Lactic Acid Fermentation of Chlorella vulgaris to Improve the Aroma of New Microalgae-Based Foods: Impact of Composition and Bacterial Growth on the Volatile Fraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Biomasses and Bacterial Strains Used for Fermentation
2.2. Set Up of Fermentations
2.3. HS-SPME/GC-MS Analysis
2.4. Statistical Analysis
3. Results
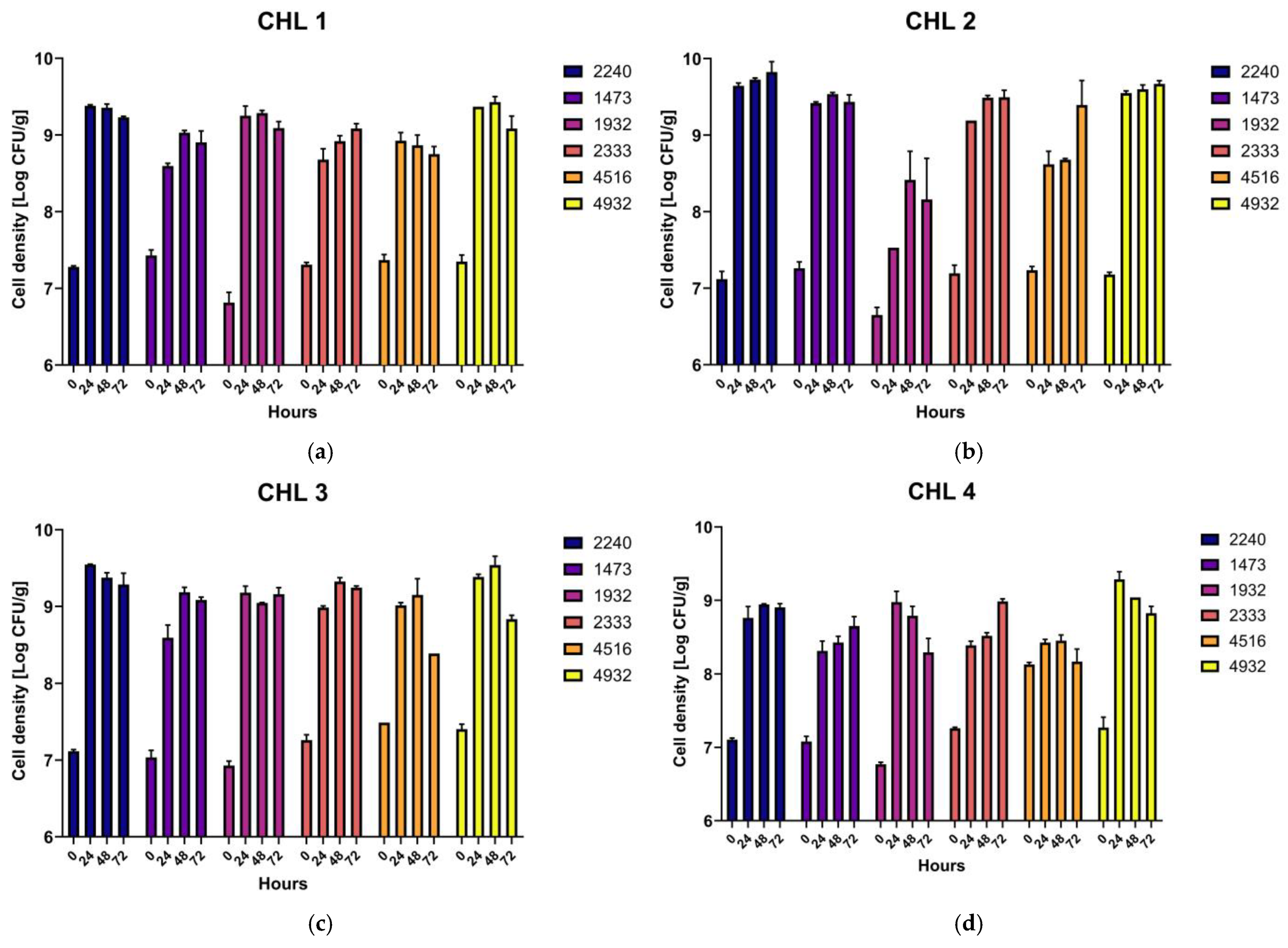
3.1. Chlorella Fermentation
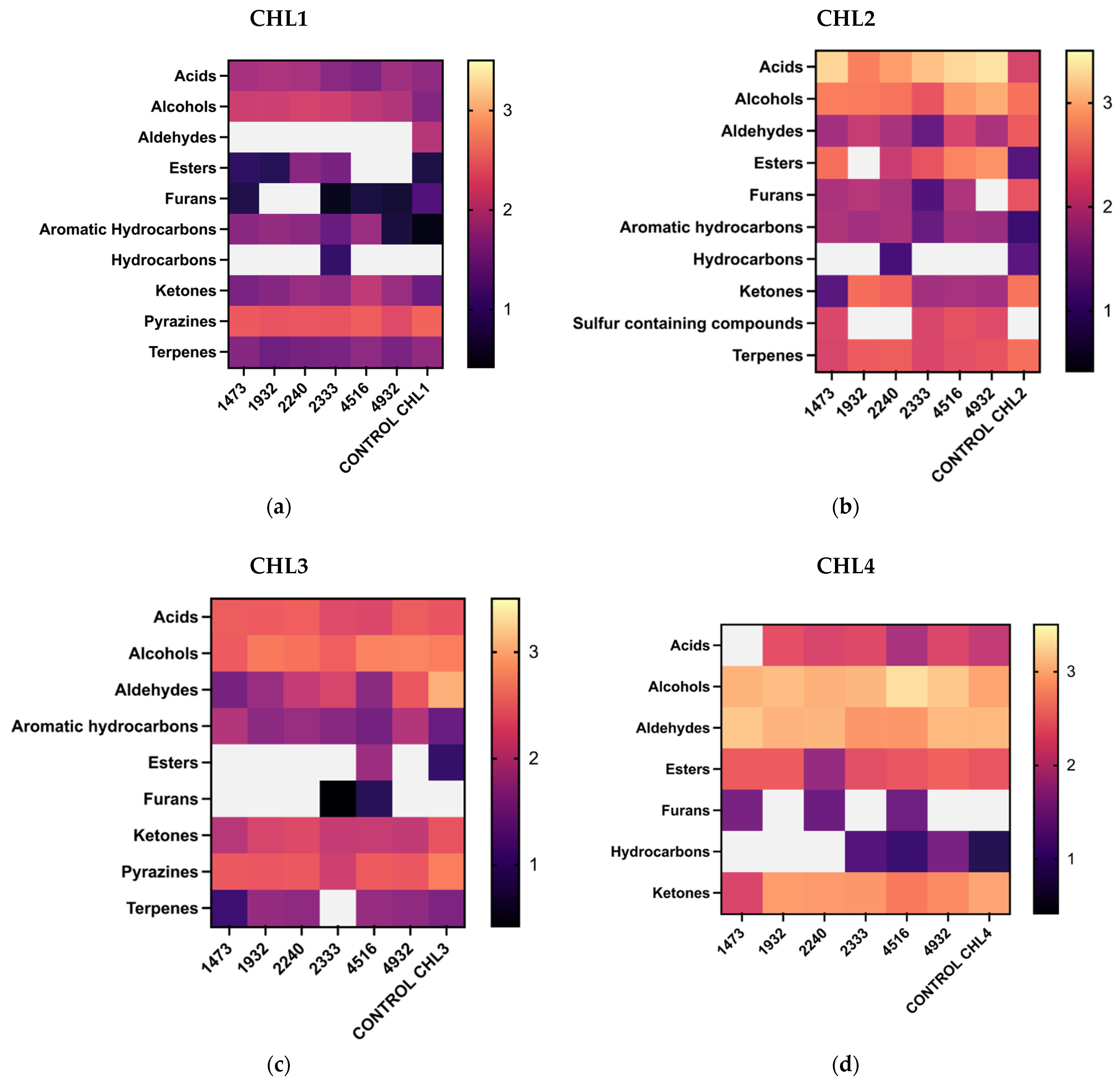
3.2. Volatile Profile Characterization of C. vulgaris and Changes in Volatile Components After Fermentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Edible Seaweeds and Spirulina Extracts for Food Application: In Vitro and In Situ Evaluation of Antimicrobial Activity towards Foodborne Pathogenic Bacteria. Foods 2020, 9, 1442. [Google Scholar] [CrossRef]
- Çelekli, A.; Özbal, B.; Bozkurt, H. Challenges in Functional Food Products with the Incorporation of Some Microalgae. Foods 2024, 13, 725. [Google Scholar] [CrossRef]
- Graham, A.E.; Ledesma-Amaro, R. The Microbial Food Revolution. Nat. Commun. 2023, 14, 2231. [Google Scholar] [CrossRef] [PubMed]
- Olaizola, M.; Grewe, C. Commercial Microalgal Cultivation Systems. In Grand Challenges in Algae Biotechnology; Hallmann, A., Rampelotto, P.H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–34. ISBN 978-3-030-25233-5. [Google Scholar]
- Cruz, J.D.; Vasconcelos, V. Legal Aspects of Microalgae in the European Food Sector. Foods 2023, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, Composition, Production, Processing and Applications of Chlorella vulgaris: A Review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Coronado-Reyes, J.A.; Salazar-Torres, J.A.; Juárez-Campos, B.; González-Hernández, J.C. Chlorella vulgaris, a Microalgae Important to Be Used in Biotechnology: A Review. Food Sci. Technol. 2022, 42, e37320. [Google Scholar] [CrossRef]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.A.; Mendes, M.A. Chlorella and Spirulina Microalgae as Sources of Functional Foods, Nutraceuticals, and Food Supplements; an Overview. MOJ Food Process. Technol. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- Van Durme, J.; Goiris, K.; De Winne, A.; De Cooman, L.; Muylaert, K. Evaluation of the Volatile Composition and Sensory Properties of Five Species of Microalgae. J. Agric. Food Chem. 2013, 61, 10881–10890. [Google Scholar] [CrossRef]
- Schüler, L.; Greque De Morais, E.; Trovão, M.; Machado, A.; Carvalho, B.; Carneiro, M.; Maia, I.; Soares, M.; Duarte, P.; Barros, A.; et al. Isolation and Characterization of Novel Chlorella vulgaris Mutants with Low Chlorophyll and Improved Protein Contents for Food Applications. Front. Bioeng. Biotechnol. 2020, 8, 469. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic Metabolism Revisited: Metabolism of Lactic Acid Bacteria in Food Fermentations and Food Spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on Fermented Foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Smid, E.J.; Kleerebezem, M. Production of Aroma Compounds in Lactic Fermentations. Annu. Rev. Food Sci. Technol. 2014, 5, 313–326. [Google Scholar] [CrossRef]
- Bhowmik, D.; Dubey, J.; Mehra, S. Probiotic Efficiency of Spirulina platensis—Stimulating Growth of Lactic Acid Bacteria. World J. Dairy Food Sci. 2009, 4, 160–163. [Google Scholar]
- Martelli, F.; Bernini, V.; Neviani, E.; Vasconcelos, V.; Urbatzka, R. Lactic Acid Fermented Microalgae and Cyanobacteria as a New Source of Lipid Reducing Compounds: Assessment through Zebrafish Nile Red Fat Metabolism Assay and Untargeted Metabolomics. Food Funct. 2024, 15, 5554–5565. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Solid-State Fermentation of Arthrospira platensis to Implement New Food Products: Evaluation of Stabilization Treatments and Bacterial Growth on the Volatile Fraction. Foods 2020, 10, 67. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; O’Toole, W.; Pot, B.; Vandamme, P.; Walter, J.; Watanabe, K.; Wuyts, S.; Felis, G.E.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Monnin, L.; Zheng, J.; Zhang, L.; Coton, M.; Sicard, D.; Walter, J. Starter Culture Development and Innovation for Novel Fermented Foods. Annu. Rev. Food Sci. Technol. 2024, 15, 211–239. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 16 October 2019).
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Gyenis, B.; Szigeti, J.; Molnár, N.; Varga, L. Use of Dried Microalgal Biomasses to Stimulate Acid Production and Growth of Lactobacillus plantarum and Enterococcus faecium in Milk. Acta Agrar. Kaposváriensis 2005, 9, 53–59. [Google Scholar]
- Ścieszka, S.; Klewicka, E. Influence of the Microalga Chlorella vulgaris on the Growth and Metabolic Activity of Lactobacillus Spp. Bacteria. Foods 2020, 9, 959. [Google Scholar] [CrossRef] [PubMed]
- Nicolotti, C.; Sanz Moxo, J.; Bottari, B.; Cirlini, M.; Bernini, V.; Gatti, M.; Urbatzka, R.; Martelli, F. The Bioactivities of Lactic Acid-Fermented Arthrospira platensis and Its Application in Functional Beverages. Beverages 2024, 10, 111. [Google Scholar] [CrossRef]
- Isleten Hosoglu, M. Aroma Characterization of Five Microalgae Species Using Solid-Phase Microextraction and Gas Chromatography–Mass Spectrometry/Olfactometry. Food Chem. 2018, 240, 1210–1218. [Google Scholar] [CrossRef]
- Lafarge, C.; Cayot, N. Insight on a Comprehensive Profile of Volatile Compounds of Chlorella vulgaris Extracted by Two “Green” Methods. Food Sci. Nutr. 2019, 7, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Urlass, S.; Wu, Y.; Nguyen, T.T.L.; Winberg, P.; Turner, M.S.; Smyth, H. Unravelling the Aroma and Flavour of Algae for Future Food Applications. Trends Food Sci. Technol. 2023, 138, 370–381. [Google Scholar] [CrossRef]
- Van De Walle, S.; Gifuni, I.; Coleman, B.; Baune, M.-C.; Rodrigues, A.; Cardoso, H.; Fanari, F.; Muylaert, K.; Van Royen, G. Innovative vs Classical Methods for Drying Heterotrophic Chlorella vulgaris: Impact on Protein Quality and Sensory Properties. Food Res. Int. 2024, 182, 114142. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Calani, L.; Cirlini, M.; Bernini, V.; Neviani, E.; Del Rio, D.; Galaverna, G.; Lazzi, C. Effect of Fermentation with Single and Co-Culture of Lactic Acid Bacteria on Okara: Evaluation of Bioactive Compounds and Volatile Profiles. Food Funct. 2021, 12, 3033–3043. [Google Scholar] [CrossRef]
- Cuellar-Bermúdez, S.P.; Barba-Davila, B.; Serna-Saldivar, S.O.; Parra-Saldivar, R.; Rodriguez-Rodriguez, J.; Morales-Davila, S.; Goiris, K.; Muylaert, K.; Chuck-Hernández, C. Deodorization of Arthrospira platensis Biomass for Further Scale-up Food Applications. J. Sci. Food Agric. 2017, 97, 5123–5130. [Google Scholar] [CrossRef]
- Colonia, B.S.O.; De Melo Pereira, G.V.; Carvalho, J.C.D.; Karp, S.G.; Rodrigues, C.; Soccol, V.T.; Fanka, L.S.; Soccol, C.R. Deodorization of Algae Biomass to Overcome Off-Flavors and Odor Issues for Developing New Food Products: Innovations, Trends, and Applications. Food Chem. Adv. 2023, 2, 100270. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Zheng, J.-H.; Ren, D.-F.; Lu, J. Mixed Fermentation of Spirulina platensis with Lactobacillus plantarum and Bacillus subtilis by Random-Centroid Optimization. Food Chem. 2018, 264, 64–72. [Google Scholar] [CrossRef]
- Cichoński, J.; Chrzanowski, G. Microalgae as a Source of Valuable Phenolic Compounds and Carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef]
- Sugisawa, H.; Nakamura, K.; Tamura, H. The Aroma Profile of the Volatiles in Marine Green Algae (Ulva pertusa). Food Rev. Int. 1990, 6, 573–589. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, M.; Qiu, D.; Zhang, J.; Zhao, N.; Feng, G.; Wu, H.; Zeng, M.; Obadina, A.O. Comparative Evaluation of Sensory and Instrumental Flavor Profiles of Four Edible Microalgae: Spirulina platensis, Chlorella pyrenoidosa, Chlamydomonas reinhardtii, and Haematococcus pluvialis. Algal Res. 2024, 82, 103628. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Meneses, A.C.D.; Araújo, P.H.H.D.; Oliveira, D.D. A Review on Enzymatic Synthesis of Aromatic Esters Used as Flavor Ingredients for Food, Cosmetics and Pharmaceuticals Industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- Krause, S.; Keller, S.; Hashemi, A.; Descharles, N.; Bonazzi, C.; Rega, B. From Flours to Cakes: Reactivity Potential of Pulse Ingredients to Generate Volatile Compounds Impacting the Quality of Processed Foods. Food Chem. 2022, 371, 131379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Song, X.; Liu, W.; Gao, X. Mixed Fermentation of Chlorella pyrenoidosa and Bacillus velezensis SW-37 by Optimization. LWT 2023, 175, 114448. [Google Scholar] [CrossRef]
- Gao, F.; Yang, L.; Chen, A.-J.; Zhou, W.-H.; Chen, D.-Z.; Chen, J.-M. Promoting Effect of Plant Hormone Gibberellin on Co-Metabolism of Sulfamethoxazole by Microalgae Chlorella pyrenoidosa. Bioresour. Technol. 2022, 351, 126900. [Google Scholar] [CrossRef]



| Name | Carbohydrates | Proteins | Fat | Fibre |
|---|---|---|---|---|
| C. vulgaris “Smooth” CHL1 | 34 | 30 | 7 | 20 |
| C. vulgaris “Premium” CHL2 | 8 | 55 | 10 | 15 |
| C. vulgaris “Honey” CHL3 | 24 | 30 | 8 | 23 |
| C. vulgaris “White” CHL4 | 55 | 32.5 | 9.5 | 9 |
| ID | Species | Growth Temperature | Isolation Matrix |
|---|---|---|---|
| UPCCO 1473 | Lacticaseibacillus rhamnosus | 37 °C | Parmigiano Reggiano |
| UPCCO 1932 | Lactobacillus delbrueckii bulgaricus | 37 °C | Curd |
| UPCCO 2240 | Lacticaseibacillus casei | 37 °C | Parmigiano Reggiano |
| UPCCO 2333 | Lacticaseibacillus paracasei | 30 °C | Parmigiano Reggiano |
| UPCCO 4516 | Leuconostoc citreum | 30 °C | Sourdough |
| UPCCO 4932 | Lactiplantibacillus plantarum | 30 °C | Minas cheese |
| CHL 1 | CHL 2 | CHL 3 | CHL 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | T0 | T3 | ΔT = T3-T0 | T0 | T3 | ΔT = T3-T0 | T0 | T3 | ΔT = T3-T0 | T0 | T3 | ΔT = T3-T0 |
| UPCCO 1473 | 7.43 ± 0.07 | 8.90 ± 0.15 | 1.48 ± 0.12 | 7.26 ± 0.08 | 9.44 ± 0.09 | 2.17 ± 0.01 | 7.04 ± 0.09 | 9.09 ± 0.04 | 2.05 ± 0.08 | 7.08 ± 0.08 | 8.65 ± 0.12 | 1.57 ± 0.07 |
| UPCCO 1932 | 6.82 ± 0.13 | 9.09 ± 0.09 | 2.27 ± 0.05 | 6.65 ± 0.10 | 8.54 ± 0.31 | 1.89 ± 0.30 | 6.93 ± 0.06 | 9.16 ± 0.08 | 2.23 ± 0.03 | 6.77 ± 0.03 | 8.30 ± 0.19 | 1.53 ± 0.23 |
| UPCCO 2240 | 7.28 ± 0.01 | 9.23 ± 0.02 | 1.95 ± 0.01 | 7.12 ± 0.09 | 9.82 ± 0.14 | 2.71 ± 0.06 | 7.12 ± 0.02 | 9.29 ± 0.15 | 2.17 ± 0.18 | 7.11 ± 0.03 | 8.91 ± 0.05 | 1.80 ± 0.04 |
| UPCCO 2333 | 7.31 ± 0.03 | 9.09 ± 0.07 | 1.78 ± 0.06 | 7.20 ± 0.10 | 9.50 ± 0.09 | 2.30 ± 0.01 | 7.26 ± 0.08 | 9.24 ± 0.02 | 1.98 ± 0.08 | 7.26 ± 0.01 | 8.99 ± 0.04 | 1.73 ± 0.04 |
| UPCCO 4516 | 7.35 ± 0.08 | 8.75 ± 0.10 | 1.38 ± 0.04 | 7.24 ± 0.05 | 9.40 ± 0.32 | 2.16 ± 0.37 | 7.49 ± 0.06 | 8.39 ± 0.10 | 0.90 ± 0.05 | 8.13 ± 0.02 | 8.17 ± 0.17 | 1.04 ± 0.21 |
| UPCCO 4932 | 7.37 ± 0.08 | 9.15 ± 0.16 | 1.73 ± 0.11 | 7.18 ± 0.02 | 9.67 ± 0.04 | 2.49 ± 0.03 | 7.41 ± 0.07 | 8.84 ± 0.05 | 1.43 ± 0.05 | 7.27 ± 0.14 | 8.83 ± 0.09 | 1.55 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolotti, C.; Cirlini, M.; Del Vecchio, L.; Hadj Saadoun, J.; Bernini, V.; Gatti, M.; Bottari, B.; Martelli, F. Lactic Acid Fermentation of Chlorella vulgaris to Improve the Aroma of New Microalgae-Based Foods: Impact of Composition and Bacterial Growth on the Volatile Fraction. Foods 2025, 14, 1511. https://doi.org/10.3390/foods14091511
Nicolotti C, Cirlini M, Del Vecchio L, Hadj Saadoun J, Bernini V, Gatti M, Bottari B, Martelli F. Lactic Acid Fermentation of Chlorella vulgaris to Improve the Aroma of New Microalgae-Based Foods: Impact of Composition and Bacterial Growth on the Volatile Fraction. Foods. 2025; 14(9):1511. https://doi.org/10.3390/foods14091511
Chicago/Turabian StyleNicolotti, Caterina, Martina Cirlini, Lorenzo Del Vecchio, Jasmine Hadj Saadoun, Valentina Bernini, Monica Gatti, Benedetta Bottari, and Francesco Martelli. 2025. "Lactic Acid Fermentation of Chlorella vulgaris to Improve the Aroma of New Microalgae-Based Foods: Impact of Composition and Bacterial Growth on the Volatile Fraction" Foods 14, no. 9: 1511. https://doi.org/10.3390/foods14091511
APA StyleNicolotti, C., Cirlini, M., Del Vecchio, L., Hadj Saadoun, J., Bernini, V., Gatti, M., Bottari, B., & Martelli, F. (2025). Lactic Acid Fermentation of Chlorella vulgaris to Improve the Aroma of New Microalgae-Based Foods: Impact of Composition and Bacterial Growth on the Volatile Fraction. Foods, 14(9), 1511. https://doi.org/10.3390/foods14091511







