Synergistic Approaches to Foodborne Pathogen Control: A Narrative Review of Essential Oils and Bacteriophages
Abstract
1. Introduction
2. Materials and Methods
3. Essential Oils
3.1. Chemical Composition and Bioactive Compounds
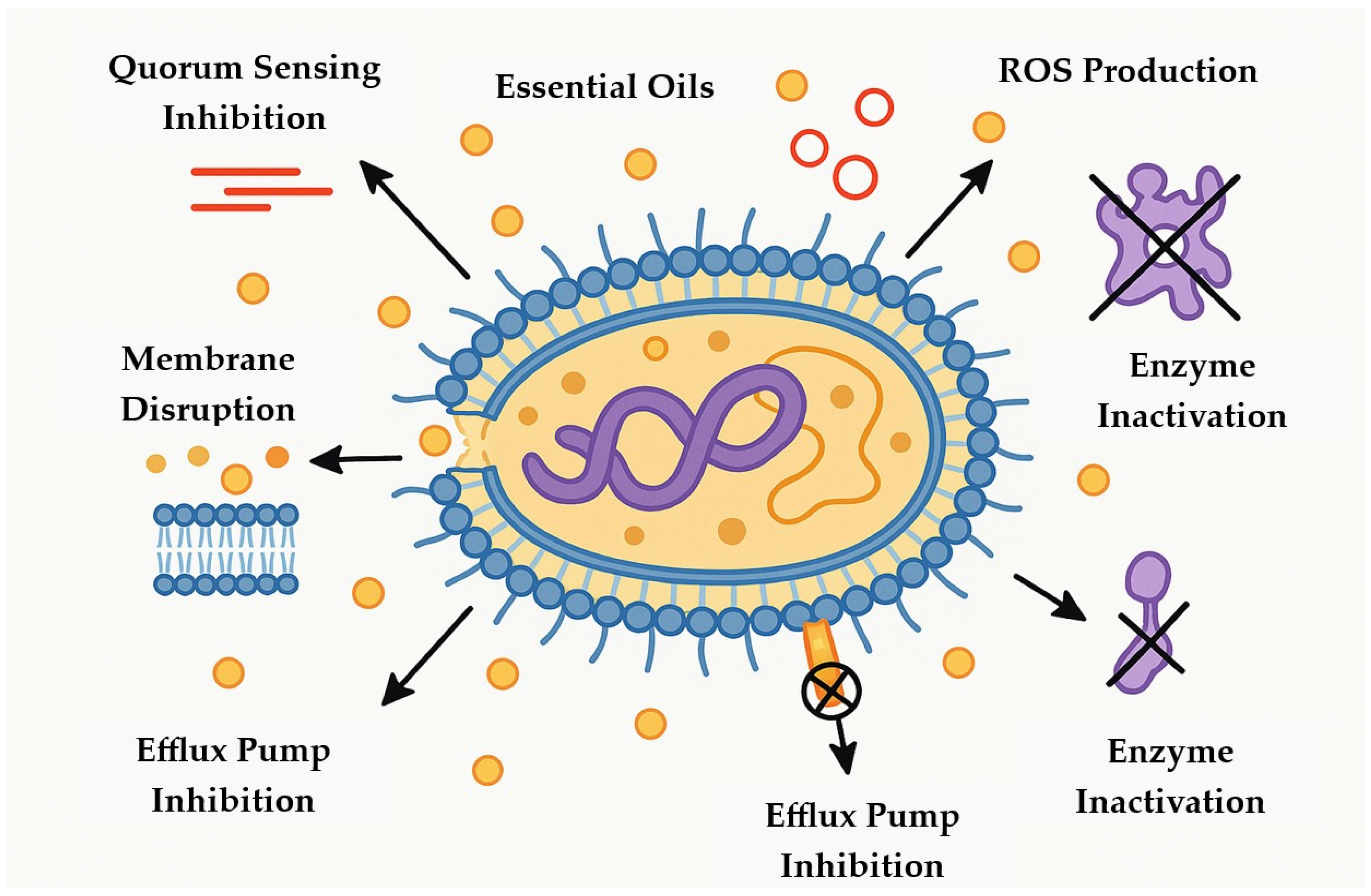
3.2. Antimicrobial Mechanisms of Action of Essential Oils
3.3. Applications of Essential Oils in Food Systems
3.4. Challenges and Limitations of EOs
4. Bacteriophages
4.1. Biological Characteristics and Classification
4.2. Mechanisms of Antibacterial Action
4.3. Applications of Bacteriophages in Food Systems
| Product | Manufacturer | Target Pathogen(s) | Application Matrix | Regulatory |
|---|---|---|---|---|
| ListShield™ [73] | Intralytix Ltd. (Columbia, SC, USA) | L. monocytogenes | Ready-to-eat foods, non-food contact equipment, surfaces, etc., in food processing plants and other food establishments | FDA, 21 Code of Federal Regulations (CFR) 172.785; FDA, Generally Recognized as Safe Notice (GRN) 528; United States Environmental Protection Agency (EPA) Reg. No. 74234-1; Israel Ministry of Health; Health Canada |
| EcoShield™ [74] | E. coli O157:H7 | Red meat surfaces | FDA, Food Contact Notification (FCN) 1018; Israel Ministry of Health; Health Canada | |
| SalmoFresh™ [75] | Salmonella spp. | Poultry, fish and shellfish, and fresh and processed fruits and vegetables | FDA, GRN 435; United States Department of Agriculture (USDA), Food Safety and Inspection Service (FSIS) Directive 7120.1; Israel Ministry of Health; Health Canada | |
| ShigaShield [76] | Shigella spp. | Food | FDA, GRN 672 | |
| PhageGuard Listex™ P100 [77] | Micreos Food Safety (Wageningen, The Netherlands) | L. monocytogenes | Cheese, fish, meat, ready-to-eat products | FDA, GRAS Notice (GRN) 198/218; Food Standards Australia New Zealand (FSANZ); EFSA; Swiss BAG; Israel Ministry of Health; Health Canada |
| PhageGuard S [78] | Salmonella spp. | Poultry, meat, cheese | FDA, GRN 468; FSANZ; Swiss BAG; Israel Ministry of Health; Health Canada | |
| PhageGuard E [79] | E. coli O157:H7 | Beef, vegetables | FDA, GRN 757 | |
| Salmonelex™ [80] | Salmonella spp. | Various foods | FDA and USDA, GRAS | |
| Bafasal® [81] | Proteon Pharmaceuticals (Mumbai, India) | Salmonella spp. | Animal feed, poultry farming | Approved in the EU |
4.4. Challenges and Limitations of Bacteriophages
5. Synergistic Applications of Essential Oils and Bacteriophages
5.1. Overview of Experimental Studies
5.2. Limitations, Considerations, and Practical Implications
6. Research Gaps and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EOs | Essential oils |
| AMR | Antimicrobial resistance |
| MDR | Multidrug-resistant |
| ROS | Reactive oxygen species |
| FDA | Food and Drug Administration |
| GRAS | Generally Recognized as Safe |
| RTE | Ready-to-eat |
| EU | European Union |
| EFSA | European Food Safety Administration |
| US | United States |
| CFR | Code of Federal Regulations |
| GRN | Generally Recognized as Safe |
| EPA | United States Environmental Protection Agency |
| FCN | Food Contact Notification |
| USDA | United States Department of Agriculture |
| FSIS | Food Safety and Inspection Services |
| FSANZ | Food Standards Australia New Zealand |
| BAG | Swiss Federal Office of Public Health (BAG) |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| FIC | Fractional Inhibitory Concentration |
References
- Antimicrobial Resistance (AMR)-ECDC. Available online: https://www.ecdc.europa.eu/en/antimicrobial-resistance (accessed on 20 January 2025).
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 5 March 2025).
- Thapa, S.P.; Shrestha, S.; Anal, A.K. Addressing the antibiotic resistance and improving the food safety in food supply chain (farm-to-fork) in Southeast Asia. Food Control 2020, 108, 106809. [Google Scholar] [CrossRef]
- Grudlewska-Buda, K.; Bauza-Kaszewska, J.; Wiktorczyk-Kapischke, N.; Budzyńska, A.; Gospodarek-Komkowska, E.; Skowron, K. Antibiotic Resistance in Selected Emerging Bacterial Foodborne Pathogens—An Issue of Concern? Antibiotics 2023, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- DRUG-RESISTANT INFECTIONS A Threat to Our Economic Future. Available online: www.worldbank.org (accessed on 5 March 2025).
- Samreen Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Lack of New Antibiotics Threatens Global Efforts to Contain Drug-Resistant Infections. Available online: https://www.who.int/news/item/17-01-2020-lack-of-new-antibiotics-threatens-global-efforts-to-contain-drug-resistant-infections (accessed on 5 March 2025).
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Łojewska, E.; Sakowicz, T. An Alternative to Antibiotics: Selected Methods to Combat Zoonotic Foodborne Bacterial Infections. Curr. Microbiol. 2021, 78, 4037. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F.; Pinto, L.; Tapia-Rodríguez, M.R. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef]
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Jadhav, S.R. Encapsulation of essential oils and their application in antimicrobial active packaging. Food Control 2022, 136, 108883. [Google Scholar] [CrossRef]
- Cui, L.; Watanabe, S.; Miyanaga, K.; Kiga, K.; Sasahara, T.; Aiba, Y.; Tan, X.-E.; Veeranarayanan, S.; Thitiananpakorn, K.; Nguyen, H.M.; et al. A Comprehensive Review on Phage Therapy and Phage-Based Drug Development. Antibiotics 2024, 13, 870. [Google Scholar] [CrossRef]
- Atterbury, R.J. Bacteriophage biocontrol in animals and meat products. Microb. Biotechnol. 2009, 2, 601. [Google Scholar] [CrossRef]
- Fernández, L.; Escobedo, S.; Gutiérrez, D.; Portilla, S.; Martínez, B.; García, P.; Rodríguez, A. Bacteriophages in the Dairy Environment: From Enemies to Allies. Antibiotics 2017, 6, 27. [Google Scholar] [CrossRef]
- Bai, J.; Jeon, B.; Ryu, S. Effective inhibition of Salmonella Typhimurium in fresh produce by a phage cocktail targeting multiple host receptors. Food Microbiol. 2019, 77, 52–60. [Google Scholar] [CrossRef]
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. Essential Oils as Multicomponent Mixtures and Their Potential for Human Health and Well-Being. Front. Pharmacol. 2022, 13, 956541. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.H.; Dar, N.A.; Alie, B.A.; Dar, S.A.; Lone, A.A.; Mir, G.H.; Fayaz, U.; Ali, S.; Tyagi, A.; El-Sheikh, M.A.; et al. Unraveling the Variability of Essential Oil Composition in Different Accessions of Bunium persicum Collected from Different Temperate Micro-Climates. Molecules 2023, 28, 2404. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Chroho, M.; Rouphael, Y.; Petropoulos, S.A.; Bouissane, L. Carvacrol and Thymol Content Affects the Antioxidant and Antibacterial Activity of Origanum compactum and Thymus zygis Essential Oils. Antibiotics 2024, 13, 139. [Google Scholar] [CrossRef]
- Rúa, J.; Del Valle, P.; De Arriaga, D.; Fernández-Álvarez, L.; García-Armesto, M.R. Combination of Carvacrol and Thymol: Antimicrobial Activity Against Staphylococcus aureus and Antioxidant Activity. Foodborne Pathog. Dis. 2019, 16, 622–629. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef]
- Shafi, A.; Akram, K. Herbal oil in healthcare. Herb. Biomol. Healthc. Appl. 2022, 205–213. [Google Scholar]
- Oseni, O.M.; Sajaditabar, R.; Mahmoud, S.S. Metabolic engineering of terpene metabolism in lavender. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 67. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Mishra, S. Plant Monoterpenoids (Prospective Pesticides). Ecofriendly Pest Manag. Food Secur. 2016, 16, 507–524. [Google Scholar]
- Mukarram, M.; Choudhary, S.; Khan, M.A.; Poltronieri, P.; Khan, M.M.A.; Ali, J.; Kurjak, D.; Shahid, M. Lemongrass Essential Oil Components with Antimicrobial and Anticancer Activities. Antioxidants 2021, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Schmidt, E.; Bail, S.; Buchbauer, G.; Stoilova, I.; Atanasova, T.; Stoyanova, A. Chemical composition, olfactory evaluation and antioxidant effects of essential oil from Mentha x piperita. Nat. Prod. Commun. 2009, 4, 1107–1112. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid Based Complement Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yusoff, K.; Lim, S.H.E.; Chong, C.M.; Lai, K.S. Membrane Disruption Properties of Essential Oils—A Double-Edged Sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Martínez, A.; Stashenko, E.E.; Sáez, R.T.; Zafra, G.; Ortiz, C. Effect of Essential Oil from Lippia origanoides on the Transcriptional Expression of Genes Related to Quorum Sensing, Biofilm Formation, and Virulence of Escherichia coli and Staphylococcus aureus. Antibiotics 2023, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J. Anti-biofilm and Virulence Factor-Reducing Activities of Essential Oils and Oil Components as a Possible Option for Bacterial Infection Control. Planta Medica 2020, 86, 520–537. [Google Scholar] [CrossRef]
- Khwaza, V.; Aderibigbe, B.A. Antibacterial Activity of Selected Essential Oil Components and Their Derivatives: A Review. Antibiotics 2025, 14, 68. [Google Scholar] [CrossRef]
- Kong, A.S.Y.; Maran, S.; Yap, P.S.X.; Lim, S.H.E.; Yang, S.K.; Cheng, W.H.; Tan, Y.H.; Lali, K.S. Anti- and Pro-Oxidant Properties of Essential Oils against Antimicrobial Resistance. Antioxidants 2022, 11, 1819. [Google Scholar] [CrossRef]
- Melkina, O.E.; Plyuta, V.A.; Khmel, I.A.; Zavilgelsky, G.B. The Mode of Action of Cyclic Monoterpenes (−)-Limonene and (+)-α-Pinene on Bacterial Cells. Biomolecules 2021, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, F.; Bojko, B.; Bessonneau, V.; Pawliszyn, J. Cinnamaldehyde Characterization as an Antibacterial Agent toward E. coli Metabolic Profile Using 96-Blade Solid-Phase Microextraction Coupled to Liquid Chromatography-Mass Spectrometry. J. Proteome Res. 2016, 15, 963–975. [Google Scholar] [CrossRef]
- Gutiérrez-Pacheco, M.M.; Torres-Moreno, H.; Flores-Lopez, M.L.; Velázquez Guadarrama, N.; Ayala-Zavala, J.F.; Ortega-Ramírez, L.A. Mechanisms and Applications of Citral’s Antimicrobial Properties in Food Preservation and Pharmaceuticals Formulations. Antibiotics 2023, 12, 1608. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.O.; Holley, R.A. Mechanisms of Bactericidal Action of Cinnamaldehyde against Listeria monocytogenes and of Eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750. [Google Scholar] [CrossRef] [PubMed]
- Aiemsaard, J.; Aiumlamai, S.; Aromdee, C.; Taweechaisupapong, S.; Khunkitti, W. The effect of lemongrass oil and its major components on clinical isolate mastitis pathogens and their mechanisms of action on Staphylococcus aureus DMST 4745. Res. Vet. Sci. 2011, 91, e31–e37. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil-Bear. Plants. 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Pierozan, M.B.; Filho, J.G.d.O.; Cappato, L.P.; Costa, A.C.; Egea, M.B. Essential Oils Against Spoilage in Fish and Seafood: Impact on Product Quality and Future Challenges. Foods 2024, 13, 3903. [Google Scholar] [CrossRef]
- Noshirvani, N. Essential Oils as Natural Food Preservatives: Special Emphasis on Antimicrobial and Antioxidant Activities. J. Food. Qual. 2024, 2024, 5807281. [Google Scholar] [CrossRef]
- Antonino, C.; Difonzo, G.; Faccia, M.; Caponio, F. Effect of edible coatings and films enriched with plant extracts and essential oils on the preservation of animal-derived foods. J. Food Sci. 2024, 89, 748–772. [Google Scholar] [CrossRef]
- Duong, T.H.; Dinh, K.B.; Luu, T.; Chapman, J.; Baji, A.; Truong, V.K. Nanoengineered sustainable antimicrobial packaging: Integrating essential oils into polymer matrices to combat food waste. Int. J. Food Sci. Technol. 2024, 59, 5887–5901. [Google Scholar] [CrossRef]
- Mani-López, E.; Lorenzo-Leal, A.C.; Palou, E.; López-Malo, A. Principles of Sensory Evaluation in Foods Containing Essential Oil. Essent. Oils Food Process. Chem. Saf. Appl. 2017, 9, 293–325. [Google Scholar]
- Fernandes, B.; Oliveira, M.C.; Marques, A.C.; dos Santos, R.G.; Serrano, C. Microencapsulation of Essential Oils and Oleoresins: Applications in Food Products. Foods 2024, 13, 3873. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef]
- EUCAST: Disk Diffusion Methodology. Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology (accessed on 28 March 2025).
- Kim, J.; Kim, S.; Wang, J.; Ahn, J. Synergistic antimicrobial activity of essential oils in combination with phage endolysin against Salmonella Typhimurium in cooked ground beef. Food Control. 2024, 157, 110187. [Google Scholar] [CrossRef]
- Iseppi, R.; Camellini, S.; Zurlini, C.; Cigognini, I.M.; Cannavacciuolo, M.; Messi, P. Essential Oils and Bacteriocin-Based Active Edible Coating: An Innovative, Natural and Sustainable Approach for the Control of Listeria monocytogenes in Seafoods. Appl. Sci. 2023, 13, 2562. [Google Scholar] [CrossRef]
- Abdallah, K.; Tharwat, A.; Gharieb, R. High efficacy of a characterized lytic bacteriophage in combination with thyme essential oil against multidrug-resistant Staphylococcus aureus in chicken products. Iran J. Vet. Res. 2021, 22, 24–32. [Google Scholar] [PubMed]
- Naureen, Z.; Dautaj, A.; Anpilogov, K.; Camilleri, G.; Dhuli, K.; Tanzi, B.; Maltese, P.E.; Cristofoli, F.; De Antoni, L.; Beccari, T.; et al. Bacteriophages presence in nature and their role in the natural selection of bacterial populations. Acta Bio. Medica Atenei Parm. 2020, 91, e2020024. [Google Scholar]
- Veesler, D.; Cambillau, C.A. Common Evolutionary Origin for Tailed-Bacteriophage Functional Modules and Bacterial Machineries. Microbiol. Mol. Biol. Rev. 2011, 75, 423. [Google Scholar] [CrossRef]
- Seul, A.; Brasilès, S.; Petitpas, I.; Lurz, R.; Campanacci, V.; Cambillau, C.; Weise, F.; Zairi, M.; Tavares, P.; Aluzat, I. Biogenesis of a Bacteriophage Long Non-Contractile Tail. J. Mol. Biol. 2021, 433, 167112. [Google Scholar] [CrossRef]
- Casjens, S.R.; Molineux, I.J. Short noncontractile tail machines: Adsorption and DNA delivery by podoviruses. Adv. Exp. Med. Biol. 2012, 726, 143–179. [Google Scholar]
- Carmody, C.M.; Goddard, J.M.; Nugen, S.R. Bacteriophage Capsid Modification by Genetic and Chemical Methods. Bioconjug. Chem. 2021, 32, 466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, T.; Yu, M.; Chen, Y.L.; Jin, M. The Life Cycle Transitions of Temperate Phages: Regulating Factors and Potential Ecological Implications. Viruses 2022, 14, 1904. [Google Scholar] [CrossRef]
- Stone, E.; Campbell, K.; Grant, I.; McAuliffe, O. Understanding and Exploiting Phage–Host Interactions. Viruses 2019, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Elois, M.A.; da Silva, R.; Pilati, G.V.T.; Rodríguez-Lázaro, D.; Fongaro, G. Bacteriophages as Biotechnological Tools. Viruses 2023, 15, 349. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Draper, L.A.; Ross, R.P.; Hill, C. The Advantages and Challenges of Using Endolysins in a Clinical Setting. Viruses 2021, 13, 680. [Google Scholar] [CrossRef]
- Borodovich, T.; Shkoporov, A.N.; Ross, R.P.; Hill, C. Phage-mediated horizontal gene transfer and its implications for the human gut microbiome. Gastroenterol. Rep. 2022, 10, goac012. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M. Bacteriophages and Food Production: Biocontrol and Bio-Preservation Options for Food Safety. Antibiotics 2022, 11, 1324. [Google Scholar] [CrossRef]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- García-Anaya, M.C.; Sepulveda, D.R.; Zamudio-Flores, P.B.; Acosta-Muñiz, C.H. Bacteriophages as additives in edible films and coatings. Trends Food Sci. Technol. 2023, 132, 150–161. [Google Scholar] [CrossRef]
- Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Escamez, P.S.F.; Girones, R.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Norrung, B.; et al. Evaluation of the safety and efficacy of ListexTM P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA J. 2016, 14. [Google Scholar] [CrossRef]
- Grigore-Gurgu, L.; Bucur, F.I.; Mihalache, O.A.; Nicolau, A.I. Comprehensive Review on the Biocontrol of Listeria monocytogenes in Food Products. Foods 2024, 13, 734. [Google Scholar] [CrossRef]
- Carter, C.D.; Parks, A.; Abuladze, T.; Li, M.; Woolston, J.; Magnone, J.; Senecal, A.; Kropinski, A.M.; Sulakvelidze, A. Bacteriophage cocktail significantly reduces Escherichia coli O157:H7 contamination of lettuce and beef, but does not protect against recontamination. Bacteriophage 2012, 2, 178. [Google Scholar] [CrossRef]
- Sukumaran, A.T.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Reduction of Salmonella on chicken breast fillets stored under aerobic or modified atmosphere packaging by the application of lytic bacteriophage preparation SalmoFreshTM. Poult. Sci. 2016, 95, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Fokas, R.; Kotsiri, Z.; Vantarakis, A. Can Bacteriophages Be Effectively Utilized for Disinfection in Animal-Derived Food Products? A Systematic Review. Pathogens 2025, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- López-Cuevas, O.; Medrano-Félix, J.A.; Castro-Del Campo, N.; Chaidez, C. Bacteriophage applications for fresh produce food safety. Int. J. Environ. Health Res. 2021, 31, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Naureen, Z.; Malacarne, D.; Anpilogov, K.; Dautaj, A.; Camilleri, G.; Cecchin, S.; Bressan, S.; Casadei, A.; Albion, E.; Sorrentino, E.; et al. Comparison between American and European legislation in the therapeutical and alimentary bacteriophage usage. Acta Bio Medica Atenei Parm. 2020, 91, e2020023. [Google Scholar]
- Turck, D.; Bohn, T.; Castenmiller, J.; de Stefaan, H.; Karen, I.H.-E.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pentieva, K. Guidance on the scientific requirements for an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2024, 22, 8961. [Google Scholar]
- ListShieldTM—Intralytix, Inc. Available online: https://www.intralytix.com/product/1?e=ListShield (accessed on 27 March 2025).
- EcoShieldTM—Intralytix, Inc. Available online: https://www.intralytix.com/product/2 (accessed on 27 March 2025).
- SalmoFreshTM—Intralytix, Inc. Available online: https://www.intralytix.com/product/3?e=SalmoFresh (accessed on 27 March 2025).
- ShigaShieldTM—Intralytix, Inc. Available online: https://www.intralytix.com/product/8?e=ShigaShield (accessed on 27 March 2025).
- Listeria Control for the Food Industry|Phageguard. Available online: https://www.phageguard.com/solutions/listeria?_gl=1*1dj4s72*_up*MQ..*_gs*MQ..&gclid=CjwKCAjw7pO_BhAlEiwA4pMQvIoPTO0HcX2yIpRC1L0K-Zn6lsKKN0kB8NjJFlx9C1UkSz3-ywH24BoCjBsQAvD_BwE (accessed on 27 March 2025).
- Salmonella Control for the Food Industry|Phageguard. Available online: https://www.phageguard.com/solutions/salmonella?_gl=1*s4nl5y*_up*MQ..*_gs*MQ..&gclid=CjwKCAjw7pO_BhAlEiwA4pMQvIoPTO0HcX2yIpRC1L0K-Zn6lsKKN0kB8NjJFlx9C1UkSz3-ywH24BoCjBsQAvD_BwE (accessed on 27 March 2025).
- Phage Technology for E. coli Control|Phageguard. Available online: https://www.phageguard.com/solutions/e-coli?_gl=1*4y4ljr*_up*MQ..*_gs*MQ..&gclid=CjwKCAjw7pO_BhAlEiwA4pMQvIoPTO0HcX2yIpRC1L0K-Zn6lsKKN0kB8NjJFlx9C1UkSz3-ywH24BoCjBsQAvD_BwE (accessed on 27 March 2025).
- Soffer, N.; Abuladze, T.; Woolston, J.; Li, M.; Hanna, L.F.; Heyse, S.; Charbonneau, D.; Sulakvelidze, A. Bacteriophages safely reduce Salmonella contamination in pet food and raw pet food ingredients. Bacteriophage 2016, 6, e1220347. [Google Scholar] [CrossRef]
- Bafasal–Proteon Pharmaceutical. Available online: https://www.proteonpharma.com/bafasal-product/ (accessed on 27 March 2025).
- Jia, H.J.; Jia, P.P.; Yin, S.; Bu, L.K.; Yang, G.; Pei, D.S. Engineering bacteriophages for enhanced host range and efficacy: Insights from bacteriophage-bacteria interactions. Front. Microbiol. 2023, 14, 1172635. [Google Scholar] [CrossRef]
- Fister, S.; Robben, C.; Witte, A.K.; Schoder, D.; Wagner, M.; Rossmanith, P. Influence of Environmental Factors on Phage–Bacteria Interaction and on the Efficacy and Infectivity of Phage P100. Front. Microbiol. 2016, 7, 1152. [Google Scholar] [CrossRef]
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The influence of external factors on bacteriophages—Review. Folia Microbiol. 2011, 56, 191. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef]
- Balcázar, J.L. How do bacteriophages promote antibiotic resistance in the environment? Clin. Microbiol. Infect. 2018, 24, 447–449. [Google Scholar] [CrossRef]
- Colavecchio, A.; Cadieux, B.; Lo, A.; Goodridge, L.D. Bacteriophages contribute to the spread of antibiotic resistance genes among foodborne pathogens of the Enterobacteriaceae family–A review. Front. Microbiol. 2017, 8, 271343. [Google Scholar] [CrossRef] [PubMed]
- Ranveer, S.A.; Dasriya, V.; Ahmad, M.F.; Dhillon, H.S.; Samtiya, M.; Shama, E.; Anand, T.; Dhewa, T.; Chaudhary, V.; Chaudhary, P.; et al. Positive and negative aspects of bacteriophages and their immense role in the food chain. Npj Sci. Food 2024, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.; Da Silva, G.J. CRISPR-Cas: Converting A Bacterial Defence Mechanism into A State-of-the-Art Genetic Manipulation Tool. Antibiotics 2019, 8, 18. [Google Scholar] [CrossRef]
- Park, W.; Park, M.; Chun, J.; Hwang, J.; Kim, S.; Choi, N.; Kim, S.M.; Kim, S.; Jung, S.; Ko, K.S.; et al. Delivery of endolysin across outer membrane of Gram-negative bacteria using translocation domain of botulinum neurotoxin. Int. J. Antimicrob. Agents 2024, 64, 107216. [Google Scholar] [CrossRef]
- Ghosh, A. Application of Essential Oil Compounds and Bacteriophage to Application of Essential Oil Compounds and Bacteriophage to Control Staphylococcus aureus. Master’s Thesis, University of Arkansas, Fayetteville, NC, USA, May 2015. Available online: https://scholarworks.uark.edu/etd (accessed on 24 March 2025).
- Ghosh, A.; Ricke, S.C.; Almeida, G.; Gibson, K.E. Combined Application of Essential Oil Compounds and Bacteriophage to Inhibit Growth of Staphylococcus aureus In Vitro. Curr. Microbiol. 2016, 72, 426–435. [Google Scholar] [CrossRef]
- Elafify, M.; Mahmoud, A.A.; Wang, X.; Zhang, S.; Ding, T.; Ahn, J. Synergistic antimicrobial efficacy of phage cocktails and essential oils against Escherichia coli. Microb. Pathog. 2025, 200, 107330. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef]
- Costa, M.J.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Bacteriophage Delivery Systems for Food Applications: Opportunities and Perspectives. Viruses 2023, 15, 1271. [Google Scholar] [CrossRef] [PubMed]
- Gaudereto, J.J.; Neto, L.V.P.; Leite, G.C.; Espinoza, E.P.S.; Martins, R.C.R.; Villas Boa Prado, G.; Rossi, F.; Guimarães, T.; Levin, A.S.; Costa, S.F. Comparison of methods for the detection of in vitro synergy in multidrug-resistant gram-negative bacteria. BMC Microbiol. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 20029. [Google Scholar] [CrossRef] [PubMed]
- Matloob, A.; Ayub, H.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Khalid, W.; Nayik, G.A.; Ramniwas, S.; et al. A Review on Edible Coatings and Films: Advances, Composition, Production Methods, and Safety Concerns. ACS Omega 2023, 8, 28932–28944. [Google Scholar] [CrossRef]
- Gupta, D.; Lall, A.; Kumar, S.; Patil, T.D.; Gaikwad, K.K. Plant-based edible films and coatings for food-packaging applications: Recent advances, applications, and trends. Sustain. Food Technol. 2024, 2, 1428–1455. [Google Scholar] [CrossRef]
- O’Donnell, S.T.; Ross, R.P.; Stanton, C. The Progress of Multi-Omics Technologies: Determining Function in Lactic Acid Bacteria Using a Systems Level Approach. Front. Microbiol. 2020, 10, 483666. [Google Scholar] [CrossRef]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 1–15. [Google Scholar] [CrossRef]
- EFSA Evaluates Bacteriophages, EFSA. Available online: https://www.efsa.europa.eu/en/news/efsa-evaluates-bacteriophages?utm_source=chatgpt.com (accessed on 28 March 2025).

| Study (Author, Year) | Target Pathogen | Essential Oil | Phage Type | Food Matrix | Method | Main Outcome |
|---|---|---|---|---|---|---|
| Ghosh, 2015 [91] | S. aureus (incl. MRSA) | Lemongrass, cinnamon, melissa, tea tree | Phage K | Raw chicken meat | In vitro + inoculated food | No synergy at 6–13 °C; higher phage effect at 25 °C |
| Ghosh et al., 2016 [92] | S. aureus (incl. MRSA) | EO compounds (alpha-pinene, 3-carene) | Phage K | In vitro | Disk diffusion | Additive/synergistic inhibition at high EO conc. (3.28%) |
| Abdallah et al., 2021 [51] | S. aureus (MDR) | Thyme oil (0.5–1%) | vB_SauM_CP9 (Myoviridae) | Chicken fillets | Surface application | Synergistic reduction (87.2%) after 120 min |
| Kim et al., 2024 [49] | S. typhimurium | Carvacrol, eugenol, thymol (½ MIC), AITC (allyl isothiocyanate) | Phage endolysin (LysPB32) | Cooked ground beef | In vitro + food trial | >2-log CFU/g reduction in meat; synergistic membrane disruption |
| Elafify et al., 2025 [93] | E. coli ATCC 15597 | Cinnamon, thymol (½ MIC) | MS2 + T7 cocktail | In vitro | Spot test, time-kill assay, fitness/mutation assays | Strong synergy; >5-log reduction; reduced resistance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fokas, R.; Giormezis, N.; Vantarakis, A. Synergistic Approaches to Foodborne Pathogen Control: A Narrative Review of Essential Oils and Bacteriophages. Foods 2025, 14, 1508. https://doi.org/10.3390/foods14091508
Fokas R, Giormezis N, Vantarakis A. Synergistic Approaches to Foodborne Pathogen Control: A Narrative Review of Essential Oils and Bacteriophages. Foods. 2025; 14(9):1508. https://doi.org/10.3390/foods14091508
Chicago/Turabian StyleFokas, Rafail, Nikolaos Giormezis, and Apostolos Vantarakis. 2025. "Synergistic Approaches to Foodborne Pathogen Control: A Narrative Review of Essential Oils and Bacteriophages" Foods 14, no. 9: 1508. https://doi.org/10.3390/foods14091508
APA StyleFokas, R., Giormezis, N., & Vantarakis, A. (2025). Synergistic Approaches to Foodborne Pathogen Control: A Narrative Review of Essential Oils and Bacteriophages. Foods, 14(9), 1508. https://doi.org/10.3390/foods14091508








