From Hen Nutrition to Baking: Effects of Pomegranate Seed and Linseed Oils on Egg White Foam Stability and Sponge Cake Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Design of Experiments
2.1.2. Sampling Procedure
2.2. Methods
2.2.1. Foaming Properties
2.2.2. Size Distribution of Gas Bubbles Suspended in Liquid
2.2.3. Assessment of the Raising Properties of Eggs
- (a)
- Weight with an accuracy of 0.01 g using a Mettler Toledo PB 602—S/FACT balance (Columbus, OH, USA);
- (b)
- The volume of the cakes was determined using seed displacement by the method of Khna et al. with some modification [33];
- (c)
- Hardness of sponge cakes was conducted according to Rozyło and Laskowski with some modifications. TA.XT plus texture analyzer equipped with a 50 mm diameter cylindrical attachment (P-100) was used for measurement [34]. For the study, 2.5 cm thick cubes were prepared from each dough sample analyzed. The samples were subjected to double compression, with the roller travel speed set at 15 mm/min during the test. The compression process was carried out at a constant deformation of the samples equal to 50% of their height.
- (a)
- The Moisture of the cakes was established by drying the samples in a conventional oven at 98 °C for 24 h according to AOAC method [35];
- (b)
- The total fat content of cakes was assessed by the use of CO2 supercritical extraction: pump pressure—9000 PSI; cell temperature—100 °C; carbon dioxide flow rate—1.3 L/min; static time—5 min; and dynamic time—45 min (TFE2000 analyser, LECO, St. Joseph, MI, USA) [36];
- (c)
- Protein content was established based on the nitrogen amount that was analyzed using the TruSpec N LECO Company Analyzer. The analysis was done by means of the Dumas method, according to PN-EN ISO 16634-1:2008 [37], where values of N% were multiplied by 6.25 in order to calculate the protein %;
- (d)
- The ash content was analyzed by ashing the samples using a muffle furnace oven at 525 °C for 12 h [38];
- (e)
- The fatty acid analysis was performed stepwise. First, lipids were extracted from cakes by means of CO2 supercritical extraction (pump pressure—9000 PSI; cell temperature 100 °C; carbon dioxide flow rate—1.3 L/min; static time—5 min; and dynamic time—45 min) using FAT Ex-tractor TFE 2000 Leco, St. Joshep, MI, USA) [36]. After the extraction, lipids were methylated using sodium methylate [39]. In detail, 0.1 mL of extracted fat was placed in the glass test tube of 2 mL capacity, and 0.5 mL of 0.025 M of sodium methylate solution was added. The mixture was heated in a closed tube at 60 °C until the mixture was clear. The analysis of fatty acids was carried out using gas chromatography (Trace GC Ultra, Thermo Electron Corporation, Waltham, MA, USA). For the analysis, a Supelcowax 10 column (dimensions 30 m × 0.25 mm × 0.25 µm) was used. Helium was used as a sample carrier with a flow rate of 5 mL/min. The injector temperature was 220 °C. The temperature of the column was kept for 3 min at 60 °C, then increased at a rate of 7 °C/min up to 200 °C and then held at this temperature for 20 min. The detector temperature was set to 250 °C and the split flow was 10 mL/min. Peak identification was done using an external standard (FIM/FAME Supelco, Poznań, Poland). Fatty acid methyl esters were identified by comparing their retention times with authentic standards (Sigma Aldrich, Poznań, Poland) as well as the Punicic Acid Standard (Larodan Fine Chemicals AB, Malmö, Sweden).
2.2.4. Statistical Analysis
3. Results
3.1. Egg Whites Foam Characteristics
3.2. Properties and Quality of Sponge Cakes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Myers, M.; Ruxton, C.H.S. Eggs: Healthy or Risky? A Review of Evidence from High Quality Studies on Hen’s Eggs. Nutrients 2023, 15, 2657. [Google Scholar] [CrossRef]
- Kostogrys, R.B.; Filipiak-Florkiewicz, A.; Dereń, K.; Drahun, A.; Czyżyńska-Cichoń, I.; Cieślik, E.; Szymczyk, B.; Franczyk-Żarów, M. Effect of Dietary Pomegranate Seed Oil on Laying Hen Performance and Physicochemical Properties of Eggs. Food Chem. 2017, 221, 1096–1103. [Google Scholar] [CrossRef]
- Puglisi, M.J.; Fernandez, M.L. The Health Benefits of Egg Protein. Nutrients 2022, 14, 2904. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, X.; Tang, Q.; Ma, M.; Jin, Y.; Sheng, L. Functional Properties and Extraction Techniques of Chicken Egg White Proteins. Foods 2022, 11, 2434. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Song, W.-L. Egg Yolk, Source of Bad Cholesterol and Good Lipids? Am. J. Clin. Nutr. 2019, 110, 548–549. [Google Scholar] [CrossRef]
- Xiao, N.; Zhao, Y.; Yao, Y.; Wu, N.; Xu, M.; Du, H.; Tu, Y. Biological Activities of Egg Yolk Lipids: A Review. J. Agric. Food Chem. 2020, 68, 1948–1957. [Google Scholar] [CrossRef]
- Gautron, J.; Dombre, C.; Nau, F.; Feidt, C.; Guillier, L. Review: Production Factors Affecting the Quality of Chicken Table Eggs and Egg Products in Europe. Animal 2022, 16, 100425. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, X.; Ding, L.; Liu, C.; Ai, M.; Jin, Y.; Isobe, K.; Handa, A.; Cai, Z. Enhancement of Emulsification Properties by Modulation of Egg White Protein Fibril Structure with Different Heating Times. Food Hydrocoll. 2023, 135, 108203. [Google Scholar] [CrossRef]
- Razi, S.M.; Fahim, H.; Amirabadi, S.; Rashidinejad, A. An Overview of the Functional Properties of Egg White Proteins and Their Application in the Food Industry. Food Hydrocoll. 2023, 135, 108183. [Google Scholar] [CrossRef]
- Li, J.; Zhai, J.; Gu, L.; Su, Y.; Gong, L.; Yang, Y.; Chang, C. Hen Egg Yolk in Food Industry—A Review of Emerging Functional Modifications and Applications. Trends Food Sci. Technol. 2021, 115, 12–21. [Google Scholar] [CrossRef]
- Xue, H.; Han, T.; Xu, M.; Yao, Y.; Wu, N.; Chen, S.; Zhang, G.; Wang, W.; Zhao, Y.; Tu, Y. Processing Technology, Principle, and Nutritional Characteristics of Preserved Eggs: A Review. Trends Food Sci. Technol. 2022, 128, 265–277. [Google Scholar] [CrossRef]
- Chege, C.G.K.; Wanyama, R.; Lundy, M.; Nguru, W.; Jäger, M. Does Retail Food Diversity in Urban Food Environments Influence Consumer Diets? Sustainability 2021, 13, 7666. [Google Scholar] [CrossRef]
- Kartikasari, L.R.; Geier, M.S.; Hughes, R.J.; Bastian, S.E.P.; Gibson, R.A. Omega-3 Fatty Acid Levels and Sensory Quality of Eggs Following Consumption of Alpha-Linolenic Acid Enriched Diets. Food Res. 2021, 5, 57–64. [Google Scholar] [CrossRef]
- Mulatsih, S.; Soesanto, I.R.H.; Retnani, Y.; Yani, A.; Mutia, R.; Tanti, A. Exploring the Potential of Omega-3 Enriched Egg Industry in Indonesia: Production, Consumer Demand, and Competitiveness. J. Ilmu Produksi Dan Teknol. Has. Peternak. 2024, 12, 75–81. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, J.; Li, F.; Zheng, J.; Xu, G. Effect of Oils in Feed on the Production Performance and Egg Quality of Laying Hens. Animals 2021, 11, 3482. [Google Scholar] [CrossRef] [PubMed]
- Filipiak-Florkiewicz, A.; Dymińska-Czyż, M.; Szymczyk, B.; Franczyk-Żarów, M.; Kostogrys, R.; Florkiewicz, A.; Lukasiewicz, M. Design of Physicochemical Properties of Eggs as a Result of Modification of the Fat Fraction of Laying Feed. Molecules 2024, 29, 1242. [Google Scholar] [CrossRef]
- Batkowska, J.; Drabik, K.; Brodacki, A.; Czech, A.; Adamczuk, A. Fatty Acids Profile, Cholesterol Level and Quality of Table Eggs from Hens Fed with the Addition of Linseed and Soybean Oil. Food Chem. 2021, 334, 127612. [Google Scholar] [CrossRef]
- Perić, J.; Drinić, M. Enriching Table Eggs with Omega-3 Fatty Acids by Using Ground Flaxseed or a Combination of Flax Cake and Flaxseed Oil in the Diet of Laying Hens. Vet. Arh. 2021, 91, 399–409. [Google Scholar] [CrossRef]
- Kralik, G.; Kralik, Z.; Grčević, M.; Galović, O.; Hanžek, D.; Biazik, E. Fatty Acid Profile of Eggs Produced by Laying Hens Fed Diets Containing Different Shares of Fish Oil. Poult. Sci. 2021, 100, 101379. [Google Scholar] [CrossRef]
- Fraeye, I.; Bruneel, C.; Lemahieu, C.; Buyse, J.; Muylaert, K.; Foubert, I. Dietary Enrichment of Eggs with Omega-3 Fatty Acids: A Review. Food Res. Int. 2012, 48, 961–969. [Google Scholar] [CrossRef]
- Pappas, A.C.; Charisi, A.; Chatziantoniou, C.-M.; Giamouri, E.; Mitsiopoulou, C.; Moschopoulos, V.; Christodoulou, C.; Papadomichelakis, G.; Kotsampasi, B.; Mitsopoulos, I.K.; et al. Effects of Dietary Pomegranate Seed Oil Addition to Diets for Laying Hens on Fatty Acid Profile of Eggs. Anim. Feed Sci. Technol. 2023, 300, 115643. [Google Scholar] [CrossRef]
- Franczyk-Zarów, M.; Kostogrys, R.B.; Szymczyk, B.; Jawień, J.; Gajda, M.; Cichocki, T.; Wojnar, L.; Chlopicki, S.; Pisulewski, P.M. Functional Effects of Eggs, Naturally Enriched with Conjugated Linoleic Acid, on the Blood Lipid Profile, Development of Atherosclerosis and Composition of Atherosclerotic Plaque in Apolipoprotein E and Low-Density Lipoprotein Receptor Double-Knockout Mice (apoE/LDLR−/−). Br. J. Nutr. 2008, 99, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Dunn-Horrocks, S.; Pichardo-Fuchs, M.; Lee, J.; Ruiz-Feria, C.; Creger, C.; Hyatt, D.; Stringfellow, K.; Sanchez, M.; Farnell, M. Effect of Omega-3 Enriched Layer Rations on Egg Quality. Int. J. Poult. Sci. 2011, 10, 8–11. [Google Scholar] [CrossRef][Green Version]
- Ding, L.; Xia, M.; Zeng, Q.; Zhao, Q.; Cai, Z.; Zhu, Z. Foaming Properties and Aggregation Mechanism of Egg White Protein with Different Physical Treatments. LWT 2022, 153, 112505. [Google Scholar] [CrossRef]
- Zin, M. Food and Nutrition Assessment; Wydawnictwo Uniwersytetu Rzeszowskiego: Rzeszów, Poland, 2009; ISBN 978-83-7338-490-3. (In Polish) [Google Scholar]
- Vigneau, E.; Loisel, C.; Devaux, M.F.; Cantoni, P. Number of Particles for the Determination of Size Distribution from Microscopic Images. Powder Technol. 2000, 107, 243–250. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Labbafi, M.; Thakur, R.K.; Vial, C.; Djelveh, G. Development of an On-Line Optical Method for Assessment of the Bubble Size and Morphology in Aerated Food Products. Food Chem. 2007, 102, 454–465. [Google Scholar] [CrossRef]
- Bernsen, J. Dynamic Thresholding of Grey-Level Images. ICPR`86: Proceedings of International Conference on Pattern Recognition, Paris, France, 27–31 October 1986; pp. 1251–1255. [Google Scholar]
- Sezgin, M.; Sankur, B. Survey over Image Thresholding Techniques and Quantitative Performance Evaluation. J. Electron. Imaging 2004, 13, 146–168. [Google Scholar] [CrossRef]
- Junker, B. Measurement of Bubble and Pellet Size Distributions: Past and Current Image Analysis Technology. Bioprocess Biosyst. Eng. 2006, 29, 185–206. [Google Scholar] [CrossRef]
- Gupta, M.; Bawa, A.S.; Semwal, A.D. Effect of Barley Flour Incorporation on the Instrumental Texture of Sponge Cake. Int. J. Food Prop. 2009, 12, 243–251. [Google Scholar] [CrossRef]
- Khan, J.; Khurshid, S.; Sarwar, A.; Aziz, T.; Naveed, M.; Ali, U.; Makhdoom, S.I.; Nadeem, A.A.; Khan, A.A.; Sameeh, M.Y.; et al. Enhancing Bread Quality and Shelf Life via Glucose Oxidase Immobilized on Zinc Oxide Nanoparticles—A Sustainable Approach towards Food Safety. Sustainability 2022, 14, 14255. [Google Scholar] [CrossRef]
- Różyło, R.; Laskowski, J. Predicting bread quality (bread loaf volume and crumb texture). Pol. J. Food Nutr. Sci. 2011, 61, 61–67. [Google Scholar] [CrossRef]
- Oxford University Press. Official Methods of Analysis of AOAC International; Oxford University Press: Oxford, UK, 2023; ISBN 978-0-19-761014-5. [Google Scholar]
- Domagała, J.; Sady, M.; Grega, T.; Pustkowiak, H.; Florkiewicz, A. The Influence of Cheese Type and Fat Extraction Method on the Content of Conjugated Linoleic Acid. J. Food Compos. Anal. 2010, 23, 238–243. [Google Scholar] [CrossRef]
- ISO 16634-1:2008; Foodstuffs—Determination of Total Nitrogen by Combustion According to the Dumas Principle and Calculation of the Total Protein Content—Part 1: Oilseeds and Animal Feed. Polish Standarisation Comitee PN-EN: Warsaw, Poland, 2008.
- ISO 2171:2010; Cereal Grains, Legume Seeds and Their Products—Determination of Ash Content by Combustion Method. Polish Standarisation Comitee PN-EN: Warsaw, Poland, 2010.
- DeMan, J.M. Determination of the Fatty Acid Composition of Milk Fat by Dual Column Temperature Programmed Gas-Liquid Chromatography. J. Dairy Sci. 1964, 47, 546–547. [Google Scholar] [CrossRef]
- Böhm, W.; Hornik, K. A Kolmogorov-Smirnov Test for r Samples. Fundam. Inform. 2012, 117, 103–125. [Google Scholar] [CrossRef]
- Ahmadinia, F.; Mohtarami, F.; Esmaiili, M.; Pirsa, S. Investigation of Physicochemical and Sensory Characteristics of Low Calorie Sponge Cake Made from Flaxseed Mucilage and Flaxseed Flour. Sci. Rep. 2023, 13, 20949. [Google Scholar] [CrossRef] [PubMed]
- Aghajanzadeh, S.; Sultana, A.; Mohammad Ziaiifar, A.; Khalloufi, S. Formation of Pores and Bubbles and Their Impacts on the Quality Attributes of Processed Foods: A Review. Food Res. Int. 2024, 188, 114494. [Google Scholar] [CrossRef]
- Diaz, J.T.; Foegeding, E.A.; Stapleton, L.; Kay, C.; Iorizzo, M.; Ferruzzi, M.G.; Lila, M.A. Foaming and Sensory Characteristics of Protein-Polyphenol Particles in a Food Matrix. Food Hydrocoll. 2022, 123, 107148. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.-M.; Sun, C.-F.; Lv, J.-H.; Yang, Y.-J. Comparative Study on Foaming Properties of Egg White with Yolk Fractions and Their Hydrolysates. Foods 2021, 10, 2238. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lim, J.; Lee, J.; Hwang, H.-S.; Lee, S. Utilization of Oleogels as a Replacement for Solid Fat in Aerated Baked Goods: Physicochemical, Rheological, and Tomographic Characterization. J. Food Sci. 2017, 82, 445–452. [Google Scholar] [CrossRef]
- Sarantidi, E.; Ainatzoglou, A.; Papadimitriou, C.; Stamoula, E.; Maghiorou, K.; Miflidi, A.; Trichopoulou, A.; Mountzouris, K.C.; Anagnostopoulos, A.K. Egg White and Yolk Protein Atlas: New Protein Insights of a Global Landmark Food. Foods 2023, 12, 3470. [Google Scholar] [CrossRef] [PubMed]
- Pycarelle, S.C.; Bosmans, G.M.; Pareyt, B.; Brijs, K.; Delcour, J.A. The Role of Intact and Disintegrated Egg Yolk Low-Density Lipoproteins during Sponge Cake Making and Their Impact on Starch and Protein Mediated Structure Setting. Foods 2021, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Kamat, V.B.; Lawrence, G.A.; Hart, C.J.; Yoell, R. Contribution of Egg Yolk Lipoproteins to Cake Structure. J. Sci. Food Agric. 1973, 24, 77–88. [Google Scholar] [CrossRef]
- Malvano, F.; Laudisio, M.; Albanese, D.; d’Amore, M.; Marra, F. Olive Oil-Based Oleogel as Fat Replacer in a Sponge Cake: A Comparative Study and Optimization. Foods 2022, 11, 2643. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Eshak, N.S.; Mohamed, H.I.; Bendary, E.S.A.; Danial, A.W. Physical Characteristics, Mineral Content, and Antioxidant and Antibacterial Activities of Punica Granatum or Citrus Sinensis Peel Extracts and Their Applications to Improve Cake Quality. Plants 2022, 11, 1740. [Google Scholar] [CrossRef] [PubMed]
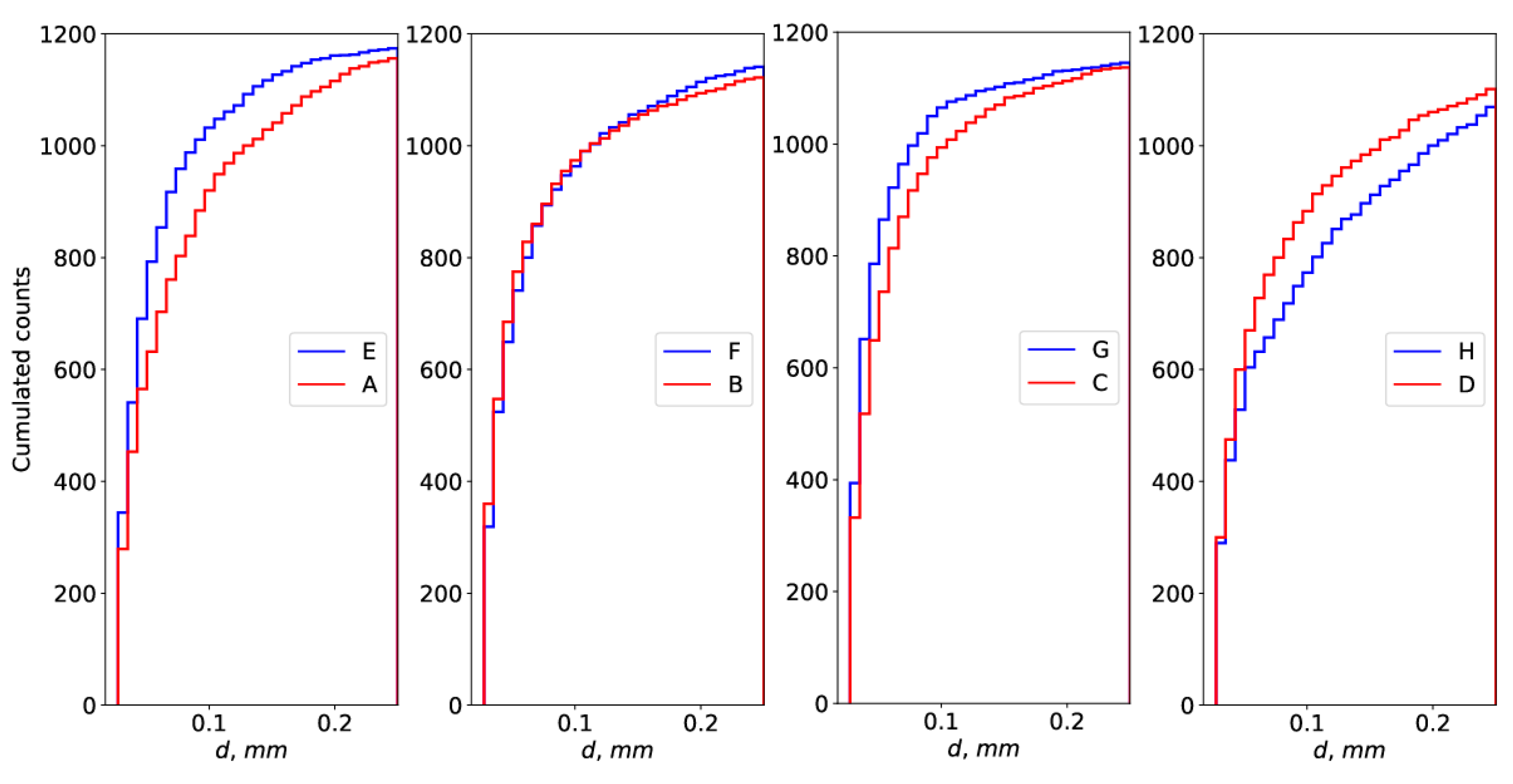
| Parameter | Experiment 1 | Experiment 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| Foaming Capacity [%] | 500.09 a ±10.04 | 553.85 a ±38.28 | 609.89 a ±13.51 | 540.07 a ±56.50 | 612.64 A ±38.31 | 590.02 A ±72.01 | 816.20 B ±91.15 | 1005.76 C ±11.83 |
| Stability of foams [%] | 94.41 a ±0.82 | 95.66 ab ±0.47 | 95.48 ab ±0.02 | 96.86 b ±0.27 | 95.35 A ±0.39 | 96.03 AB ±0.75 | 97.70 C ±0.05 | 97.00 B ±0.14 |
| Foam index [%] | 4.72 a ±0.04 | 5.29 a ±0.39 | 5.80 a ±0.98 | 5.23 a ±0.56 | 5.84 A ±0.38 | 5.66 A ±0.73 | 7.97 B ±0.88 | 9.75 C ±0.12 |
| Percentage of gas in the foam [%] | 83.33 a ±0.00 | 83.97 a ±0.90 | 85.00 a ±2.35 | 84.31 a ±1.38 | 85.49 AB ±0.59 | 84.52 A ±1.68 | 88.19 BC ±0.98 | 90.00 C ±0.00 |
| Foam density [dm−3] | 163.37 a ±1.77 | 155.66 a ±13.22 | 145.14 a ±21.34 | 148.76 a ±19.88 | 141.75 B ±9.04 | 148.46 B ±10.65 | 111.37 A ±7.57 | 90.18 A ±0.16 |
| Parameter | Experiment 1 | Experiment 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| de [mm] | 0.082 | 0.083 | 0.079 | 0.098 | 0.065 | 0.078 | 0.070 | 0.072 |
| d32 [mm] | 0.187 | 0.187 | 0.187 | 0.188 | 0.187 | 0.187 | 0.187 | 0.186 |
| PDI | 0.44 | 0.45 | 0.42 | 0.52 | 0.35 | 0.42 | 0.37 | 0.39 |
| Parameter | Experiment 1 | Experiment 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| Cake weight [g] | 338.00 a ±4.15 | 334.92 a ±4.72 | 337.69 a ±5.52 | 326.10 a ±7.07 | 329.12 A ±10.28 | 324.36 A ±11.59 | 332.04 A ±1.63 | 326.38 A ±2.87 |
| Cake volume [cm3] | 1075.00 b ±106.06 | 835.00 a ±35.35 | 815.00 a ±49.49 | 1091.50 b ±4.94 | 1000.00 A ±155.56 | 1075.00 A ±7.07 | 1015.00 A ±21.21 | 1055.00 A ±21.21 |
| Hardness [N] | 387.85 a ±76.618 | 406.07 a ±123.21 | 522.36 a ±117.19 | 1032.08 b ±189.19 | 392.52 A ±57.59 | 295.29 A ±136.30 | 478.17 A ±51.40 | 679.06 A ±171.84 |
| Parameter | Sponge Cakes | |||
|---|---|---|---|---|
| A vs. E | B vs. F | C vs. G | D vs. H | |
| Cake weight [g] | 0.341 | 0.145 | 0.061 | 0.962 |
| Volume of the cake [cm3] | 0.629 | 0.011 | 0.034 | 0.141 |
| Hardness [N] | 0.557 | 0.850 | 0.137 | 0.202 |
| Parameter | Experiment 1 | Experiment 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| Dry matter | 68.51 a ±0.65 | 64.60 a ±1.14 | 67.21 a ±2.52 | 66.33 a ±0.75 | 69.20 C ±1.22 | 66.27 B ±1.21 | 65.43 A ±0.32 | 67.69 C ±0.28 |
| Protein | 13.49 c ±0.10 | 13.34 b ±0.03 | 13.39 bc ±0.02 | 13.12 a ±0.01 | 13.63 B ±0.10 | 13.11 A ±0.11 | 13.31 A ±0.09 | 13.75 B ±0.11 |
| Fat | 12.05 c ±0.07 | 11.25 b ±0.07 | 10.85 b ±0.35 | 10.30 a ±0.00 | 9.97 A ±0.04 | 11.30 B ±0.14 | 11.10 B ±0.14 | 12.25 C ±0.21 |
| Ash | 1.33 a ±0.00 | 1.27 a ±0.00 | 1.25 a ±0.03 | 1.37 a ±0.00 | 1.35 A ±0.03 | 1.33 A ±0.01 | 1.23 A ±0.01 | 1.20 A ±0.01 |
| Acid | Experiment 1 | Experiment 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| Saturated Fatty Acids | ||||||||
| C14:0 tetradecanoic (myristic acid) | 0.46 b ±0.03 | 0.39 a ±0.06 | 0.45 b ±0.02 | 0.42 ab ±0.00 | 0.52 A ±0.02 | 0.48 A ±0.02 | 0.47 A ±0.01 | 0.49 A ±0.01 |
| C15:0 pentadecanoic (pentadecylic acid) | 0.09 a ±0.00 | 0.07 a ±0.00 | 0.09 a ±0.00 | 0.09 a ±0.00 | 0.11 B ±0.01 | 0.08 A ±0.00 | 0.09 A ±0.01 | 0.09 A ±0.01 |
| C16:0 hexadecanoic (palmitic acid) | 16.85 a ±0.47 | 17.59 b ±0.04 | 17.17 b ±0.35 | 16.94 a ±0.02 | 16.65 A ±0.13 | 17.10 A ±0.33 | 16.74 A ±0.18 | 17.11 A ±0.00 |
| C17:0 heptadecanoic (margaric acid) | 0.21 a ±0.02 | 0.15 a ±0.00 | 0.19 a ±0.00 | 0.24 b ±0.01 | 0.23 A ±0.04 | 0.23 A ±0.05 | 0.24 A ±0.01 | 0.25 A ±0.00 |
| C18:0 octadecanoic (stearic acid) | 7.73 a ±0.61 | 7.90 a ±0.39 | 7.7 a ±0.67 | 7.95 a ±0.41 | 6.21 A ±0.22 | 8.07 B ±0.21 | 6.93 A ±0.74 | 7.59 B ±0.07 |
| C20:0 eicosanoic acid (arachidic acid) | 0.33 a ±0.07 | 0.3 a ±0.06 | 0.32 a ±0.03 | 0.39 a ±0.01 | 0.38 A ±0.07 | 0.36 A ±0.00 | 0.36 A ±0.00 | 0.38 A ±0.01 |
| C22:0 docosanoic (behenic acid) | 0.08 b ±0.01 | 0.00 a ±0.00 | 0.08 b ±0.00 | 0.12 b ±0.00 | 0.05 A ±0.07 | 0.11 B ±0.01 | 0.10 B ±0.00 | 0.13 B ±0.00 |
| Monounsaturated Fatty Acids | ||||||||
| C14:1 9-tetradecenoic (myristoleic acid) | 0.05 a ±0.00 | 0.04 a ±0.02 | 0.05 a ±0.01 | 0.05 a ±0.00 | 0.07 B ±0.00 | 0.06 A ±0.00 | 0.06 A ±0.00 | 0.06 A ±0.00 |
| C16:1 trans-3-hexadecenoic | 1.15 a ±0.07 | 1.10 a ±0.07 | 1.0 a ±0.05 | 0.9 a ±0.02 | 1.45 B ±0.05 | 1.16 B ±0.09 | 1.23 AB ±0.00 | 1.08 A ±0.00 |
| C16:1 9-cis-Hexadecenoic (palimitoleic acid) | 4.06 b ±0.51 | 2.86 a ±0.15 | 2.84 a ±0.34 | 3.05 a ±0.04 | 3.86 B ±0.05 | 3.32 A ±0.21 | 2.84 A ±0.34 | 2.97 A ±0.01 |
| C17:1 10-heptadecenoic acid | 0.22 a ±0.07 | 0.19 a ±0.02 | 0.17 a ±0.00 | 0.22 a ±0.04 | 0.25 A ±0.07 | 0.19 A ±0.02 | 0.21 A ±0.03 | 0.21 A ±0.01 |
| C18:1 cis-9-Octadecenoic (oleic acid) | 42.36 a ±0.95 | 43.09 a ±0.16 | 41.76 a ±0.76 | 39.94 a ±0.40 | 40.18 A ±0.91 | 41.80 A ±0.18 | 42.72 A ±1.01 | 41.08 B ±0.26 |
| C22:1 (13Z)-docos-13-enoic (erucic acid) | 0.12 b ±0.04 | 0.00 a ±0.00 | 0.13 b ±0.01 | 0.14 b ±0.01 | 0.12 A ±0.04 | 0.13 A ±0.00 | 0.13 A ±0.00 | 0.14 A ±0.02 |
| Polyunsaturated Fatty Acids | ||||||||
| C16:2 Hexadecadienoic acid | 0.08 a ±0.00 | 0.08 a ±0.00 | 0.08 a ±0.00 | 0.09 b ±0.01 | 0.09 B ±0.00 | 0.09 B ±0.01 | 0.09 B ±0.00 | 0.08 A ±0.01 |
| C18:2 n−6 cis,cis-9,12-octadecadienoic (linoleic acid) | 16.75 a ±0.00 | 16.63 a ±0.01 | 16.92 a ±0.07 | 16.81 a ±0.12 | 17.36 B ±0.22 | 16.55 A ±0.12 | 16.8 A ±0.19 | 16.67 A ±0.06 |
| C18:2 Conjugated linoleic acids—CLA | 0.12 a ±0.00 | 0.74 b ±0.01 | 1.49 c ±0.04 | 2.23 b ±0.01 | 0.10 A ±0.02 | 1.21 B ±0.02 | 1.66 C ±0.09 | 2.21 D ±0.02 |
| C18:3 n-3 cis,cis,cis-9,12,15-octadecatrienoic (α-linolenic acid) | 8.08 b ±0.41 | 7.31 a ±0.19 | 7.75 b ±0.02 | 7.86 ab ±0.17 | 7.44 B ±0.55 | 7.17 A ±0.35 | 7.37 A ±0.21 | 6.84 A ±0.07 |
| C18:3 Conjugated linolenic acid - CLnA | 0.00 a ±0.00 | 0.23 b ±0.08 | 0.46 c ±0.35 | 0.84 d ±0.04 | 0.00 A ±0.00 | 0.32 B ±0.06 | 0.62 C ±0.01 | 0.97 D ±0.02 |
| C20:2 eicosadienoic | 0.07 a ±0.02 | 0.06 a ±0.02 | 0.07 a ±0.01 | 0.08 a ±0.01 | 0.06 A ±0.02 | 0.08 A ±0.01 | 0.08 A ±0.00 | 0.09 A ±0.01 |
| C20:3 n-6 cis,cis,cis-8,11,14-eicosatrienoic dihomo-γ-linolenic acid | 0.05 a ±0.00 | 0.10 b ±0.02 | 0.02 a ±0.02 | 0.05 a ±0.01 | 0.06 A ±0.02 | 0.05 A ±0.01 | 0.04 A ±0.01 | 0.06 A ±0.00 |
| C20:4 n-6 5,8,11,14-all-cis-eicosatetraenoic (arachidonic acid) | 0.43 ab ±0.15 | 0.39 b ±0.01 | 0.33 a ±0.19 | 0.42 ab ±0.06 | 0.17 A ±0.03 | 0.57 B ±0.12 | 0.34 B ±0.24 | 0.52 B ±0.03 |
| C22:6 n-3 docosahexaenoic —DHA (cervonic acid) | 0.21 b ±0.06 | 0.20 b ±0.02 | 0.18 b ±0.11 | 0.21 b ±0.05 | 0.00 A ±0.00 | 0.17 B ±0.03 | 0.08 A ±0.07 | 0.14 B ±0.02 |
| Other C18:2, C18:3, CLA | 0.45 a ±0.07 | 0.53 a ±0.05 | 0.64 b ±0.00 | 0.85 b ±0.07 | 0.59 B ±0.06 | 0.69 A ±0.03 | 0.77 B ±0.04 | 0.82 B ±0.00 |
| Saturated fatty acids—SFA (%) | 25.77 a ±0.92 | 26.42 a ±0.31 | 26.03 a ±0.96 | 26.17 a ±0.41 | 24.16 A ±0.34 | 26.41 A ±0.60 | 24.9 A ±0.92 | 26.03 A ±0.09 |
| Monounsaturated fatty acids— MUFA (%) | 47.98 b ±0.63 | 47.29 b ±0.11 | 46.01 a ±1.19 | 44.38 a ±0.26 | 49.94 B ±0.67 | 46.66 B ±0.53 | 47.20 B ±0.71 | 45.54 A ±0.28 |
| Polyunsaturated fatty acids— PUFA (%) | 26.24 a ±0.28 | 26.28 a ±0.21 | 27.95 b ±0.23 | 29.45 b ±0.14 | 25.89 A ±0.32 | 26.92 A ±0.07 | 27.86 B ±0.21 | 28.42 C ±0.19 |
| Parameter | Sponge Cake | |||
|---|---|---|---|---|
| A vs. E | B vs. F | C vs. G | D vs. H | |
| PUFA | ||||
| C16:2 | 0.422 | 0.422 | 0.000 | 0.422 |
| C18:2 n-6 | 0.095 | 0.148 | 0.360 | 0.067 |
| C18:2 | 0.000 | 0.000 | 0.006 | 0.003 |
| C18:3 n-3 | 0.417 | 0.141 | 0.785 | 0.003 |
| C18:3 | 0.001 | 0.018 | 0.023 | 0.003 |
| C20:2 n-9 | 0.542 | 0.591 | 0.422 | 0.051 |
| C20:3 n-6 | 0.830 | 0.422 | 0.062 | 0.183 |
| C20:4 n-6 | 0.039 | 0.406 | 0.821 | 0.300 |
| C22:6 n-3 | 0.034 | 0.518 | 0.138 | 0.709 |
| Other C18:2, C18:3, CLA | 0.063 | 0.048 | 0.040 | 0.000 |
| MUFA | ||||
| C14:1 | 0.037 | 0.422 | 0.422 | 0.422 |
| C16:1 | 0.007 | 0.918 | 0.121 | 0.666 |
| C16:1 | 0.004 | 0.203 | 0.960 | 0.660 |
| C17:1 | 0.690 | 0.633 | 0.516 | 0.057 |
| C18:1 | 0.026 | 0.497 | 0.658 | 0.356 |
| C22:1 | 0.591 | 0.899 | 0.000 | 0.492 |
| SFA | ||||
| C14:0 | 0.019 | 0.685 | 0.245 | 0.191 |
| C15:0 | 0.311 | 0.292 | 0.311 | 0.698 |
| C16:0 | 0.094 | 0.586 | 0.023 | 0.832 |
| C17:0 | 0.903 | 0.759 | 0.016 | 0.008 |
| C18:0 | 0.035 | 0.529 | 0.246 | 0.818 |
| C20:0 | 0.840 | 0.751 | 0.384 | 0.142 |
| C22:0 | 0.332 | 0.167 | 0.002 | 0.012 |
| Share of PUFA | 0.004 | 0.085 | 0.017 | 0.164 |
| Share of MUFA | 0.008 | 0.153 | 0.875 | 0.642 |
| Share of SFA | 0.034 | 0.498 | 0.164 | 0.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukasiewicz, M.; Dymińska-Czyż, M.; Szymczyk, B.; Franczyk-Żarów, M.; Kostogrys, R.; Florkiewicz, A.; Ptaszek, P.; Zięć, G.; Filipiak-Florkiewicz, A. From Hen Nutrition to Baking: Effects of Pomegranate Seed and Linseed Oils on Egg White Foam Stability and Sponge Cake Quality. Foods 2025, 14, 1417. https://doi.org/10.3390/foods14081417
Lukasiewicz M, Dymińska-Czyż M, Szymczyk B, Franczyk-Żarów M, Kostogrys R, Florkiewicz A, Ptaszek P, Zięć G, Filipiak-Florkiewicz A. From Hen Nutrition to Baking: Effects of Pomegranate Seed and Linseed Oils on Egg White Foam Stability and Sponge Cake Quality. Foods. 2025; 14(8):1417. https://doi.org/10.3390/foods14081417
Chicago/Turabian StyleLukasiewicz, Marcin, Maja Dymińska-Czyż, Beata Szymczyk, Magdalena Franczyk-Żarów, Renata Kostogrys, Adam Florkiewicz, Paweł Ptaszek, Gabriela Zięć, and Agnieszka Filipiak-Florkiewicz. 2025. "From Hen Nutrition to Baking: Effects of Pomegranate Seed and Linseed Oils on Egg White Foam Stability and Sponge Cake Quality" Foods 14, no. 8: 1417. https://doi.org/10.3390/foods14081417
APA StyleLukasiewicz, M., Dymińska-Czyż, M., Szymczyk, B., Franczyk-Żarów, M., Kostogrys, R., Florkiewicz, A., Ptaszek, P., Zięć, G., & Filipiak-Florkiewicz, A. (2025). From Hen Nutrition to Baking: Effects of Pomegranate Seed and Linseed Oils on Egg White Foam Stability and Sponge Cake Quality. Foods, 14(8), 1417. https://doi.org/10.3390/foods14081417







