Improving Sensory Differentiation: Refining the ‘Fruitiness’ Descriptor in Extra Virgin Olive Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Sample Preparation
2.3. Chemical Analysis
2.4. Sensory Analysis
2.5. Volatile Compound Analysis
2.6. Volatile Compound Identification
2.7. Volatile Compound Relative Quantification
2.8. Statistical Analysis
3. Results and Discussion
3.1. Exploratory Analysis
3.2. Classification Analysis
3.3. Volatile Compounds
3.4. Sensory Analysis Results
4. Conclusions
- Green fruity EVOOs: Characterized by high aromatic intensity with predominant green sensory notes and elevated concentrations of related volatile compounds.
- Fruity EVOOs: Exhibit considerable aromatic intensity with a balance of green and fruity notes, indicating optimal maturity.
- Ripe fruity EVOOs: Show a significant decrease in aromatic intensity, with a limited range of aromatic notes and reduced concentrations of positive aromas.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rébufa, C.; Pinatel, C.; Artaud, J.; Girard, F. A Comparative Study of the Main International Extra Virgin Olive Oil Competitions: Their Impact on Producers and Consumers. Trends Food Sci. Technol. 2021, 107, 445–454. [Google Scholar] [CrossRef]
- Rodríguez-Cohard, J.C.; Sánchez-Martínez, J.D.; Gallego-Simón, V.J. Olive Crops and Rural Development: Capital, Knowledge and Tradition. Reg. Sci. Policy Pract. 2019, 11, 935–949. [Google Scholar] [CrossRef]
- European Commission of Agriculture and Rural Development. Olive Oil. An Overview of the Production and Marketing of Olive Oil in the EU. Available online: https://agriculture.ec.europa.eu/farming/crop-productions-and-plant-based-products/olive-oil_en (accessed on 7 April 2025).
- Commision Delegated Regulation (EU) 2022/2104 of 29 July 2022 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Marketing Standards for Olive Oil, and Repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. Available online: https://eur-lex.europa.eu/eli/reg_del/2022/2104/oj/eng (accessed on 10 March 2025).
- International Olive Council (IOC). Sensory Analysis of Olive Oil. Method for the Organoleptic Assessment of Virgin Olive Oil. Madrid, 2018; Volume T.20, Doc. No 15, Rev. 10. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T20-Doc.-15-REV-10-2018-Eng.pdf (accessed on 10 March 2025).
- International Olive Council (IOC). Organoleptic assessment of extra virgin olive oil applying to use a Designation of Origin. Madrid 2005, 20, 22. [Google Scholar]
- Genovese, A.; Caporaso, N.; Sacchi, R. Flavor Chemistry of Virgin Olive Oil: An Overview. Appl. Sci. 2021, 11, 1639. [Google Scholar] [CrossRef]
- García-Pizarro, A.; Romero, A.; Martí, E.; Hermoso, J.F.; Ninot, A.; Aceña, L.; Mestres, M. Feasibility of Using Secondary Attributes in Sensory Analysis to Characterize Protected Designation of Origin of Olive Oil. Agronomy 2024, 14, 2218. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Mulinacci, N. Virgin Olive Oil Volatile Compounds: Composition, Sensory Characteristics, Analytical Approaches, Quality Control, and Authentication. J. Agric. Food Chem. 2021, 69, 2013–2040. [Google Scholar] [CrossRef]
- Chaji, S.; Bajoub, A.; Cravotto, C.; Voss, M.; Tabasso, S.; Hanine, H.; Cravotto, G. Metabolomics in Action: Towards Producing Authentic Virgin Olive Oil Rich in Bioactive Compounds and with Distinctive Organoleptic Features. LWT-Food Sci. Technol. 2024, 191, 115681. [Google Scholar] [CrossRef]
- Caporaso, N.; Genovese, A.; Pérez-Jiménez, M.A.; Olivero-David, R.; Sacchi, R. Impact of Olive Harvesting Date on Virgin Olive Oil Volatile Composition in four Spanish Varieties. Eur. J. Lipid Sci. Tech. 2021, 123, 2000350. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive Oil Volatile Compounds, Flavour Development and Quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative Study of Virgin Olive Oil Sensory Defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Neugebauer, A.; Granvogl, M.; Schieberle, P. Characterization of the Key Odorants in High-Quality Extra Virgin Olive Oils and Certified Off-Flavor Oils to Elucidate Aroma Compounds Causing a Rancid Off-Flavor. J. Agric. Food Chem. 2020, 68, 5927–5937. [Google Scholar] [CrossRef]
- Brkić Bubola, K.; Lukić, I.; Krapac, M.; Koprivnjak, O. Exploring the Connection between the Occurrence and Intensity of “Grubby” Defect and Volatile Composition of Olive Oil. Foods 2023, 12, 4473. [Google Scholar] [CrossRef]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Penco-Valenzuela, J.M.; Calderón-Santiago, M.; Priego-Capote, F. Metabolic Patterns in the Lipoxygenase Pathway Associated to Fruitiness Attributes of Extra Virgin Olive Oil. J. Food Compos. Anal. 2022, 109, 104478. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Aparicio-Ruiz, R.; Morales, M.T.; García-González, D.L. Contribution of Specific Volatile Markers to Green and Ripe Fruity Attributes in Extra Virgin Olive Oils Studied with Three Analytical Methods. Food Chem. 2022, 399, 133942. [Google Scholar] [CrossRef]
- International Olive Council. World Catalogue of Olive Varieties; International Olive Oil Council: Madrid, Spain, 2000. [Google Scholar]
- Uceda, M.; Frías, L. Harvest Dates. In Evolution of the Fruit Oil Content, Oil Composition and Oil Quality, Proceedings of II Seminario Oleícola International; International Olive Council: Córboba, Spain, 1975; pp. 25–46. [Google Scholar]
- Generalitat de Catalunya. Gencat Laboratori Agroalimentari. 2025. Available online: https://agricultura.gencat.cat/ca/ambits/alimentacio/laboratori-agroalimentari/index.html#googtrans(ca|en) (accessed on 8 April 2025).
- European Union. Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive Residue Oil and on the Relevant Methods of Analysis. Off. J. Eur. Communities 1991, 248, 1–83, and updates. Consolidated version 22/11/2022. Available online: http://data.europa.eu/eli/reg/1991/2568/2022-11-24 (accessed on 10 March 2025).
- Romero Aroca, A.J. Caracterización y diferenciación de los aceites vírgenes de oliva de la comarca del Priorat (Tarragona), dentro del mercado global de aceites de la variedad “Arbequina”. Ph.D. Thesis, University of Lleida, Lleida, Spain, 4 November 2011. [Google Scholar]
- Aparicio-Ruiz, R.; Ortiz Romero, C.; Casadei, E.; García-González, D.L.; Servili, M.; Selvaggini, R.; Lacoste, F.; Escobessa, J.; Vichi, S.; Quintanilla-Casas, B.; et al. Collaborative Peer Validation of a Harmonized SPME-GC-MS Method for Analysis of Selected Volatile Compounds in Virgin Olive Oils. Food Control 2022, 135, 108756. [Google Scholar] [CrossRef]
- Acree, T.; Arn, H. Flavornet and Human Odor Space. Available online: http://www.flavornet.org (accessed on 10 March 2025).
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/ (accessed on 10 March 2025).
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 10 March 2025).
- Merck. Available online: https://www.sigmaaldrich.com/ES/es?utm_source=google&utm_medium=cpc&utm_campaign=20856389750&utm_content=158103336913&gad_source=1&gclid=EAIaIQobChMIpMmsqLSUjAMV7y0GAB1WfyMZEAAYASAAEgIB1vD_BwE (accessed on 10 March 2025).
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Virgin Olive Oil Odour Notes: Their Relationships with Volatile Compounds from the Lipoxygenase Pathway and Secoiridoid Compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Aparicio, R.; Luna, G. Characterisation of Monovarietal Virgin Olive Oils. Eur. J. Lipid Sci. Technol. 2002, 104, 614–627. [Google Scholar] [CrossRef]
- Rekik, O.; Mansour, A.B.; Gomes Da Silva, M.D.R.; Bouaziz, M. Identification of Trace Volatile and Phenolic Compounds in Olive Oils with Trees Growing in Different Area Conditions: Using SPME/GC-MS. Food Anal. Methods 2021, 14, 2494–2510. [Google Scholar] [CrossRef]
- Benkhoud, H.; M’Rabet, Y.; Gara ali, M.; Mezni, M.; Hosni, K. Essential Oils as Flavoring and Preservative Agents: Impact on Volatile Profile, Sensory Attributes, and the Oxidative Stability of Flavored Extra Virgin Olive Oil. J. Food Process. Preserv. 2022, 46, 15379. [Google Scholar] [CrossRef]
- Casadei, E.; Valli, E.; Aparicio-Ruiz, R.; Ortiz-Romero, C.; García-González, D.L.; Vichi, S.; Quintanilla-Casas, B.; Tres, A.; Bendini, A.; Toschi, T.G. Peer Inter-Laboratory Validation Study of a Harmonized SPME-GC-FID Method for the Analysis of Selected Volatile Compounds in Virgin Olive Oils. Food Control 2021, 123, 107823. [Google Scholar] [CrossRef]
- Lobo-Prieto, A.; Tena, N.; Aparicio-Ruiz, R.; Morales, M.T.; García-González, D.L. Tracking Sensory Characteristics of Virgin Olive Oils During Storage: Interpretation of their Changes from a Multiparametric Perspective. Molecules 2020, 25, 1686. [Google Scholar] [CrossRef] [PubMed]
- Angerosa, F.; Basti, C.; Vito, R.; Lanza, B. Effect of Fruit Stone Removal on the Production of Virgin Olive Oil Volatile Compounds. Food Chem. 1999, 67, 295–299. [Google Scholar] [CrossRef]
- Inarejos-García, A.M.; Santacatterina, M.; Salvador, M.D.; Fregapane, G.; Gómez-Alonso, S. PDO Virgin Olive Oil Quality—Minor Components and Organoleptic Evaluation. Food Res. Int. 2010, 43, 2138–2146. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Aguilar, I.; Zamora, R. Phenolic Trapping of Lipid Oxidation Products 4-oxo-2-alkenals. Food Chem. 2018, 240, 822–830. [Google Scholar] [CrossRef]
- Beltrán, G.; Del Rio, C.; Sánchez, S.; Martínez, L. Influence of Harvest Date and Crop Yield on the Fatty Acid Composition of Virgin Olive Oils from cv. Picual. J. Agric. Food Chem. 2004, 52, 3434–3440. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, C.H.; Ulrich, D.; Lestari, R.; Schippel, K.; Ebert, G. Identification of Potent Odorants in Different Cultivars of Snake Fruit [Salacca zalacca (Gaert.) Voss] Using Gas Chromatography-Olfactometry. J. Agric. Food Chem. 2005, 53, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Reiners, J.; Grosch, W. Odorants of Virgin Olive Oils with Different Flavor Profiles. J. Agric. Food Chem. 1998, 46, 2754–2768. [Google Scholar] [CrossRef]
- Boudebouz, A.; Romero, A.; Hermoso, J.F.; Boqué, R.; Mestres, M. Processing Factors that Affect the Balance of Alcohols and Alkyl Esters during ‘Arbequina’ Olive Oil Production: Separation and Clarification Steps. LWT-Food Sci. Technol. 2021, 149, 111842. [Google Scholar] [CrossRef]
- Di Lecce, G.; Piochi, M.; Pacetti, D.; Frega, N.G.; Bartolucci, E.; Scortichini, S.; Fiorini, D. Eleven Monovarietal Extra Virgin Olive Oils from Olives Grown and Processed under the Same Conditions: Effect of the Cultivar on the Chemical Composition and Sensory Traits. Foods 2020, 9, 904. [Google Scholar] [CrossRef]
- Franco, M.N.; Sánchez, J.; De Miguel, C.; Martínez, M.; Martín-Vertedor, D. Influence of the Fruit’s Ripeness on Virgin Olive Oil Quality. J. Oleo Sci. 2015, 64, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.T.; Aparicio, R.; Calvente, J.J. Influence of Olive Ripeness on the Concentration of Green Aroma Compounds in Virgin Olive Oil. Flavour Fragr. J. 1996, 11, 171–178. [Google Scholar] [CrossRef]
- Rodrigues, N.; Peres, A.M.; Baptista, P.; Pereira, J.A. Olive Oil Sensory Analysis as a Tool to Preserve and Valorize the Heritage of Centenarian Olive Trees. Plants 2022, 11, 257. [Google Scholar] [CrossRef] [PubMed]
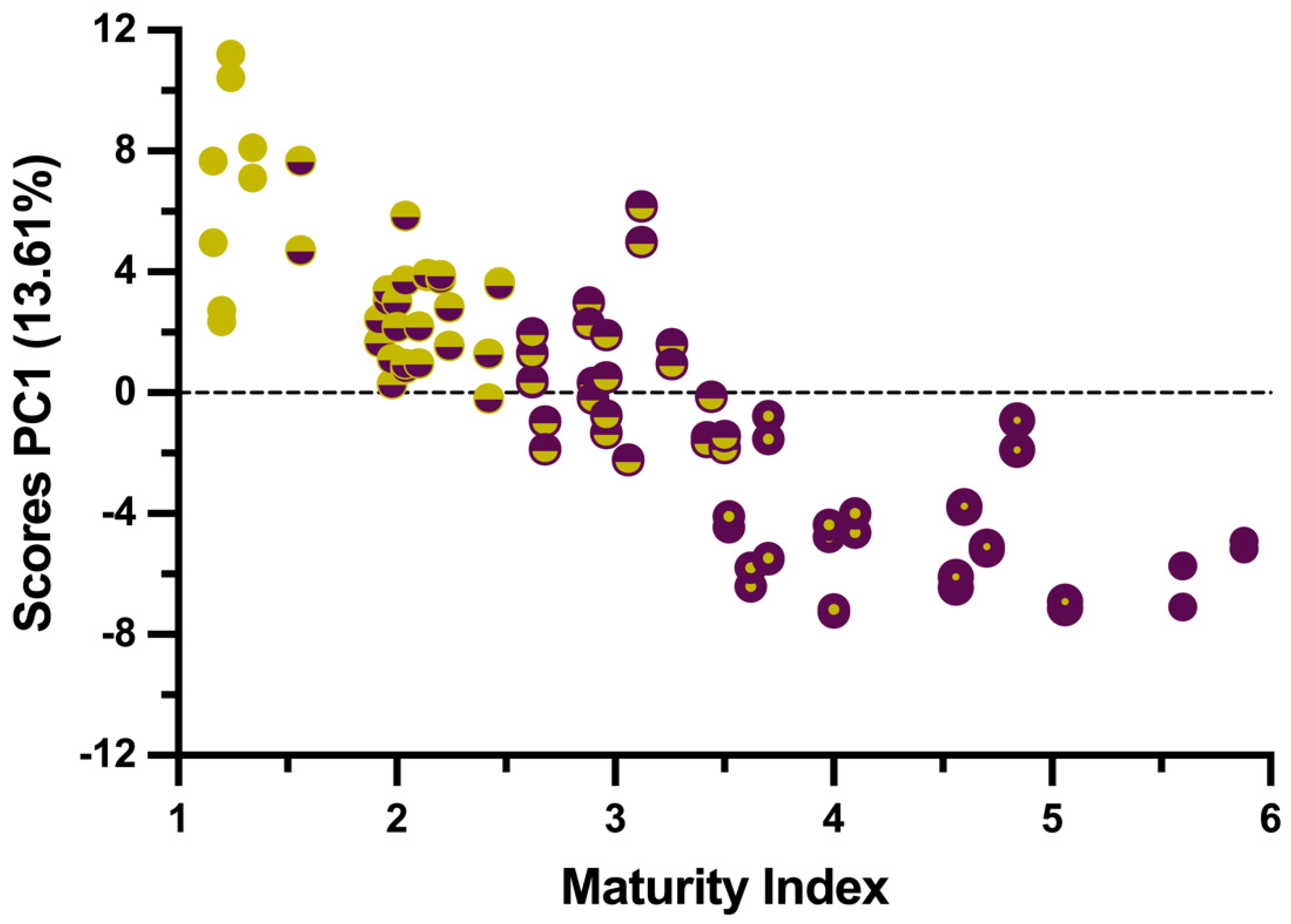
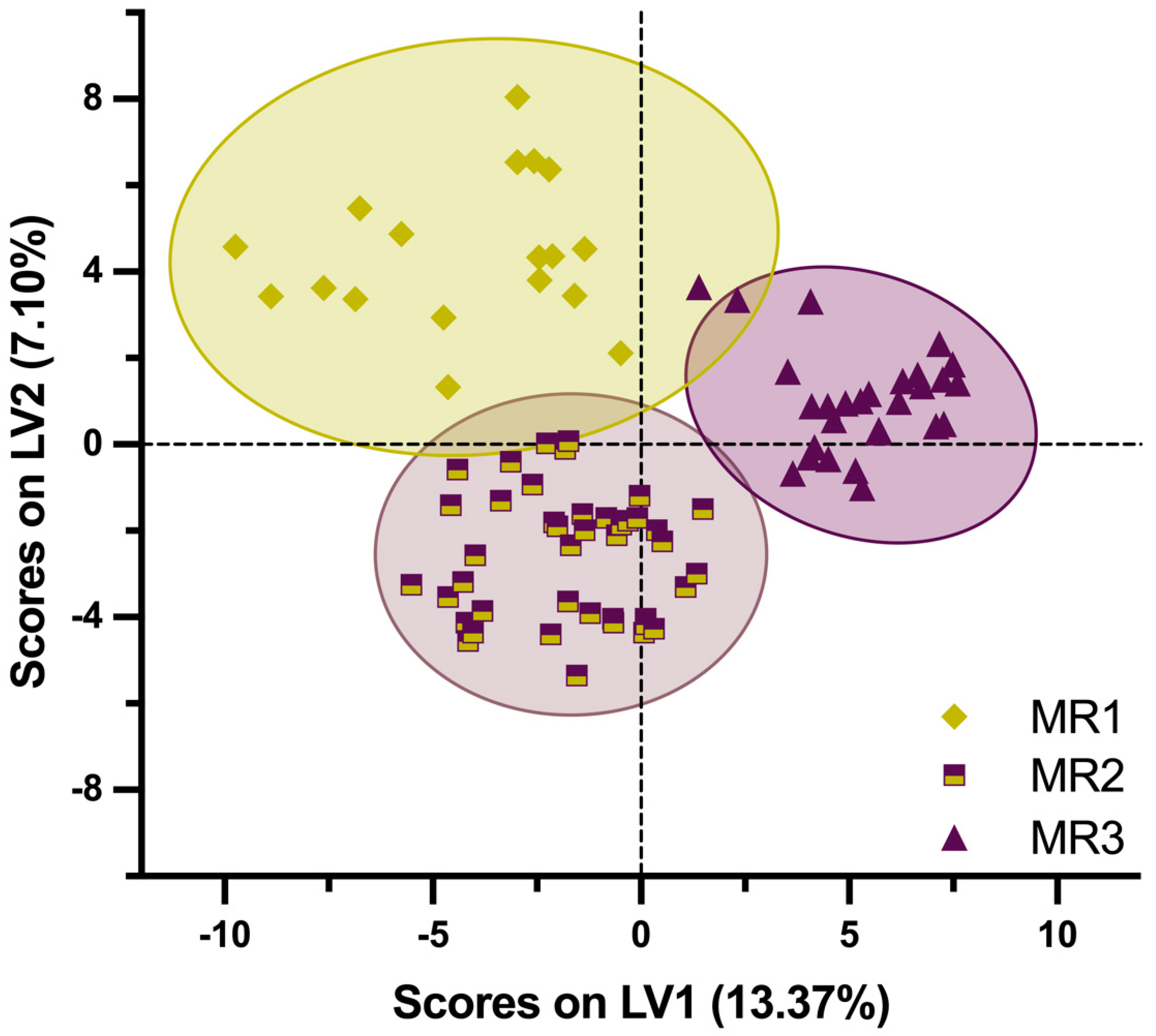
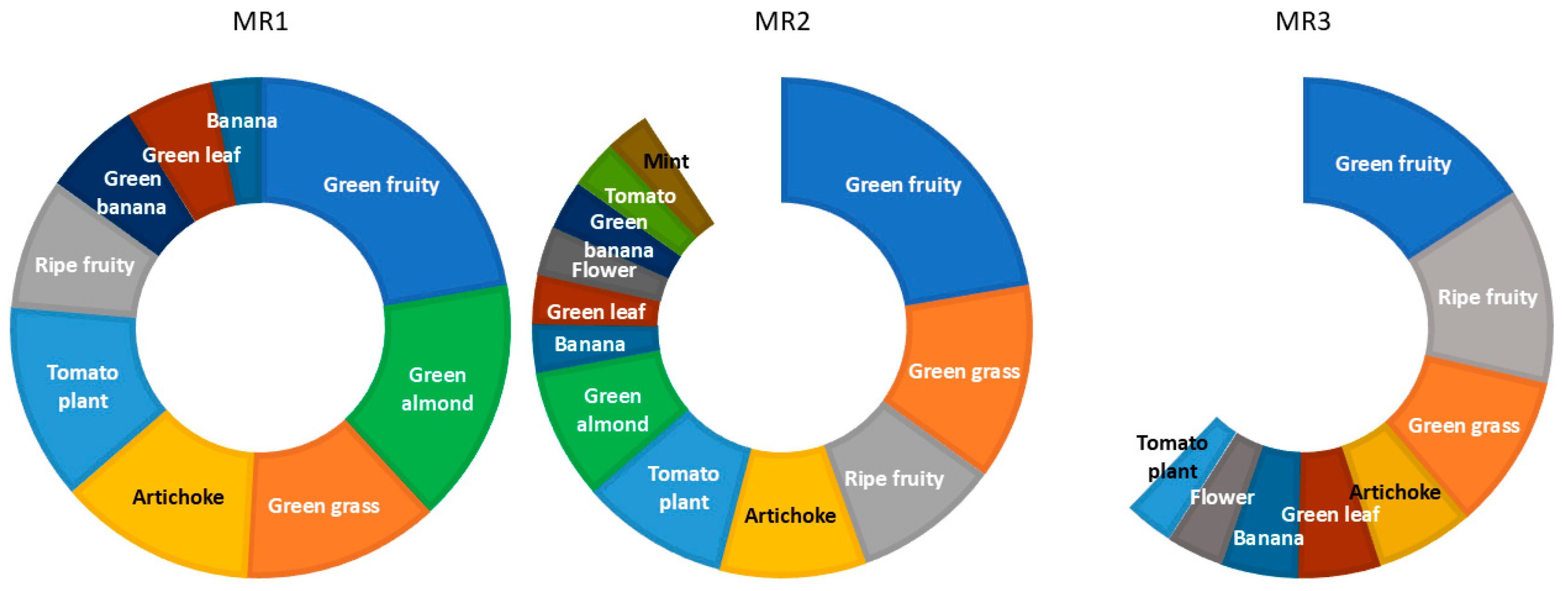
| Maturity Range | From MI | To MI | No. of Samples | Mean MI |
|---|---|---|---|---|
| MR1 | 0.00 | 2.00 | 9 | 1.60 |
| MR2 | 2.01 | 3.50 | 21 | 2.72 |
| MR3 | 3.51 | 7.00 | 15 | 4.55 |
| RI 1 | Volatile Compound | Odor Descriptor | A′ MR1 | A′ MR2 | A′ MR3 | VIP 2 for |
|---|---|---|---|---|---|---|
| <800 | Pentane | Odorless | 5.5 a | 3.2 a | 21.7 b | MR3 |
| <800 | 1,4-pentadiene | Mild hydrocarbon | 62.5 b | 59.0 b | 22.7 a | MR2 |
| <800 | Heptane | Odorless | 17.3 b | 4.4 a | 16.6 b | - |
| <800 | Dimethyl sulfide | Cabbage, sulfur | 1.0 a | 2.6 a | 6.9 b | MR3 |
| <800 | 2-methyl-heptane | Gasoline-like | 15.6 b | 7.0 ab | 1.2 a | - |
| 801 | Octane | Odorless | 14.6 ab | 14.5 a | 19.7 b | - |
| 816 | Acetone | Solvent-like, sweet | 15.2 a | 33.6 ab | 57.4 b | - |
| 823 | Methyl acetate | Fruity, sweet | 4.2 b | 1.4 a | 04.7 b | - |
| 837 | 2-propenal | Pungent, acrid | <LOD | <LOD | 0.6 | MR3 |
| 904 | 2-butanone | Sweet, acetone-like | 2.4 ab | 0.7 a | 4.3 b | MR3 |
| 912 | 2-methylbutanal | Malty | <LOD | <LOD | 1.8 | MR3 |
| 915 | 3-methylbutanal | Malty | <LOD | <LOD | 1.1 | MR3 |
| 937 | Benzene | Sweet, solvent-like | 1.6 b | 0.3 a | 2.8 b | MR3 |
| 947 | Ethanol | Sweet | 36.7 b | 19.2 a | 24.3 ab | MR1 |
| 956 | 3,4-diethyl-1,5-hexadiene (RS/SR) | Mild hydrocarbon-like | 40.2 b | 38.4 b | 15.4 a | MR2 |
| 962 | 3,4-diethyl-1,5-hexadiene (Meso) | Hydrocarbon-like | 34.4 b | 32.1 b | 13.2 a | MR1, MR2 |
| 978 | 3-pentanone | Fruity, sweet | 15.4 a | 21.9 b | 15.3 a | MR2 |
| 1000 | Decane | Alkane, odorless | 1.6 ab | 1.0 a | 2.6 b | MR3 |
| 1007 | (Z)-3-ethyl-1,5-octadiene | Hydrocarbon-like | 174.1 b | 166.4 b | 67.5 a | MR2 |
| 1020 | 1-penten-3-one | Green, pungent | 290.6 b | 294.3 b | 135.3 a | MR2 |
| 1035 | Toluene | Paint | 113.5 b | 50.3 a | 137.7 b | MR3 |
| 1047 | 2-methyl-2-butenal | Green, pungent | 1.5 a | 1.1 a | <LOD | MR1 |
| 1061 | α-fenchene | Camphor | 0.2 | <LOD | <LOD | MR1 |
| 1072 | Butyl acetate | Fruity, pear | <LOD | <LOD | 0.6 | - |
| 1074 | (E,E)-3,7-decadiene | Green, waxy | 51.8 b | 53.3 b | 17.1 a | MR2 |
| 1081 | Hexanal | Green, grass | 326.9 ab | 345.4 b | 230.6 a | MR2 |
| 1083 | 4,8-dimethyl-1,7-nonadiene | Herbal, green, balsamic | 43.7 a | 35.5 a | <LOD | - |
| 1110 | (Z)-2-pentenal | Green, pleasant | 13.5 c | 9.6 b | 4.8 a | MR1 |
| 1123 | Ethylbenzene | Gasoline-like | 8.1 b | 5.0 a | 16.4 b | MR3 |
| 1128 | p-xylene | Solvent-like | 2.0 a | 0.9 a | 10.3 b | MR3 |
| 1135 | m-xylene | Plastic | <LOD | <LOD | 18.3 | MR3 |
| 1137 | (Z)-3-hexenal | Leaf-like | 184.4 b | 66.7 a | 56.0 a | MR1 |
| 1142 | (E)-3-hexenal | Green, grass | 729.8 c | 186.5 b | 58.9 a | MR1 |
| 1160 | 1-butanol | Medicine, mild sweet | <LOD | <LOD | 00.4 | MR3 |
| 1168 | Methyl 3-methyl-2-butenoate | Overripe fruity, papaya | <LOD | 01.1 a | 13.6 b | MR3 |
| 1171 | 1-penten-3-ol | Butter, pungent | 74.4 b | 67.1 b | 36.1 a | MR1, MR2 |
| 1207 | (Z)-2-hexenal | Green | 314.6 c | 148.3 b | 59.1 a | MR1 |
| 1226 | (E)-2-hexenal | Green apple-like, bitter almond | 2009.7 a | 3619.1 b | 1481.2 a | MR2 |
| 1249 | (Z)-β-ocimene | Citrus, herb, flower | 0.5 a | 44.8 b | 27.9 b | MR2 |
| 1259 | 3-octanone | Mushroom-like, earthy | <LOD | <LOD | 00.4 | MR3 |
| 1276 | Hexyl acetate | Fruity, sweet | 4.4 a | 12.2 b | 6.1 ab | MR2 |
| 1282 | 1,2,4-trimethylbenzene | Plastic | 2.8 b | 1.5 a | 3.1 b | - |
| 1298 | Acetoin | Butter, cream | 0.1 a | 0.3 a | 1.3 b | - |
| 1321 | (Z)-3-hexenyl acetate | Green, apple | 69.8 b | 74.4 b | 24.7 a | MR2 |
| 1330 | (E)-2-pentenol | Mushroom | 8.9 b | 7.9 b | 3.3 a | MR1, MR2 |
| 1339 | (Z)-2-pentenol | Green, plastic, rubber | 104.5 b | 91.4 b | 36.7 a | MR1, MR2 |
| 1374 | 1-hexanol | Resin, flower, green | 21.4 a | 56.3 b | 26.6 a | MR2 |
| 1382 | (Z)-3-hexenol | Leaf-like, grass | 1.8 b | 2.4 b | 0.4 a | MR2 |
| 1389 | Methyl 3-hydroxy-3-methylbutanoate | Ester | <LOD | 0.6 a | 5.3 b | MR3 |
| 1391 | 2-methyl-2-cyclohexenone | Mild wood | 1.4 b | 0.1 a | <LOD | MR1 |
| 1404 | (E)-3-hexenol | Moss, fresh | 182.4 b | 188.1 b | 54.9 a | MR2 |
| 1411 | (Z,E)-2,4-hexadienal | Green | 81.1 c | 42.4 b | 18.5 a | MR1 |
| 1418 | (E,E)-2,4-hexadienal | Green | 186.1 c | 102.5 b | 43.7 a | MR1 |
| 1427 | (E)-2-hexenol | Herb, leaf, walnut | 12.8 a | 57.1 b | 17.4 a | MR1 |
| 1490 | Copaene | Wood, spice | 0.1 a | 0.7 a | <LOD | MR2 |
| 1498 | α-cubebene | Herb, wax, mango | 0.8 a | 8.3 b | 0.5 a | MR2 |
| 1510 | 2-ethyl-1-hexanol | Rose, green | 0.6 a | 4.6 b | 0.6 a | MR2 |
| 1537 | 2,4-dimethyl-2-hexene | Slight hydrocarbon | 8.5 b | 1.8 a | 0.6 a | - |
| 1564 | Propanoic acid | Pungent, rancid | 0.5 a | 0.9 b | 1.4 b | MR3 |
| 1565 | Valeric anhydride | Rancid | 7.5 b | 2.0 a | 0.1 a | MR1 |
| 1592 | 2-methyl propanoic acid | Rancid, butter, cheese | 0.4 a | 0.6 a | 3.1 b | MR3 |
| 1616 | 4-oxo-2-hexenal | Metallic, green | 46.0 c | 9.9 b | 2.3 a | MR1 |
| 1662 | Dimethyl sulfoxide | Garlic | 0.9 a | 1.7 b | 0.7 a | MR2 |
| 1738 | α-muurolene | Wood | <LOD | 0.4 | <LOD | MR2 |
| 1753 | α-farnesene | Wood, sweet | <LOD | 0.9 a | 4.1 b | MR3 |
| 1768 | 2-cyclohexen-1,4-dione | Slight sweet | 5.3 c | 1.5 b | 0.4 a | MR1 |
| 1787 | Methoxy-phenyl-oxime | Flower | 14.4 b | 27.0 c | 5.1 a | MR2 |
| 1803 | 5-ethyl-2(5H)-furanone | Spice, caramel | 28.9 c | 7.7 b | 2.3 a | MR1 |
| 1815 | 2-methyl-2-butenoic acid | Spicy, rancid | 0.9 | <LOD | <LOD | MR1 |
| 1854 | Anethole | Anise, fennel | 0.5 a | 0.5 a | 2.2 b | MR3 |
| 1961 | 2-phenylethanol | Honey, rose, lilac | 6.3 b | 5.2 b | 1.6 a | - |
| 1996 | 3-hexenoic acid | Cheese, green, grassy | 3.0 b | 0.8 a | 0.3 a | MR1 |
| >20,000 | γ-nonalactone | Coconut, peach | 55.5 c | 17.9 b | 1.8 a | MR1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Pizarro, Á.; Romero, A.; Schorn-García, D.; Ezenarro, J.; Mestres, M.; Aceña, L. Improving Sensory Differentiation: Refining the ‘Fruitiness’ Descriptor in Extra Virgin Olive Oil. Foods 2025, 14, 1390. https://doi.org/10.3390/foods14081390
García-Pizarro Á, Romero A, Schorn-García D, Ezenarro J, Mestres M, Aceña L. Improving Sensory Differentiation: Refining the ‘Fruitiness’ Descriptor in Extra Virgin Olive Oil. Foods. 2025; 14(8):1390. https://doi.org/10.3390/foods14081390
Chicago/Turabian StyleGarcía-Pizarro, Ángel, Agustí Romero, Daniel Schorn-García, Jokin Ezenarro, Montserrat Mestres, and Laura Aceña. 2025. "Improving Sensory Differentiation: Refining the ‘Fruitiness’ Descriptor in Extra Virgin Olive Oil" Foods 14, no. 8: 1390. https://doi.org/10.3390/foods14081390
APA StyleGarcía-Pizarro, Á., Romero, A., Schorn-García, D., Ezenarro, J., Mestres, M., & Aceña, L. (2025). Improving Sensory Differentiation: Refining the ‘Fruitiness’ Descriptor in Extra Virgin Olive Oil. Foods, 14(8), 1390. https://doi.org/10.3390/foods14081390









