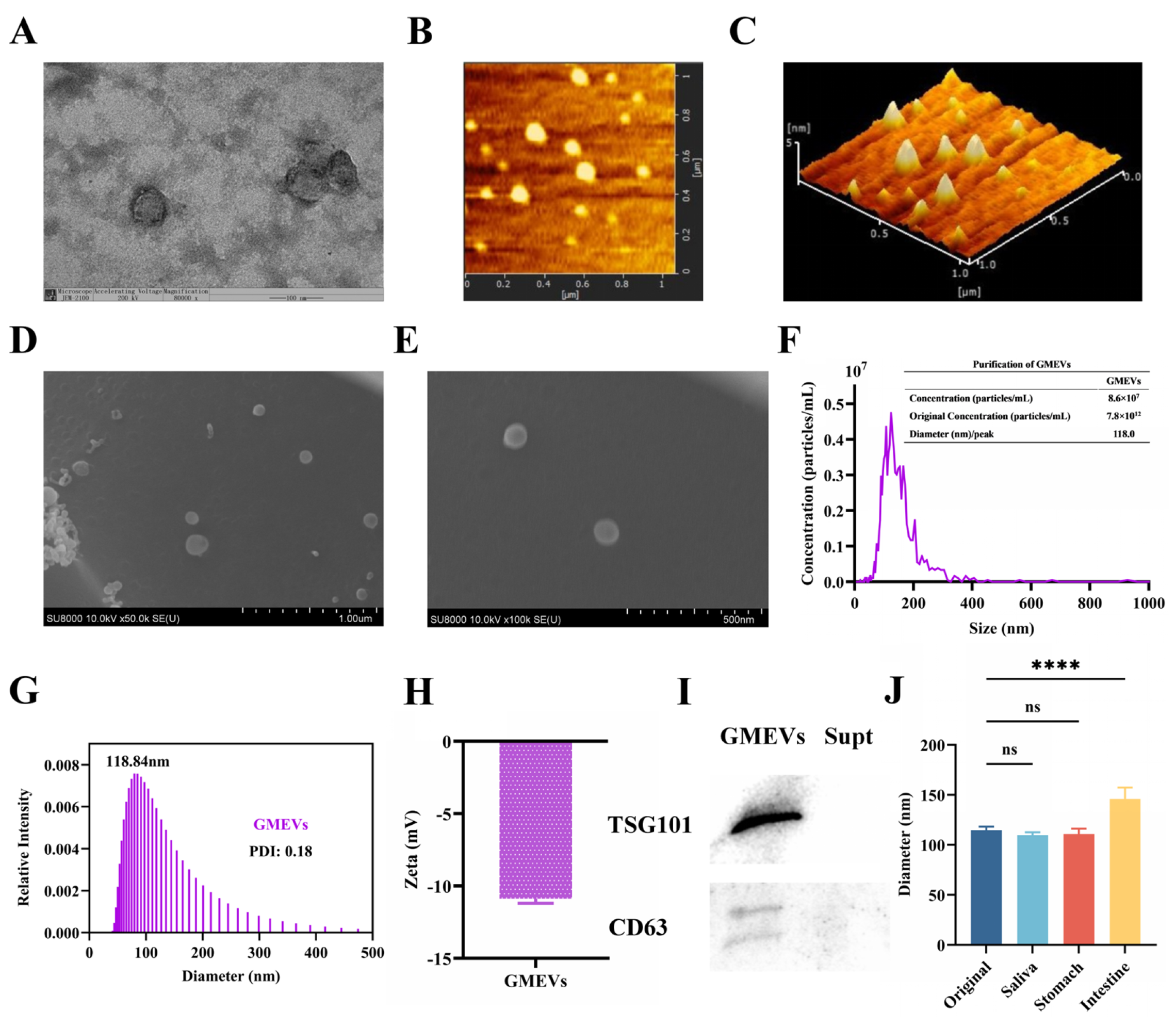
3.1. Identification and Analysis of GMEVs
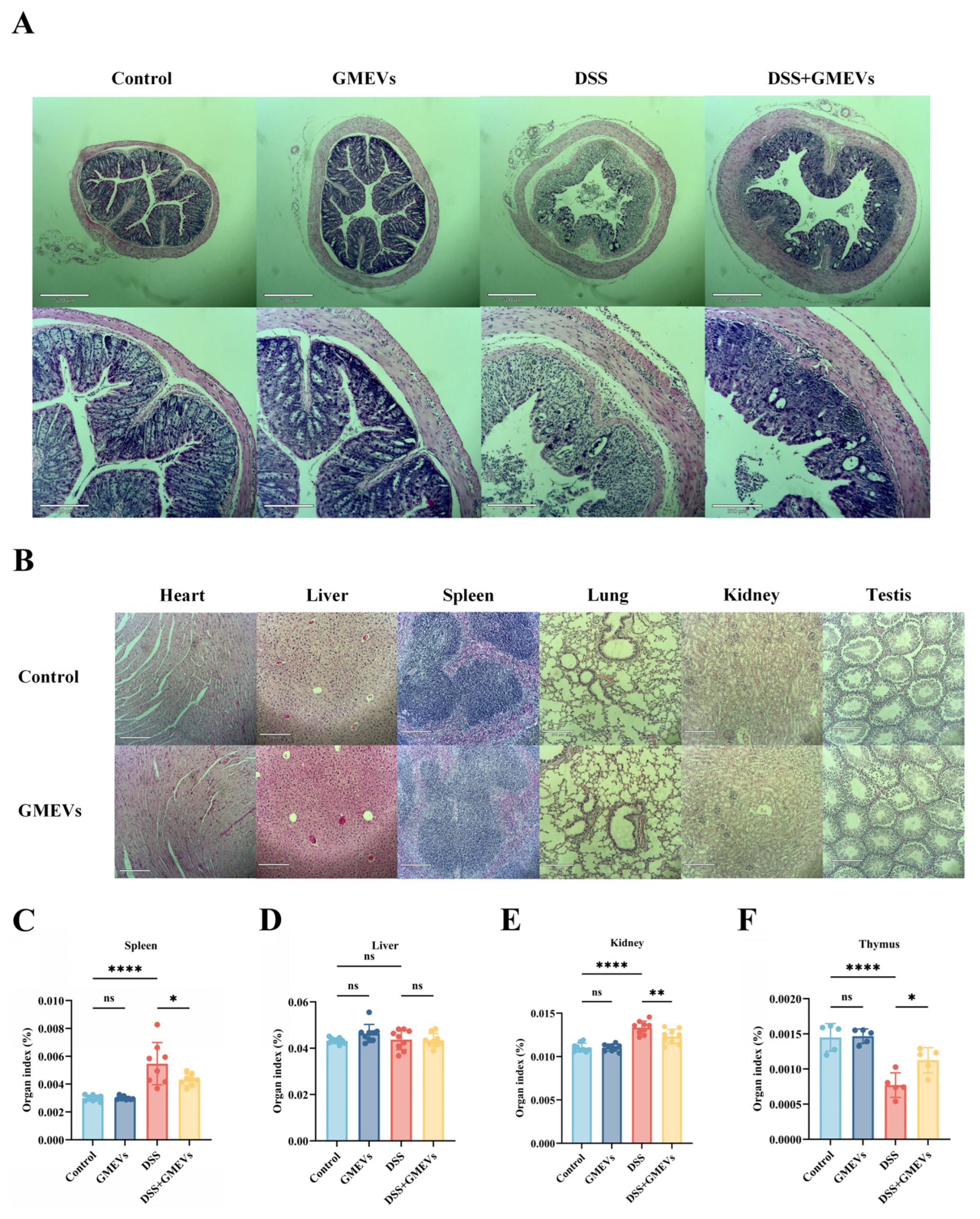
3.2. GMEVs Relieved DSS-Induced Colitis Symptoms in Mice
3.3. GMEVs Attenuated DSS-Induced Histopathological Changes in Mice
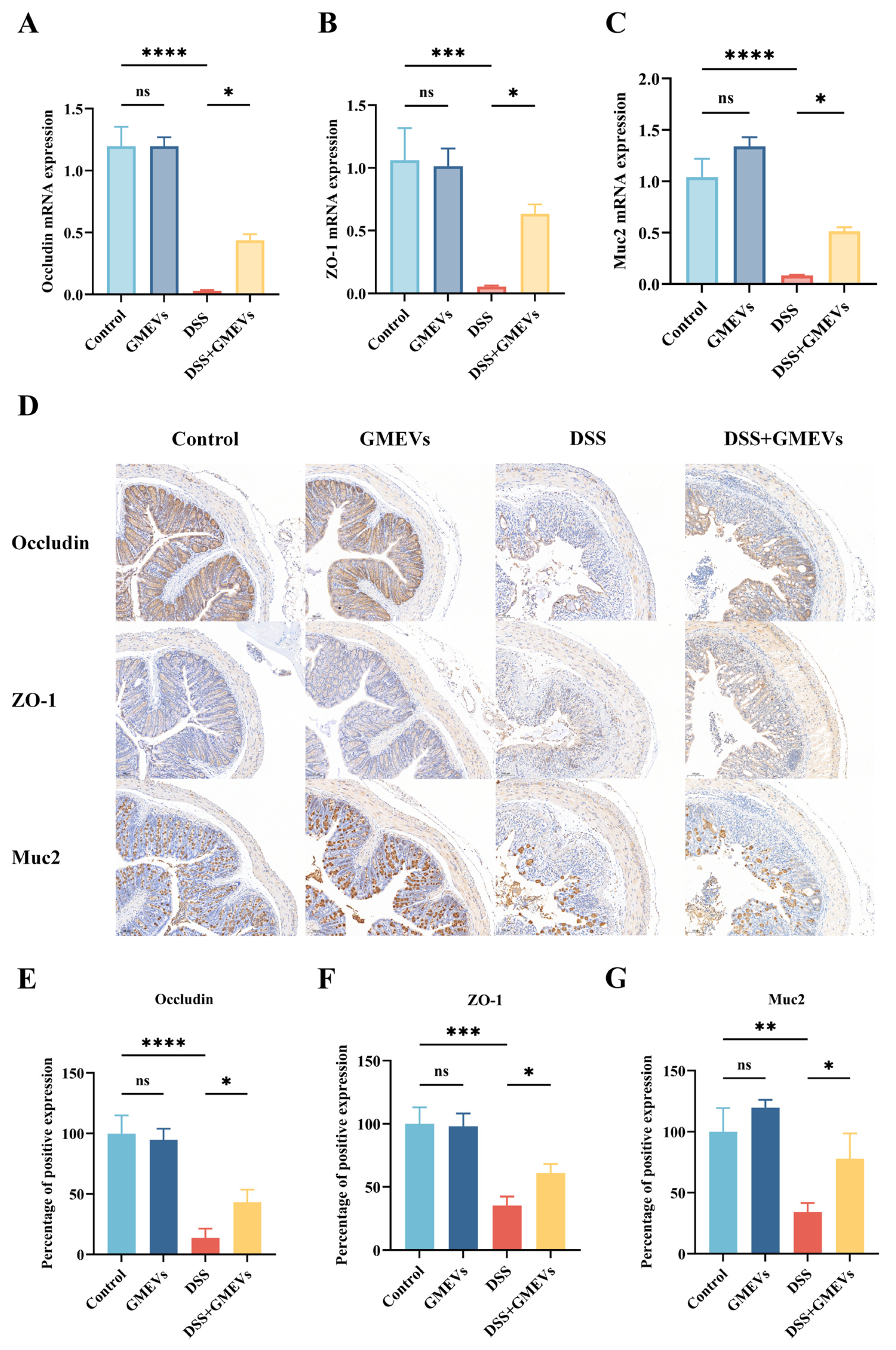
3.4. GMEVs Improved DSS-Induced Intestinal Barrier Damage
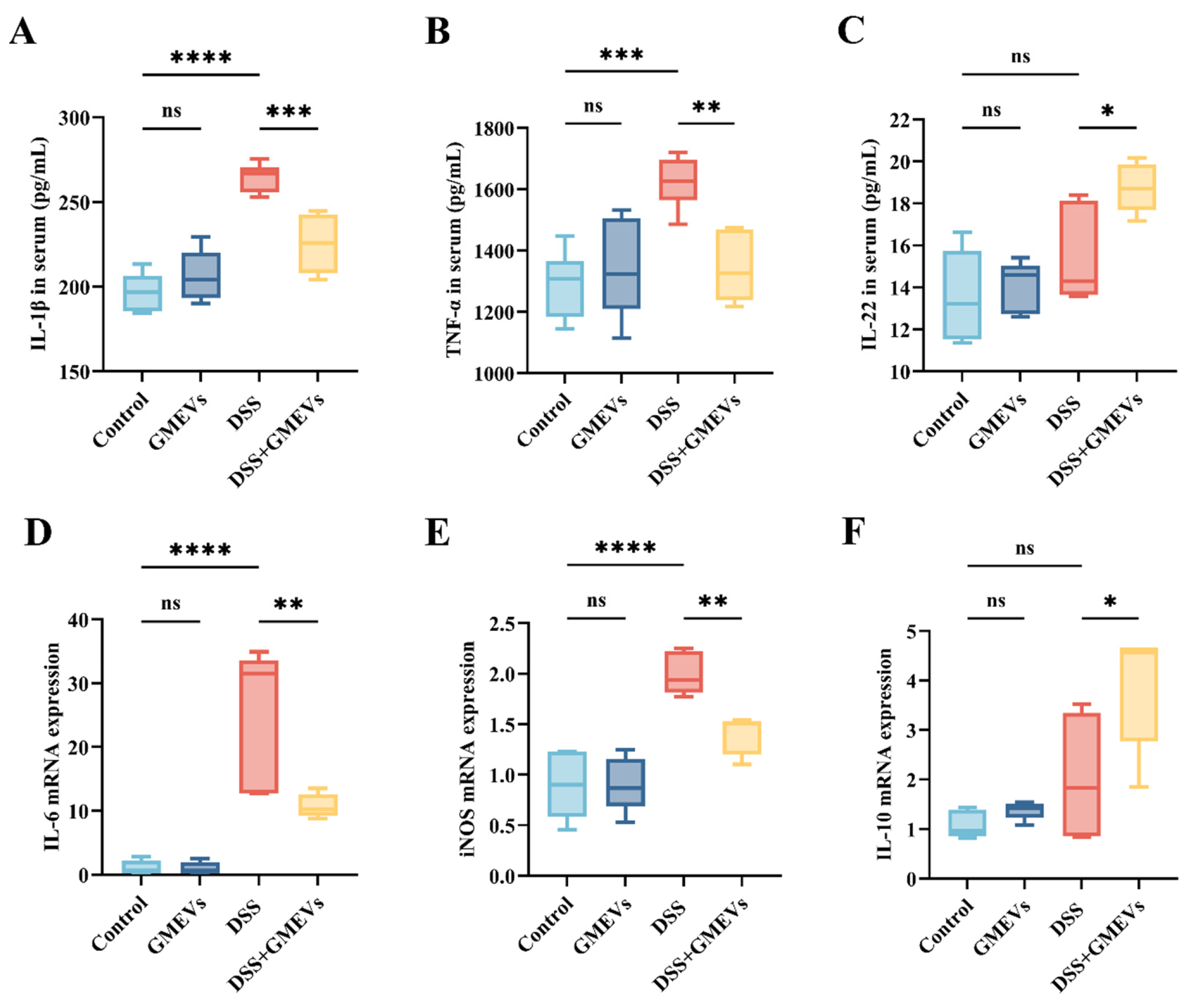
3.5. GMEVs Modulated Inflammatory Cytokine Secretion
3.6. GMEVs Ameliorated Colitis by Modulating the NF-κB Signaling Pathway
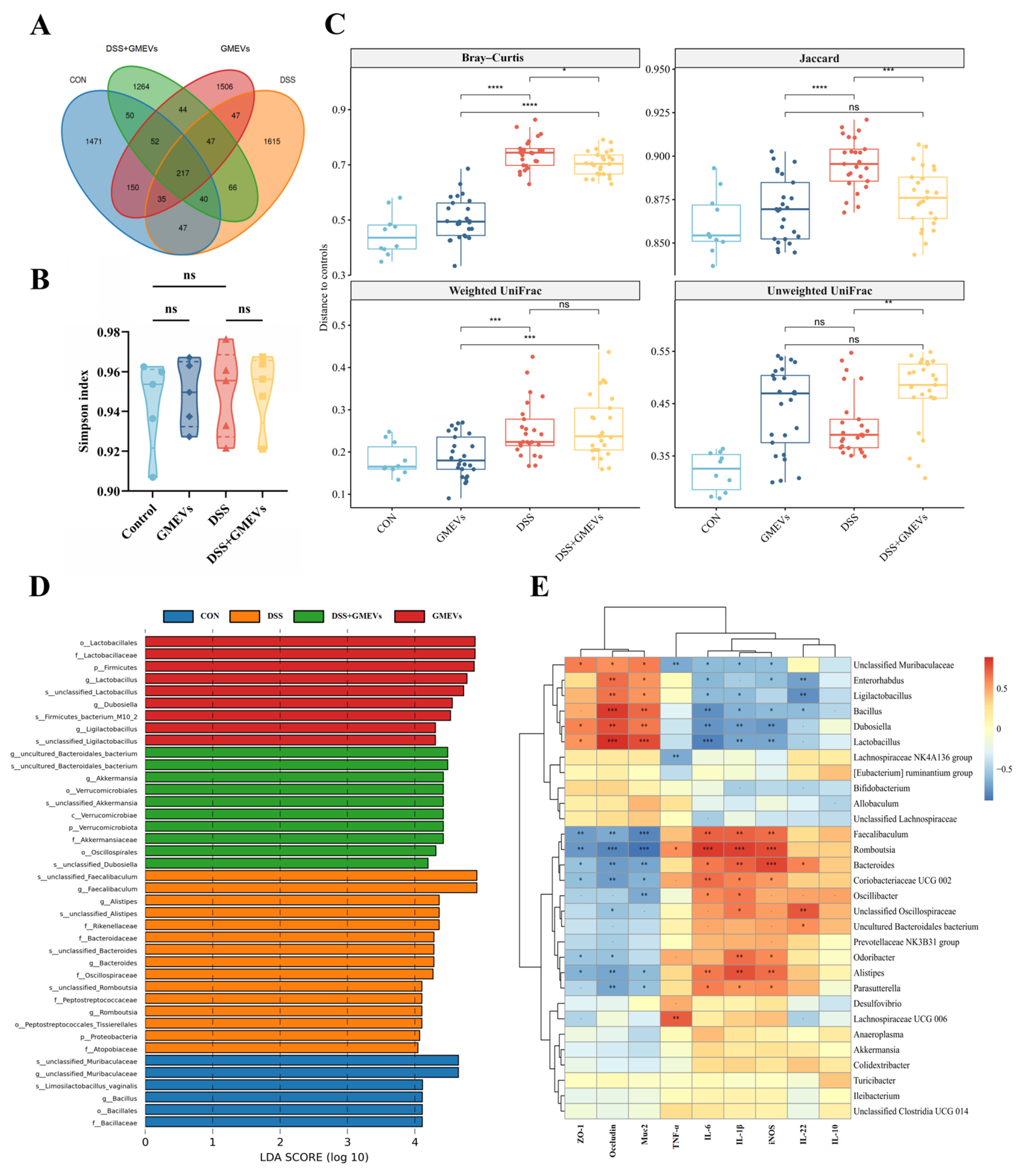
3.7. GMEVs Regulated Gut Microbiota Dysbiosis in DSS-Induced Colitis Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaplan, G.G.; Windsor, J.W. The Four Epidemiological Stages in the Global Evolution of Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative Colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Al-Bawardy, B.; Shivashankar, R.; Proctor, D.D. Novel and Emerging Therapies for Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 651415. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.R.; van Deventer, S. 25 Years of Anti-TNF Treatment for Inflammatory Bowel Disease: Lessons from the Past and a Look to the Future. Gut 2021, 70, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Dhawan, P.; Srivastava, A.S.; Singh, A.B. Inflammatory Bowel Disease: Therapeutic Limitations and Prospective of the Stem Cell Therapy. World J. Stem. Cells 2020, 12, 1050–1066. [Google Scholar] [CrossRef]
- Sicilia, B.; Arias, L.; Hontoria, G.; García, N.; Badia, E.; Gomollón, F. Are Steroids Still Useful in Immunosuppressed Patients with Inflammatory Bowel Disease? A Retrospective, Population-Based Study. Front. Med. 2021, 8, 651685. [Google Scholar] [CrossRef]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.-P.; Raffatellu, M. Mucosal Immunity to Pathogenic Intestinal Bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The Gut Microbiota Shapes Intestinal Immune Responses during Health and Disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Tang, W.; Liu, J.; Ma, Y.; Wei, Y.; Liu, J.; Wang, H. Impairment of Intestinal Barrier Function Induced by Early Weaning via Autophagy and Apoptosis Associated with Gut Microbiome and Metabolites. Front. Immunol. 2021, 12, 804870. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Y.; Yang, Y.; Guo, M.; Zhang, T.; Zong, B.; Huang, S.; Suo, L.; Ma, B.; Wang, X.; et al. Gut Microbiota-Derived Metabolites Contribute Negatively to Hindgut Barrier Function Development at the Early Weaning Goat Model. Anim. Nutr. 2022, 10, 111–123. [Google Scholar] [CrossRef]
- Cacho, N.T.; Lawrence, R.M. Innate Immunity and Breast Milk. Front. Immunol. 2017, 8, 584. [Google Scholar] [CrossRef]
- Field, C.J. The Immunological Components of Human Milk and Their Effect on Immune Development in Infants. J. Nutr. 2005, 135, 1–4. [Google Scholar] [CrossRef]
- Bui, T.M.; Mascarenhas, L.A.; Sumagin, R. Extracellular Vesicles Regulate Immune Responses and Cellular Function in Intestinal Inflammation and Repair. Tissue Barriers 2018, 6, e1431038. [Google Scholar] [CrossRef] [PubMed]
- Heidari, N.; Abbasi-Kenarsari, H.; Namaki, S.; Baghaei, K.; Zali, M.R.; Ghaffari Khaligh, S.; Hashemi, S.M. Adipose-Derived Mesenchymal Stem Cell-Secreted Exosome Alleviates Dextran Sulfate Sodium-Induced Acute Colitis by Treg Cell Induction and Inflammatory Cytokine Reduction. J. Cell Physiol. 2021, 236, 5906–5920. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, M.; Nazimek, K.; Bryniarski, K. Extracellular Vesicles-Oral Therapeutics of the Future. Int. J. Mol. Sci. 2022, 23, 7554. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, M.; Liu, X.; Wu, W.; Su, L.; Li, Y.; Liu, G.; Yan, X. General and Mild Modification of Food-Derived Extracellular Vesicles for Enhanced Cell Targeting. Nanoscale 2021, 13, 3061–3069. [Google Scholar] [CrossRef]
- Sanwlani, R.; Fonseka, P.; Chitti, S.V.; Mathivanan, S. Milk-Derived Extracellular Vesicles in Inter-Organism, Cross-Species Communication and Drug Delivery. Proteomes 2020, 8, 11. [Google Scholar] [CrossRef]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with Immune Modulatory Features Are Present in Human Breast Milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef]
- Xie, M.-Y.; Hou, L.-J.; Sun, J.-J.; Zeng, B.; Xi, Q.-Y.; Luo, J.-Y.; Chen, T.; Zhang, Y.-L. Porcine Milk Exosome MiRNAs Attenuate LPS-Induced Apoptosis through Inhibiting TLR4/NF-κB and P53 Pathways in Intestinal Epithelial Cells. J. Agric. Food Chem. 2019, 67, 9477–9491. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human Milk miRNAs Primarily Originate from the Mammary Gland Resulting in Unique miRNA Profiles of Fractionated Milk. Sci. Rep. 2016, 6, 20680. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, Functions, and Clinical Relevance of Extracellular Vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef] [PubMed]
- González, M.I.; Gallardo, B.; Cerón, C.; Aguilera-Jiménez, E.; Cortes-Canteli, M.; Peinado, H.; Desco, M.; Salinas, B. Isolation of Goat Milk Small Extracellular Vesicles by Novel Combined Bio-Physical Methodology. Front. Bioeng. Biotechnol. 2023, 11, 1197780. [Google Scholar] [CrossRef]
- Lu, X.; Ren, K.; Pan, L.; Liu, X. Sheep (Ovis aries) Milk Exosomal miRNAs Attenuate Dextran Sulfate Sodium-Induced Colitis in Mice via TLR4 and TRAF-1 Inhibition. J. Agric. Food Chem. 2024, 72, 21030–21040. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wu, S.; Zhang, K.; Xu, Z.; Quan, F. Goat Milk Exosomal microRNAs Alleviate LPS-Induced Intestinal Inflammation in Mice. Int. J. Biol. Macromol. 2024, 268, 131698. [Google Scholar] [CrossRef]
- Santoro, J.; Nuzzo, S.; Franzese, M.; Salvatore, M.; Grimaldi, A.M. Goat Milk Extracellular Vesicles: Separation Comparison of Natural Carriers for Theragnostic Application. Heliyon 2024, 10, e27621. [Google Scholar] [CrossRef]
- Yenuganti, V.R.; Afroz, S.; Khan, R.A.; Bharadwaj, C.; Nabariya, D.K.; Nayak, N.; Subbiah, M.; Chintala, K.; Banerjee, S.; Reddanna, P.; et al. Milk Exosomes Elicit a Potent Anti-Viral Activity against Dengue Virus. J. Nanobiotechnol. 2022, 20, 317. [Google Scholar] [CrossRef]
- Franzoni, G.; Mecocci, S.; De Ciucis, C.G.; Mura, L.; Dell’Anno, F.; Zinellu, S.; Fruscione, F.; De Paolis, L.; Carta, T.; Anfossi, A.G.; et al. Goat Milk Extracellular Vesicles: Immuno-Modulation Effects on Porcine Monocyte-Derived Macrophages in Vitro. Front. Immunol. 2023, 14, 1209898. [Google Scholar] [CrossRef]
- Wu, S.; Su, W.; Wang, K.; Li, H.; Huang, S.; Tie, S.; Tan, M. Milk-Derived Exosome as Delivery System for Lutein Encapsulation in Alleviating Dry Eye Disease. Chem. Eng. J. 2024, 486, 149898. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice From DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Kim, H.-J.; Eom, J.-Y.; Choi, S.-H.; Seo, H.-J.; Kwun, I.-S.; Chun, I.-J.; Sung, J.; Lim, J.-H.; Kim, J.; Song, B.-J.; et al. Plum Prevents Intestinal and Hepatic Inflammation in the Acute and Chronic Models of Dextran Sulfate Sodium-Induced Mouse Colitis. Mol. Nutr. Food Res. 2022, 66, 2101049. [Google Scholar] [CrossRef]
- Lee, S.H. Intestinal Permeability Regulation by Tight Junction: Implication on Inflammatory Bowel Diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hara, H. Role of Flavonoids in Intestinal Tight Junction Regulation. J. Nutr. Biochem. 2011, 22, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Mees, S.T.; Mennigen, R.; Spieker, T.; Rijcken, E.; Senninger, N.; Haier, J.; Bruewer, M. Expression of Tight and Adherens Junction Proteins in Ulcerative Colitis Associated Colorectal Carcinoma: Upregulation of Claudin-1, Claudin-3, Claudin-4, and Beta-Catenin. Int. J. Color. Dis. 2009, 24, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in Inflammatory Bowel Disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Zelová, H.; Hošek, J. TNF-α Signalling and Inflammation: Interactions between Old Acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 Balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Khan, F.H.; Dervan, E.; Bhattacharyya, D.D.; McAuliffe, J.D.; Miranda, K.M.; Glynn, S.A. The Role of Nitric Oxide in Cancer: Master Regulator or not? Int. J. Mol. Sci. 2020, 21, 9393. [Google Scholar] [CrossRef]
- Gao, B.; Xiang, X. Interleukin-22 from Bench to Bedside: A Promising Drug for Epithelial Repair. Cell. Mol. Immunol. 2019, 16, 666–667. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Kole, A.; Maloy, K.J. Control of Intestinal Inflammation by Interleukin-10. In Interleukin-10 in Health and Disease; Fillatreau, S., OGarra, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 380, pp. 19–38. ISBN 978-3-662-43492-5. [Google Scholar]
- Zhao, N.; Liu, C.; Li, N.; Zhou, S.; Guo, Y.; Yang, S.; Liu, H. Role of Interleukin-22 in Ulcerative Colitis. Biomed. Pharmacother. 2023, 159, 114273. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, K.M.; Guruvayoorappan, C. Protective Effect of Acacia Ferruginea against Ulcerative Colitis via Modulating Inflammatory Mediators, Cytokine Profile and NF-κB Signal Transduction Pathways. J. Environ. Pathol. Toxicol. Oncol. 2014, 33, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Dong, H.; Wu, L.; Feng, X.; Zhou, Z.; Zhao, C.; Liu, H.; Wu, H. Herb-Partitioned Moxibustion Regulates the TLR2/NF-κB Signaling Pathway in a Rat Model of Ulcerative Colitis. Evid. Based Complement. Alternat. Med. 2015, 2015, 949065. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The Microbiome in Inflammatory Bowel Disease: Current Status and the Future Ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef]
- Swidsinski, A.; Ladhoff, A.; Pernthaler, A.; Swidsinski, S.; Loening-Baucke, V.; Ortner, M.; Weber, J.; Hoffmann, U.; Schreiber, S.; Dietel, M.; et al. Mucosal Flora in Inflammatory Bowel Disease. Gastroenterology 2002, 122, 44–54. [Google Scholar] [CrossRef]
- Sylvestre, M.; Di Carlo, S.E.; Peduto, L. Stromal Regulation of the Intestinal Barrier. Mucosal Immunol. 2023, 16, 221–231. [Google Scholar] [CrossRef]
- Snoeck, V.; Goddeeris, B.; Cox, E. The Role of Enterocytes in the Intestinal Barrier Function and Antigen Uptake. Microbes Infect. 2005, 7, 997–1004. [Google Scholar] [CrossRef]
- Yao, D.; Dai, W.; Dong, M.; Dai, C.; Wu, S. MUC2 and Related Bacterial Factors: Therapeutic Targets for Ulcerative Colitis. EBioMedicine 2021, 74, 103751. [Google Scholar] [CrossRef]
- Li, B.; Hock, A.; Wu, R.Y.; Minich, A.; Botts, S.R.; Lee, C.; Antounians, L.; Miyake, H.; Koike, Y.; Chen, Y.; et al. Bovine Milk-Derived Exosomes Enhance Goblet Cell Activity and Prevent the Development of Experimental Necrotizing Enterocolitis. PLoS ONE 2019, 14, e0211431. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the Gut Barrier Integrity by a Microbial Metabolite through the Nrf2 Pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef]
- Tong, L.; Zhang, S.; Liu, Q.; Huang, C.; Hao, H.; Tan, M.S.; Yu, X.; Lou, C.K.L.; Huang, R.; Zhang, Z.; et al. Milk-Derived Extracellular Vesicles Protect Intestinal Barrier Integrity in the Gut-Liver Axis. Sci. Adv. 2023, 9, eade5041. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, e17023. [Google Scholar] [CrossRef]
- Zhu, Z.; Liao, L.; Gao, M.; Liu, Q. Garlic-Derived Exosome-like Nanovesicles Alleviate Dextran Sulphate Sodium-Induced Mouse Colitis via the TLR4/MyD88/NF-κB Pathway and Gut Microbiota Modulation. Food Funct. 2023, 14, 7520–7534. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut Microbiota and IBD: Causation or Correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of Intestinal Epithelial Cells in the Maintenance of Gut Homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef]
- Tong, L.; Hao, H.; Zhang, X.; Zhang, Z.; Lv, Y.; Zhang, L.; Yi, H. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Alters the Gut Microbiota and Enhances Intestinal Immunity in Mice. Mol. Nutr. Food Res. 2020, 64, 1901251. [Google Scholar] [CrossRef]
- Zhou, F.; Paz, H.A.; Sadri, M.; Cui, J.; Kachman, S.D.; Fernando, S.C.; Zempleni, J. Dietary Bovine Milk Exosomes Elicit Changes in Bacterial Communities in C57BL/6 Mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G618–G624. [Google Scholar] [CrossRef]
- Choi, S.-I.; Shin, Y.C.; Lee, J.S.; Yoon, Y.C.; Kim, J.M.; Sung, M.-K. N-Acetylglucosamine and Its Dimer Ameliorate Inflammation in Murine Colitis by Strengthening the Gut Barrier Function. Food Funct. 2023, 14, 8533–8544. [Google Scholar] [CrossRef]
- Fujiki, Y.; Tanaka, T.; Yakabe, K.; Seki, N.; Akiyama, M.; Uchida, K.; Kim, Y.-G. Hydrogen Gas and the Gut Microbiota Are Potential Biomarkers for the Development of Experimental Colitis in Mice. Gut Microbiome 2024, 5, e3. [Google Scholar] [CrossRef]
- Ikeda, E.; Yamaguchi, M.; Kawabata, S. Gut Microbiota-Mediated Alleviation of Dextran Sulfate Sodium-Induced Colitis in Mice. Gastro Hep Adv. 2024, 3, 461–470. [Google Scholar] [CrossRef]
- Jangid, A.; Fukuda, S.; Seki, M.; Horiuchi, T.; Suzuki, Y.; Taylor, T.D.; Ohno, H.; Prakash, T. Association of Colitis with Gut-Microbiota Dysbiosis in Clathrin Adapter AP-1B Knockout Mice. PLoS ONE 2020, 15, e0228358. [Google Scholar] [CrossRef]
- Khan, S.; Waliullah, S.; Godfrey, V.; Khan, M.A.W.; Ramachandran, R.A.; Cantarel, B.L.; Behrendt, C.; Peng, L.; Hooper, L.V.; Zaki, H. Dietary Simple Sugars Alter Microbial Ecology in the Gut and Promote Colitis in Mice. Sci. Transl. Med. 2020, 12, eaay6218. [Google Scholar] [CrossRef]
- Li, C.; Peng, K.; Xiao, S.; Long, Y.; Yu, Q. The Role of Lactobacillus in Inflammatory Bowel Disease: From Actualities to Prospects. Cell Death Discov. 2023, 9, 361. [Google Scholar] [CrossRef]
- Shi, Y.-J.; Sheng, K.-W.; Zhao, H.-N.; Liu, C.; Wang, H. Toll-Like Receptor 2 Deficiency Exacerbates Dextran Sodium Sulfate-Induced Intestinal Injury through Marinifilaceae-Dependent Attenuation of Cell Cycle Signaling. Front. Biosci. 2024, 29, 338. [Google Scholar] [CrossRef]
- Ibrahim, A.; Hugerth, L.W.; Hases, L.; Saxena, A.; Seifert, M.; Thomas, Q.; Gustafsson, J.-Å.; Engstrand, L.; Williams, C. Colitis-Induced Colorectal Cancer and Intestinal Epithelial Estrogen Receptor Beta Impact Gut Microbiota Diversity. Int. J. Cancer 2019, 144, 3086–3098. [Google Scholar] [CrossRef]
- Steidler, L.; Hans, W.; Schotte, L.; Neirynck, S.; Obermeier, F.; Falk, W.; Fiers, W.; Remaut, E. Treatment of Murine Colitis by Lactococcus Lactis Secreting Interleukin-10. Science 2000, 289, 1352–1355. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, F.; Cao, R.; Ni, X.; Xin, Z.; Deng, J.; Wu, G.; Ren, W.; Yin, Y.; Deng, B. Cecropin A Alleviates Inflammation Through Modulating the Gut Microbiota of C57BL/6 Mice with DSS-Induced IBD. Front. Microbiol. 2019, 10, 1595. [Google Scholar] [CrossRef]
- Kim, M.-J.; Jung, D.-R.; Lee, J.-M.; Kim, I.; Son, H.; Kim, E.S.; Shin, J.-H. Microbial Dysbiosis Index for Assessing Colitis Status in Mouse Models: A Systematic Review and Meta-Analysis. iScience 2024, 27, 108657. [Google Scholar] [CrossRef]
- Wang, J.; Han, L.; Liu, Z.; Zhang, W.; Zhang, L.; Jing, J.; Gao, A. Genus unclassified_Muribaculaceae and Microbiota-Derived Butyrate and Indole-3-Propionic Acid Are Involved in Benzene-Induced Hematopoietic Injury in Mice. Chemosphere 2023, 313, 137499. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The Role of the Gut Microbiome and Its Metabolites in Metabolic Diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef]
- Li, C.-R.; Peng, M.-J.; Tan, Z.-J. Progress in Research of Intestinal Microbiota Related Short Chain Fatty Acids. World Chin. J. Dig. 2024, 30, 562–570. [Google Scholar] [CrossRef]
- Hou, S.; Yu, J.; Li, Y.; Zhao, D.; Zhang, Z. Advances in Fecal Microbiota Transplantation for Gut Dysbiosis-Related Diseases. Adv. Sci. 2025, 12, 2413197. [Google Scholar] [CrossRef]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.P.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; et al. Specific Bacteria and Metabolites Associated with Response to Fecal Microbiota Transplantation in Patients with Ulcerative Colitis. Gastroenterology 2019, 156, 1440–1454.e2. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kelly, C.R.; Grinspan, A.; Mullish, B.H.; Hurtado, J.; Carrellas, M.; Marcus, J.; Marchesi, J.R.; McDonald, J.A.K.; Gerardin, Y.; et al. Inflammatory Bowel Disease Outcomes Following Fecal Microbiota Transplantation for Recurrent C. Difficile Infection. Inflamm. Bowel Dis. 2021, 27, 1371–1378. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, Y.; Chang, H.; Tun, H.-M.; Xia, X.; Peng, Y.; Qin, N. Goat Milk-Derived Extracellular Vesicles Alleviate Colitis Potentially Through Improved Gut Microbiota in Mice. Foods 2025, 14, 1514. https://doi.org/10.3390/foods14091514
Wang X, Liu Y, Chang H, Tun H-M, Xia X, Peng Y, Qin N. Goat Milk-Derived Extracellular Vesicles Alleviate Colitis Potentially Through Improved Gut Microbiota in Mice. Foods. 2025; 14(9):1514. https://doi.org/10.3390/foods14091514
Chicago/Turabian StyleWang, Xinru, Yi Liu, Hong Chang, Hein-Min Tun, Xiaodong Xia, Ye Peng, and Ningbo Qin. 2025. "Goat Milk-Derived Extracellular Vesicles Alleviate Colitis Potentially Through Improved Gut Microbiota in Mice" Foods 14, no. 9: 1514. https://doi.org/10.3390/foods14091514
APA StyleWang, X., Liu, Y., Chang, H., Tun, H.-M., Xia, X., Peng, Y., & Qin, N. (2025). Goat Milk-Derived Extracellular Vesicles Alleviate Colitis Potentially Through Improved Gut Microbiota in Mice. Foods, 14(9), 1514. https://doi.org/10.3390/foods14091514






