The Textural Properties of Extra Virgin Olive Oil (EVOO)-Hydrocolloid Beads and the Quality Parameters of Bosana EVOO as a Preservation Liquid During Bead Shelf Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extra Virgin Olive Oil
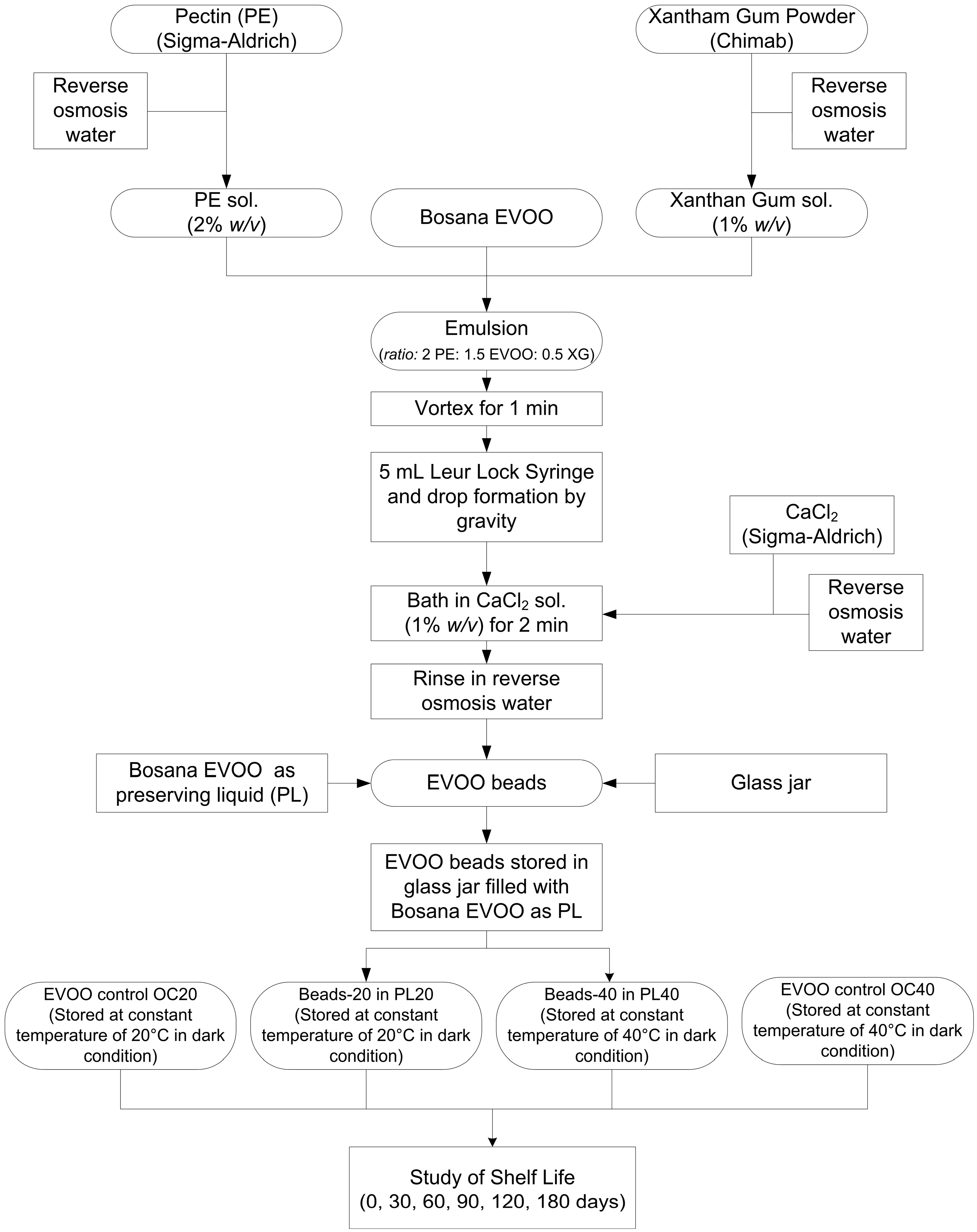
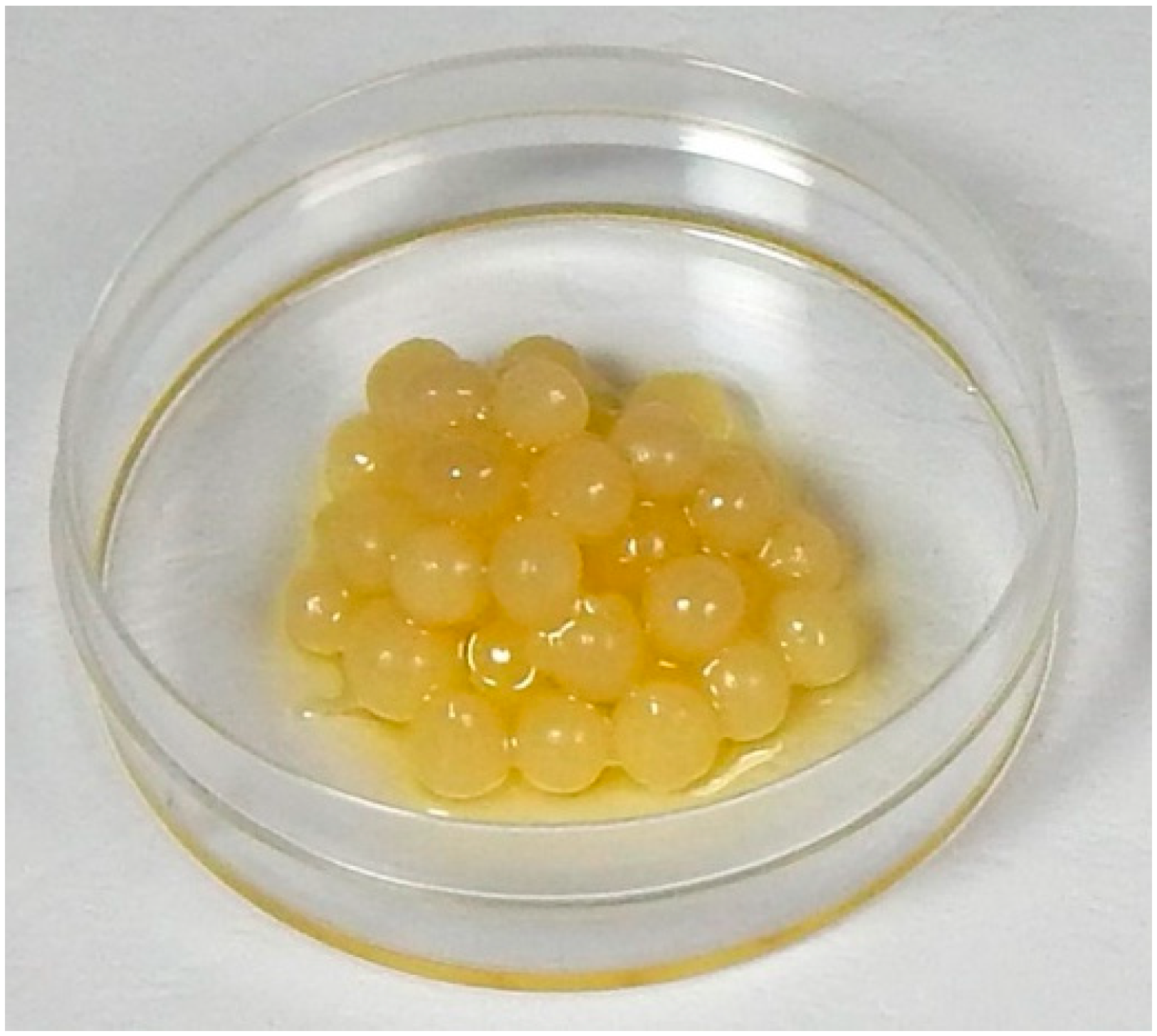
2.3. Preparation of EVOO beads
2.4. Texture Profile Analysis (TPA) of EVOO beads
2.5. Free Acidity and Peroxide Value of Preserving Oil
2.6. Total Phenolic Content (TPC) of Preserving Oil
2.7. α-Tocopherol Determination by HPLC of Preserving Oil
2.8. Statistical Analysis
3. Results
3.1. Texture Profile Analysis of beads
3.2. Determination of Acidity and Peroxide Values of Preserving Liquid
3.3. Determination of Total Phenolic Content of Preserving Liquid
3.4. α-Tocopherol Content of Preserving Liquid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delshadi, R.; Bahrami, A.; Tafti, A.G.; Barba, F.J.; Williams, L.L. Micro and Nano-Encapsulation of Vegetable and Essential Oils to Develop Functional Food Products with Improved Nutritional Profiles. Trends Food Sci. Technol. 2020, 104, 72–83. [Google Scholar] [CrossRef]
- Bhuva, S.S.; Dhamsaniya, N.K. Encapsulation of Vegetable Oils for Enhancing Oxidative Stability of PUFA. Bhuva Dhamsaniya Biol. Forum-Int. J. 2023, 15, 271–284. [Google Scholar]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Fessi, H.; Elaissari, A. Plant Oils: From Chemical Composition to Encapsulated Form Use. Int. J. Pharm. 2021, 601, 120538. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Sacanella, E.; Tahiri, I.; Casas, R. Chapter 17—Mediterranean Diet and Role of Olive Oil. In Olives and Olive Oil in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2021; pp. 205–214. ISBN 978-0-12-819528-4. [Google Scholar]
- Conte, P.; Squeo, G.; Difonzo, G.; Caponio, F.; Fadda, C.; Del Caro, A.; Urgeghe, P.P.; Montanari, L.; Montinaro, A.; Piga, A. Change in Quality during Ripening of Olive Fruits and Related Oils Extracted from Three Minor Autochthonous Sardinian Cultivars. Emir. J. Food Agric. 2019, 31, 196–205. [Google Scholar] [CrossRef]
- Patricia Blanch, G.; Flores, G.; Gómez-Jiménez, M.C.; Luisa Ruiz del Castillo, M. Effect of the Treatment of the Olive Tree (Olea Europaea L.) on the Phenolic Content and Antioxidant Properties in Olive Fruits. J. Food Nutr. Res. 2018, 6, 49–55. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A.; Del Caro, A.; Piga, A.; Vacca, V.; Caboni, M.F.; Toschi, T.G. Preliminary Characterisation of Virgin Olive Oils Obtained from Different Cultivars in Sardinia. Eur. Food Res. Technol. 2006, 222, 354–361. [Google Scholar] [CrossRef]
- Kiritsakis, A.; Kanavouras, A.; Kiritsakis, K. Chemical Analysis, Quality Control and Packaging Issues of Olive Oil. Eur. J. Lipid Sci. Technol. 2002, 104, 628–638. [Google Scholar] [CrossRef]
- Nasopoulou, C.; Karantonis, H.C.; Detopoulou, M.; Demopoulos, C.A.; Zabetakis, I. Exploiting the Anti-Inflammatory Properties of Olive (Olea Europaea) in the Sustainable Production of Functional Food and Neutraceuticals. Phytochem. Rev. 2014, 13, 445–458. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Gallardo-Gomez, M.; Simal-Gandara, J.; Carpena, M.; Lorenzo, J.M.; Lourenço-Lopes, C.; Barba, F.J.; Prieto, M.A. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Sánchez-Tainta, A. Virgin Olive Oil: A Mediterranean Diet Essential. In The Prevention of Cardiovascular Disease Through the Mediterranean Diet; Academic Press: Cambridge, MA, USA, 2017; pp. 59–87. [Google Scholar] [CrossRef]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the Olive-Derived Polyphenol Oleuropein on Human Health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Gotsis, E.; Anagnostis, P.; Mariolis, A.; Vlachou, A.; Katsiki, N.; Karagiannis, A. Health Benefits of the Mediterranean Diet: An Update of Research over the Last 5 Years. Angiology 2015, 66, 304–318. [Google Scholar] [CrossRef]
- Campestre, C.; Angelini, G.; Gasbarri, C.; Angerosa, F. The Compounds Responsible for the Sensory Profile in Monovarietal Virgin Olive Oils. Molecules 2017, 22, 1833. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Lerma-García, M.J.; Simó-Alfonso, E.F.; Chiavaro, E.; Bendini, A.; Lercker, G.; Cerretani, L. Study of Chemical Changes Produced in Virgin Olive Oils with Different Phenolic Contents during an Accelerated Storage Treatment. J. Agric. Food Chem. 2009, 57, 7834–7840. [Google Scholar] [CrossRef]
- Lozano-Sánchez, J.; Bendini, A.; Quirantes-Piné, R.; Cerretani, L.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Monitoring the Bioactive Compounds Status of Extra-Virgin Olive Oil and Storage by-Products over the Shelf Life. Food Control 2013, 30, 606–615. [Google Scholar] [CrossRef]
- Polavarapu, S.; Oliver, C.M.; Ajlouni, S.; Augustin, M.A. Physicochemical Characterisation and Oxidative Stability of Fish Oil and Fish Oil-Extra Virgin Olive Oil Microencapsulated by Sugar Beet Pectin. Food Chem. 2011, 127, 1694–1705. [Google Scholar] [CrossRef]
- Deiana, P.; Santona, M.; Dettori, S.; Culeddu, N.; Dore, A.; Molinu, M.G. Multivariate Approach to Assess the Chemical Composition of Italian Virgin Olive Oils as a Function of Variety and Harvest Period. Food Chem. 2019, 300, 125243. [Google Scholar] [CrossRef]
- Deiana, P.; Motroni, A.; Filigheddu, M.R.; Dettori, S.; Nieddu, G.; Mercenaro, L.; Alfei, B.; Culeddu, N.; Santona, M. Effect of Pedoclimatic Variables on Analytical and Organoleptic Characteristics in Olive Fruit and Virgin Olive Oil. Eur. J. Agron. 2023, 148, 126856. [Google Scholar] [CrossRef]
- Calvo, P.; Hernández, T.; Lozano, M.; González-Gómez, D. Microencapsulation of Extra-Virgin Olive Oil by Spray-Drying: Influence of Wall Material and Olive Quality. Eur. J. Lipid Sci. Technol. 2010, 112, 852–858. [Google Scholar] [CrossRef]
- Chan, L.W.; Lim, L.T.; Heng, P.W.S. Microencapsulation of Oils Using Sodium Alginate. J. Microencapsul. 2000, 17, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P.; Thirawong, N.; Puttipipatkhachorn, S. Morphology and Buoyancy of Oil-Entrapped Calcium Pectinate Gel Beads. AAPS J. 2004, 6, 2–8. [Google Scholar] [CrossRef]
- Velázquez-Gutiérrez, S.K.; Alpizar-Reyes, E.; Guadarrama-Lezama, A.Y.; Báez-González, J.G.; Alvarez-Ramírez, J.; Pérez-Alonso, C. Influence of the Wall Material on the Moisture Sorption Properties and Conditions of Stability of Sesame Oil Hydrogel Beads by Ionic Gelation. LWT 2021, 140, 110695. [Google Scholar] [CrossRef]
- Patel, A.R. Structuring Edible Oils with Hydrocolloids: Where Do We Stand? Food Biophys. 2018, 13, 113–115. [Google Scholar] [CrossRef]
- Turasan, H.; Sahin, S.; Sumnu, G. Encapsulation of Rosemary Essential Oil. LWT 2015, 64, 112–119. [Google Scholar] [CrossRef]
- Vergallo, C. Nutraceutical Vegetable Oil Nanoformulations for Prevention and Management of Diseases. Nanomaterials 2020, 10, 1232. [Google Scholar] [CrossRef]
- Kurek, M.; Descours, E.; Poldan, P.; Julou, A.; Pitois, A.; Klepac, D.; Vallet, N.; Galić, K. Possibility of Storing Olive Oil in Antioxidant Biobased Pouches Made of Chitosan and Gelatin. Food Hydrocoll. 2024, 151, 109835. [Google Scholar] [CrossRef]
- Gutiérrez-luna, K.; Ansorena, D.; Astiasarán, I. Use of Hydrocolloids and Vegetable Oils for the Formulation of a Butter Replacer: Optimization and Oxidative Stability. LWT 2022, 153, 112538. [Google Scholar] [CrossRef]
- Rezagholizade-shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive Compound Encapsulation: Characteristics, Applications in Food Systems, and Implications for Human Health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, R.; Liang, H. Food Hydrocolloids: Structure, Properties, and Applications. Foods 2024, 13, 1077. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as Thickening and Gelling Agents in Food: A Critical Review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion Stabilizing Properties of Pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Rolin, C.P. Industrial Gums; Whistler, R., Bemiller, J.N., Eds.; Elsevier: London, UK, 1993; pp. 257–293. ISBN 978-0-08-092654-4. [Google Scholar]
- Lazaridou, A.; Biliaderis, C.G. Edible Films and Coatings with Pectin; Springer: Berlin, Germany, 2020; ISBN 9783030534219. [Google Scholar]
- Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Saif Alrasbi, A.N.; Jawad, M.; Koca, E.; Aydemir, L.Y.; Alamoudi, J.A.; Almoshari, Y.; Mohan, S. Structural, Mechanical, Barrier and Antioxidant Properties of Pectin and Xanthan Gum Edible Films Loaded with Grapefruit Essential Oil. Heliyon 2024, 10, e25501. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, N.; Genovese, A.; Burke, R.; Barry-Ryan, C.; Sacchi, R. Effect of Olive Mill Wastewater Phenolic Extract, Whey Protein Isolate and Xanthan Gum on the Behaviour of Olive O/W Emulsions Using Response Surface Methodology. Food Hydrocoll. 2016, 61, 66–76. [Google Scholar] [CrossRef]
- Lagoueyte, N.; Paquin, P. Effects of Microfluidization on the Functional Properties of Xanthan Gum. Food Hydrocoll. 1998, 12, 365–371. [Google Scholar] [CrossRef]
- Sun, C.; Gunasekaran, S.; Richards, M.P. Effect of Xanthan Gum on Physicochemical Properties of Whey Protein Isolate Stabilized Oil-in-Water Emulsions. Food Hydrocoll. 2007, 21, 555–564. [Google Scholar] [CrossRef]
- de Morais Lima, M.; Bianchini, D.; Guerra Dias, A.; da Rosa Zavareze, E.; Prentice, C.; da Silveira Moreira, A. Biodegradable Films Based on Chitosan, Xanthan Gum, and Fish Protein Hydrolysate. J. Appl. Polym. Sci. 2017, 134, 44899. [Google Scholar] [CrossRef]
- Bascuas, S.; Hernando, I.; Moraga, G.; Quiles, A. Structure and Stability of Edible Oleogels Prepared with Different Unsaturated Oils and Hydrocolloids. Int. J. Food Sci. Technol. 2020, 55, 1458–1467. [Google Scholar] [CrossRef]
- Bouyer, E.; Mekhloufi, G.; Huang, N.; Rosilio, V.; Agnely, F. β-Lactoglobulin, Gum Arabic, and Xanthan Gum for Emulsifying Sweet Almond Oil: Formulation and Stabilization Mechanisms of Pharmaceutical Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 77–87. [Google Scholar] [CrossRef]
- Cortes, H.; Caballero-Florán, I.H.; Mendoza-Muñoz, N.; Escutia-Guadarrama, L.; Figueroa-González, G.; Reyes-Hernández, O.D.; González-Del Carmen, M.; Varela-Cardoso, M.; González-Torres, M.; Florán, B.; et al. Xanthan Gum in Drug Release. Cell. Mol. Biol. 2020, 66, 199–207. [Google Scholar] [CrossRef]
- Paba, A.; Chessa, L.; Cabizza, R.; Daga, E.; Urgeghe, P.P.; Testa, M.C.; Comunian, R. Zoom on Starter Lactic Acid Bacteria Development into Oxytetracycline Spiked Ovine Milk during the Early Acidification Phase. Int. Dairy J. 2019, 96, 15–20. [Google Scholar] [CrossRef]
- Petri, D.F.S. Xanthan Gum: A Versatile Biopolymer for Biomedical and Technological Applications. J. Appl. Polym. Sci. 2015, 132, 42035. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, T.V.R. Xanthan Gum Based Edible Coating Enriched with Cinnamic Acid Prevents Browning and Extends the Shelf-Life of Fresh-Cut Pears. LWT 2015, 62, 791–800. [Google Scholar] [CrossRef]
- Barbosa de Almeida, C.; Catelam, K.T.; Lopes Cornélio, M.; Lopes Filho, J.F. Morphological and Structural Characteristics of Zein Biofilms with Added Xanthan Gum. Food Technol. Biotechnol. 2010, 48, 19–27. [Google Scholar]
- Dahdah, P.; Cabizza, R.; Farbo, M.G.; Fadda, C.; Mara, A.; Hassoun, G.; Piga, A. Improving the Rheological Properties of Dough Obtained by Partial Substitution of Wheat Flour with Freeze-Dried Olive Pomace. Foods 2024, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Dahdah, P.; Cabizza, R.; Farbo, M.G.; Fadda, C.; Del Caro, A.; Montanari, L.; Hassoun, G.; Piga, A. Effect of Partial Substitution of Wheat Flour with Freeze-Dried Olive Pomace on the Technological, Nutritional, and Sensory Properties of Bread. Front. Sustain. Food Syst. 2024, 8, 1400339. [Google Scholar] [CrossRef]
- Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. Off. J. Eur. Communities 1991, L248/1, 1–83.
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Gimeno, E.; Castellote, A.I.; Lamuela-Raventós, R.M.; de la Torre, M.C.; López-Sabater, M.C. Rapid Determination of Vitamin E in Vegetable Oils by Reversed-Phase High-Performance Liquid Chromatography. J. Chromatogr. A 2000, 881, 251–254. [Google Scholar] [CrossRef]
- Patel, A.R.; Cludts, N.; Bin Sintang, M.D.; Lesaffer, A.; Dewettinck, K. Edible Oleogels Based on Water Soluble Food Polymers: Preparation, Characterization and Potential Application. Food Funct. 2014, 5, 2833–2841. [Google Scholar] [CrossRef]
- Patel, A.R.; Cludts, N.; Bin Sintang, M.D.; Lewille, B.; Lesaffer, A.; Dewettinck, K. Polysaccharide-Based Oleogels Prepared with an Emulsion-Templated Approach. ChemPhysChem 2014, 15, 3435–3439. [Google Scholar] [CrossRef]
- Giri, T.K.; Choudhary, C.; Alexander, A.; Ajazuddin, A.; Badwaik, H.; Tripathy, M.; Tripathi, D.K. Sustained Release of Diltiazem Hydrochloride from Cross-Linked Biodegradable IPN Hydrogel Beads of Pectin and Modified Xanthan Gum. Indian J. Pharm. Sci. 2013, 75, 619–627. [Google Scholar]
- Garrido, J.I.; Lozano, J.E.; Genovese, D.B. Effect of Formulation Variables on Rheology, Texture, Colour, and Acceptability of Apple Jelly: Modelling and Optimization. LWT 2015, 62, 325–332. [Google Scholar] [CrossRef]
- Goulao, L.F.; Oliveira, C.M. Cell Wall Modifications during Fruit Ripening: When a Fruit Is Not the Fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Fraeye, I.; De Roeck, A.; Duvetter, T.; Verlent, I.; Hendrickx, M.; Van Loey, A. Influence of Pectin Properties and Processing Conditions on Thermal Pectin Degradation. Food Chem. 2007, 105, 555–563. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive Oil Volatile Compounds, Flavour Development and Quality: A Critical Review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Morelló, J.R.; Motilva, M.J.; Tovar, M.J.; Romero, M.P. Changes in Commercial Virgin Olive Oil (Cv Arbequina) during Storage, with Special Emphasis on the Phenolic Fraction. Food Chem. 2004, 85, 357–364. [Google Scholar] [CrossRef]
- Chabni, A.; Bañares, C.; Torres, C.F. Study of the Oxidative Stability via Oxitest and Rancimat of Phenolic-Rich Olive Oils Obtained by a Sequential Process of Dehydration, Expeller and Supercritical CO2 Extractions. Front. Nutr. 2024, 11, 1494091. [Google Scholar] [CrossRef]
- Migliorini, M.; Cherubini, C.; Cecchi, L.; Zanoni, B. Degradazione Dei Composti Fenolici Durante La Conservazione Dell’olio Extra Vergine Di Oliva. Riv. Ital. delle Sostanze Grasse 2013, 90, 71–80. [Google Scholar]
- Krichene, D.; Salvador, M.D.; Fregapane, G. Stability of Virgin Olive Oil Phenolic Compounds during Long-Term Storage (18 Months) at Temperatures of 5–50 °C. J. Agric. Food Chem. 2015, 63, 6779–6786. [Google Scholar] [CrossRef]
- Castillo-Luna, A.; Criado-Navarro, I.; Ledesma-Escobar, C.A.; López-Bascón, M.A.; Priego-Capote, F. The Decrease in the Health Benefits of Extra Virgin Olive Oil during Storage Is Conditioned by the Initial Phenolic Profile. Food Chem. 2021, 336, 127730. [Google Scholar] [CrossRef] [PubMed]
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Modelling Virgin Olive Oil Potential Shelf-Life from Antioxidants and Lipid Oxidation Progress. Antioxidants 2022, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Caipo, L.; Sandoval, A.; Sepúlveda, B.; Fuentes, E.; Valenzuela, R.; Metherel, A.H.; Romero, N. Effect of Storage Conditions on the Quality of Arbequina Extra Virgin Olive Oil and the Impact on the Composition of Flavor-Related Compounds (Phenols and Volatiles). Foods 2021, 10, 2161. [Google Scholar] [CrossRef]
- Verleyen, T.; Kamal-Eldin, A.; Dobarganes, C.; Verhe, R.; Dewettinck, K.; Huyghebaert, A. Modeling of α-Tocopherol Loss and Oxidation Products Formed during Thermoxidation in Triolein and Tripalmitin Mixtures. Lipids 2001, 36, 719–726. [Google Scholar] [CrossRef] [PubMed]

| Beads | Hardness (N) | Springiness | Cohesiveness | Chewiness (N) |
|---|---|---|---|---|
| Temperature | ||||
| 20 °C | 0.014 ± 0.006 a | 0.93 ± 0.06 a | 0.28 ± 0.02 a | 3.71 × 10−3 ± 0.002 a |
| 40 °C | 0.013 ± 0.005 a | 0.86 ± 0.07 a | 0.27 ± 0.04 b | 3.35 × 10−3 ± 0.002 a |
| p-value | 0.66 | 0.22 | 0.000 | 0.13 |
| Day | ||||
| 0 | 0.015 ± 0.006 a | 0.98 ± 0.02 a | 0.29 ± 0.01 a | 4.47 × 10−3 ± 0.002 a |
| 30 | 0.014 ± 0.006 a | 0.94 ± 0.06 ab | 0.29 ± 0.03 a | 4.25 × 10−3 ± 0.002 ab |
| 60 | 0.014 ± 0.003 a | 0.92 ± 0.07 bc | 0.28 ± 0.02 a | 4.11 × 10−3 ± 0.002 ab |
| 90 | 0.014 ± 0.006 a | 0.90 ± 0.06 bc | 0.28 ± 0.03 a | 3.52 × 10−3 ± 0.002 bc |
| 120 | 0.014 ± 0.005 a | 0.90 ± 0.06 c | 0.26 ± 0.04 b | 3.26 × 10−3 ± 0.002 c |
| 150 | 0.012 ± 0.006 a | 0.87 ± 0.08 d | 0.26 ± 0.04 b | 2.88 × 10−3 ± 0.001 c |
| 180 | 0.012 ± 0.005 a | 0.86 ± 0.07 d | 0.25 ± 0.04 b | 2.74 × 10−3 ± 0.001 c |
| p-value | 0.17 | 0.000 | 0.000 | 0.000 |
| Temperature × Day | ||||
| 0 × 20 | 0.015 ± 0.006 a | 1.05 ± 0.02 a | 0.29 ± 0.05 a | 4.56 × 10−3 ± 0.002 a |
| 0 × 40 | 0.015 ± 0.006 a | 1.05 ± 0.02 a | 0.29 ± 0.05 a | 4.56 × 10−3 ± 0.002 a |
| 30 × 20 | 0.014 ± 0.006 a | 1.05 ± 0.14 a | 0.29 ± 0.03 a | 4.50 × 10−3 ± 0.002 a |
| 30 × 40 | 0.014 ± 0.006 a | 1.05 ± 0.13 a | 0.28 ± 0.03 a | 3.92 × 10−3 ± 0.002 a |
| 60 × 20 | 0.014 ± 0.004 a | 1.05 ± 0.10 a | 0.28 ± 0.01 a | 4.00 × 10−3 ± 0.002 a |
| 60 × 40 | 0.014 ± 0.003 a | 1.05 ± 0.10 a | 0.28 ± 0.02 a | 4.31 × 10−3 ± 0.002 a |
| 90 × 20 | 0.014 ± 0.006 a | 0.94 ± 0.11 a | 0.28 ± 0.03 a | 3.38 × 10−3 ± 0.002 a |
| 90 × 40 | 0.014 ± 0.006 a | 0.95 ± 0.03 a | 0.28 ± 0.03 a | 3.56 × 10−3 ± 0.001 a |
| 120 × 20 | 0.014 ± 0.005 a | 0.92 ± 0.08 a | 0.28 ± 0.03 a | 3.38 × 10−3 ± 0.002 a |
| 120 × 40 | 0.012 ± 0.004 a | 0.91 ± 0.07 a | 0.24 ± 0.02 b | 3.15 × 10−3 ± 0.001 a |
| 150 × 20 | 0.012 ± 0.006 a | 0.92 ± 0.07 a | 0.28 ± 0.03 a | 2.88 × 10−3 ± 0.002 a |
| 150 × 40 | 0.012 ± 0.006 a | 0.83 ± 0.07 b | 0.23 ± 0.03 b | 2.50 × 10−3 ± 0.001 a |
| 180 × 20 | 0.012 ± 0.006 a | 0.91 ± 0.08 a | 0.28 ± 0.02 a | 3.13 × 10−3 ± 0.001 a |
| 180 × 40 | 0.012 ± 0.004 a | 0.82 ± 0.04 b | 0.23 ± 0.04 b | 2.25 × 10−3 ± 0.001 a |
| p-value | 0.97 | 0.19 | 0.000 | 0.39 |
| Preserving Liquids | Free Acidity (% Oleic Acid) | Peroxide Value (meq O2/kg) | TPC (mg GAE/kg) | α-Tocopherol (mg/kg) |
|---|---|---|---|---|
| Temperature | ||||
| 20 °C | 0.45 ± 0.15 b | 14.23± 3.36 b | 206.5 ± 31.3 a | 204.6 ± 25.2 a |
| 40 °C | 0.50 ± 0.18 a | 20.06 ± 5.62 a | 191.9 ± 38.1 b | 182.5 ± 39.8 b |
| p-value | 0.000 | 0.000 | 0.000 | 0.000 |
| Day | ||||
| 0 | 0.28 ± 0.01 g | 9.86± 0.08 g | 251.6 ± 1.7 a | 243.6 ± 1.7 a |
| 30 | 0.36 ± 0.04 f | 13.27 ± 2.52 f | 205.5 ± 15.8 b | 219.4 ± 6.5 b |
| 60 | 0.41 ± 0.04 e | 15.07 ± 3.70 e | 197.7 ± 14.9 c | 187.5 ± 11.8 c |
| 90 | 0.46 ± 0.07 d | 18.56 ± 4.57 d | 185.0 ± 7.0 d | 184.1 ± 14.4 d |
| 120 | 0.54 ± 0.09 c | 19.70 ± 4.84 c | 175.9 ± 7.9 e | 179.8 ± 16.8 e |
| 150 | 0.61 ± 0.08 b | 20.68 ± 4.30 b | 168.3 ± 8.9 f | 163.1 ± 23.6 f |
| 180 | 0.80 ± 0.07 a | 22.89 ± 3.75 a | 158.1 ± 13.2 g | 152.4 ± 22.8 g |
| p-value | 0.000 | 0.000 | 0.000 | 0.000 |
| Temperature × Day | ||||
| 0 × 20 | 0.28 ± 0.01 i | 9.86 ± 0.09 l | 251.6 ± 1.9 a | 243.7 ± 1.9 a |
| 0 × 40 | 0.28 ± 0.01 i | 9.86 ± 0.09 l | 251.6 ± 1.9 a | 243.7 ± 1.9 a |
| 30 × 20 | 0.32 ± 0.02 h | 11.12 ± 0.58 i | 219.2 ± 1.1 b | 225.0 ± 1.4 b |
| 30 × 40 | 0.39 ± 0.01 g | 15.43 ± 0.30 g | 191.8 ± 0.6 d | 213.0 ± 1.1 c |
| 60 × 20 | 0.38 ± 0.02 g | 11.89 ± 0.69 i | 210.5 ± 2.2 c | 197.6 ± 1.3 d |
| 60 × 40 | 0.45 ± 0.03 f | 18.25 ± 0.28 e | 184.9 ± 1.7 e | 177.3 ± 1.7 f |
| 90 × 20 | 0.41 ± 0.02 g | 14.60 ± 0.15 h | 191.0 ± 1.4 d | 196.5 ± 1.2 d |
| 90 × 40 | 0.52 ± 0.02 e | 22.52 ± 0.03 c | 179.0 ± 1.4 f | 171.7 ± 1.9 g |
| 120 × 20 | 0.46 ± 0.02 f | 15.52 ± 0.24 g | 182.6 ± 0.2 e | 194.4 ± 1.2 d |
| 120 × 40 | 0.61 ± 0.04 d | 23.88 ± 0.62 b | 169.1 ± 1.6 g | 165.3 ± 1.3 h |
| 150 × 20 | 0.54 ± 0.03 e | 16.96 ± 0.21 f | 175.9 ± 1.4 f | 183.5 ± 1.9 e |
| 150 × 40 | 0.68 ± 0.02 c | 24.40 ± 0.16 b | 160.6 ± 1.5 h | 142.7 ± 1.9 i |
| 180 × 20 | 0.75 ± 0.01 b | 19.65 ± 0.21 d | 169.6 ± 0.8 g | 172.1 ± 1.4 g |
| 180 × 40 | 0.86 ± 0.02 a | 26.12 ± 0.54 a | 146.7 ± 0.5 i | 132.7 ± 1.2 l |
| p-value | 0.001 | 0.000 | 0.000 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farbo, M.G.; Avitabile, E.; Fadda, C.; Cabizza, R. The Textural Properties of Extra Virgin Olive Oil (EVOO)-Hydrocolloid Beads and the Quality Parameters of Bosana EVOO as a Preservation Liquid During Bead Shelf Life. Foods 2025, 14, 1472. https://doi.org/10.3390/foods14091472
Farbo MG, Avitabile E, Fadda C, Cabizza R. The Textural Properties of Extra Virgin Olive Oil (EVOO)-Hydrocolloid Beads and the Quality Parameters of Bosana EVOO as a Preservation Liquid During Bead Shelf Life. Foods. 2025; 14(9):1472. https://doi.org/10.3390/foods14091472
Chicago/Turabian StyleFarbo, Maria Grazia, Elisabetta Avitabile, Costantino Fadda, and Roberto Cabizza. 2025. "The Textural Properties of Extra Virgin Olive Oil (EVOO)-Hydrocolloid Beads and the Quality Parameters of Bosana EVOO as a Preservation Liquid During Bead Shelf Life" Foods 14, no. 9: 1472. https://doi.org/10.3390/foods14091472
APA StyleFarbo, M. G., Avitabile, E., Fadda, C., & Cabizza, R. (2025). The Textural Properties of Extra Virgin Olive Oil (EVOO)-Hydrocolloid Beads and the Quality Parameters of Bosana EVOO as a Preservation Liquid During Bead Shelf Life. Foods, 14(9), 1472. https://doi.org/10.3390/foods14091472







