Comparison of Ultraviolet A, B and C Treatments in Preserving the Quality and Nutritional Integrity of Fresh-Cut Spinach
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of Spinach
2.2. UV Treatments, Storage, and Experimental Design
2.3. Determination of Weight Loss, Dry Matter Content, Total Soluble Solids, Electrical Conductivity, pH, and Respiration Rate
- : CO2 volumetric concentration, mg kg−1, 10−6 L L−1;
- : Molecular weight of CO2 gas, 44.01 g mol−1;
- : Flask volume, L;
- m: The weight of fresh-cut spinach leaves, kg;
- : Duration of the experiment, h;
- : CO2 concentration in the initial, mg kg−1, 10−6 L L−1;
- : CO2 concentration at the end of experiment, mg kg−1, 10−6 L L−1;
- : Molar volume of gas, L mol−1;
- R: Gas constant, 0.08206 L mol−1 K−1;
- T: Temperature, K;
- P: Pressure, atm.
2.4. Determination of SPAD Value and Color Parameters (L*, a*, b*, Chroma, and Hue Angle)
2.5. Determination of Ash Content and Mineral Composition
2.6. Statistical Analysis
3. Results
3.1. Changes in Weight Loss, Dry Matter Content, Total Soluble Solids, Electrical Conductivity, pH, and Respiration Rate
3.2. Changes in SPAD and Color Parameters (L*, a*, b*, Chroma, and Hue Angle)
3.3. Changes in Ash Content and Mineral Composition
3.4. Hierarchical Cluster Analysis
4. Discussion
4.1. Weight Loss, Dry Matter Content, Total Soluble Solids, Electrical Conductivity, pH, and Repiration Rate
4.2. SPAD and Color Parameters
4.3. Ash Content and Mineral Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palumbo, M.; Attolico, G.; Capozzi, V.; Cozzolino, R.; Corvino, A.; de Chiara, M.L.V.; Cefola, M. Emerging postharvest technologies to enhance the shelf-life of fruit and vegetables: An overview. Foods 2022, 11, 3925. [Google Scholar] [CrossRef] [PubMed]
- Allende, A.; McEvoy, J.L.; Luo, Y.; Artes, F.; Wang, C.Y. Effectiveness of two-sided UV-C treatments in inhibiting natural microflora and extending shelf-life of minimally processed “Red Oak Leaf” lettuce. Food Microbiol. 2006, 23, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Batool, S.; Khalid, N.; Ali, S.; Raza, M.A.; Li, X.; Xinhua, Z. Recent trends in hydrogen-associated treatments for maintaining the postharvest quality of fresh and fresh-cut fruits and vegetables: A review. Food Control 2024, 156, 110114. [Google Scholar] [CrossRef]
- Du, Y.; Tian, Q.; Li, G.; Yi, J.; Hu, X.; Jiang, Y. Advanced application of slightly acidic electrolyzed water for fresh-cut fruits and vegetables preservation. Food Res. Int. 2024, 195, 114996. [Google Scholar] [CrossRef]
- Patil, M.; Sharma, S.; Sridhar, K.; Anurag, R.K.; Grover, K.; Dharni, K.; Sharma, M. Effect of postharvest treatments and storage temperature on physiological, nutritional, and shelf-life of broccoli (Brassica oleracea) microgreens. Sci. Hortic. 2024, 327, 112805. [Google Scholar] [CrossRef]
- Sonntag, F.; Liu, H.; Neugart, S. Nutritional and physiological effects of postharvest UV radiation on vegetables: A review. J. Agric. Food Chem. 2023, 71, 9951–9972. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Zhao, J.; Liu, L.; Zhao, L. Research progress on physical preservation technology of fresh-cut fruits and vegetables. Horticulturae 2024, 10, 1098. [Google Scholar] [CrossRef]
- Civello, P.M.; Villarreal, N.; Lobato, M.E.G.; Martínez, G.A. Physiological effects of postharvest uv treatments: Recent progress. Stewart Postharvest Rev. 2014, 10, 1–6. [Google Scholar]
- Zhang, W.; Jiang, W. UV treatment improved the quality of postharvest fruits and vegetables by inducing resistance. Trends Food Sci. Technol. 2019, 92, 71–80. [Google Scholar] [CrossRef]
- Nigro, F.; Ippolito, A.; Lima, G. Use of UV-C light to reduce Botrytis storage rot of table grapes. Postharvest Biol. Technol. 1998, 13, 171–181. [Google Scholar] [CrossRef]
- Charles, M.T.; Makhlouf, J.; Arul, J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit: II. Modification of fruit surface and changes in fungal colonization. Postharvest Biol. Technol. 2008, 47, 21–26. [Google Scholar] [CrossRef]
- Costa, L.; Vicente, A.R.; Civello, P.M.; Chaves, A.R.; Martinez, G.A. UV-C treatment delays postharvest senescence in broccoli florets. Postharvest Biol. Technol. 2006, 39, 204–210. [Google Scholar] [CrossRef]
- Wei, Z.F.; Luo, M.; Zhao, C.J.; Li, C.Y.; Gu, C.B.; Wang, W.; Fu, Y.J. UV-induced changes of active components and antioxidant activity in postharvest pigeon pea [Cajanus cajan (L.) Millsp.] leaves. J. Agric. Food Chem. 2013, 61, 1165–1171. [Google Scholar] [CrossRef]
- Darré, M.; Valerga, L.; Araque, L.C.O.; Lemoine, M.L.; Demkura, P.V.; Vicente, A.R.; Concellón, A. Role of UV-B irradiation dose and intensity on color retention and antioxidant elicitation in broccoli florets (Brassica oleracea var. italica). Postharvest Biol. Technol. 2017, 128, 76–82. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Use of postharvest UV-B and UV-C radiation treatments to revalorize broccoli byproducts and edible florets. Innov. Food Sci. Emerg. Technol. 2017, 43, 77–83. [Google Scholar] [CrossRef]
- Dyshlyuk, L.; Babich, O.; Prosekov, A.; Ivanova, S.; Pavsky, V.; Chaplygina, T. The effect of postharvest ultraviolet irradiation on the content of antioxidant compounds and the activity of antioxidant enzymes in tomato. Heliyon 2020, 6, e03288. [Google Scholar] [CrossRef]
- Battistoni, B.; Amorós, A.; Tapia, M.L.; Escalona, V. Effect of LED lights on the antioxidant properties of baby spinach leaves (Spinacia oleracea L.). Rev. FCA UNCuyo 2021, 53, 98–108. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, L.; Liu, W.; Lin, Q.; Wang, Z.; Guan, W. Effects of UV-C on antioxidant capacity, antioxidant enzyme activity and colour of fresh-cut red cabbage during storage. Int. J. Food Sci. Technol. 2017, 52, 626–634. [Google Scholar] [CrossRef]
- Woo, H.J.; Kang, J.H.; Lee, C.H.; Song, K.B. Inactivation of Listeria monocytogenes, Escherichia coli O157:H7, and pre-existing bacteria on spinach by combined treatment of Cudrania tricuspidata leaf extract washing and ultraviolet-C irradiation. Food Bioprocess Technol. 2020, 13, 1229–1239. [Google Scholar] [CrossRef]
- Han, C.; Zhen, W.; Chen, Q.; Fu, M. UV-C irradiation inhibits surface discoloration and delays quality degradation of fresh-cut stem lettuce. LWT-Food Sci. Technol. 2021, 147, 111533. [Google Scholar] [CrossRef]
- Allende, A.; Artés, F. UV-C radiation as a novel technique for keeping quality of fresh processed ‘Lollo Rosso’ lettuce. Food Res. Int. 2003, 36, 739–746. [Google Scholar] [CrossRef]
- Alegria, C.; Pinheiro, J.; Duthoit, M.; Gonçalves, E.M.; Moldão-Martins, M.; Abreu, M. Fresh-cut carrot (cv. Nantes) quality as affected by abiotic stress (heat shock and UV-C irradiation) pre-treatments. LWT-Food Sci. Technol. 2012, 48, 197–203. [Google Scholar] [CrossRef]
- Hassenberg, K.; Huyskens-Keil, S.; Herppich, W.B. Impact of postharvest UV-C and ozone treatments on microbiological properties of white asparagus (Asparagus officinalis L.). J. Appl. Bot. Food Qual. 2013, 85, 174. [Google Scholar]
- Alfaro, L.; Soler-Segura, R.; Jacquin, C.; Juan, M.; Elorrieta, M.A.; Valenzuela, J.L. Yellow bell pepper fruit response to postharvest application of ultraviolet radiation. Acta Hortic. 2016, 1194, 815–822. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Escalona, V.H.; Robles, P.A.; Martínez-Hernández, G.B.; Artés, F. Effect of UV-C radiation on quality of minimally processed spinach leaves. J. Sci. Food Agric. 2009, 89, 414–421. [Google Scholar] [CrossRef]
- Collazo, C.; Noguera, V.; Aguiló-Aguayo, I.; Abadias, M.; Colás-Medà, P.; Nicolau, I.; Viñas, I. Assessing water-assisted UV-C light and its combination with peroxyacetic acid and Pseudomonas graminis CPA-7 for the inactivation and inhibition of Listeria monocytogenes and Salmonella enterica in fresh-cut ‘Iceberg’ lettuce and baby spinach leaves. Int. J. Food Microbiol. 2019, 297, 11–20. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-thermal technologies for food processing. Front. Nutr. 2021, 8, 657090. [Google Scholar] [CrossRef] [PubMed]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, applications, and comparison of thermal (pasteurization, sterilization, and aseptic packaging) against non-thermal (ultrasounds, UV radiation, ozonation, high hydrostatic pressure) technologies in food processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]
- Tchonkouang, R.D.; Lima, A.R.; Quintino, A.C.; Cristofoli, N.L.; Vieira, M.C. UV-C light: A promising preservation technology for vegetable-based nonsolid food products. Foods 2023, 12, 3227. [Google Scholar] [CrossRef]
- Shweta, M.; Agrawal, S.B. Interactive effects between supplemental ultraviolet-B radiation and heavy metals on the growth and biochemical characteristics of Spinacia oleracea L. Braz. J. Plant Physiol. 2006, 18, 307–314. [Google Scholar] [CrossRef]
- Sakalauskienė, S.; Januškaitienė, I.; Juknys, R.; Miliauskienė, J. The effects of UV-B radiation on phytochemical properties of Spinacia oleracea. Rural Dev. 2013, 2013, 223. [Google Scholar] [CrossRef]
- Kasım, M.U.; Kasım, R. Yellowing of fresh-cut spinach (Spinacia oleracea L.) leaves delayed by UV-B applications. Inf. Process. Agric. 2017, 4, 214–219. [Google Scholar]
- Jeong, Y.J.; Ha, J.W. Combined treatment of UV-A radiation and acetic acid to control foodborne pathogens on spinach and characterization of their synergistic bactericidal mechanisms. Food Control 2019, 106, 106698. [Google Scholar] [CrossRef]
- Demircan, B.; Velioglu, Y.S.; Giuffrè, A.M. Effects of washing with boric acid solutions on residual boric acid content, microbiological load, and quality of fresh-cut spinach. Heliyon 2024, 10, e31974. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Bazoni, C.H.; Ida, E.I.; Barbin, D.F.; Kurozawa, L.E. Near-infrared spectroscopy as a rapid method for evaluation of physicochemical changes of stored soybeans. J. Stored Prod. Res. 2017, 73, 1–6. [Google Scholar] [CrossRef]
- Saltveit, M.E. Respiratory Metabolism. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 73–91. [Google Scholar]
- Kibar, B.; Kibar, H. Determination of the nutritional and seed properties of some wild edible plants consumed as vegetable in the Middle Black Sea Region of Turkey. S. Afr. J. Bot. 2017, 108, 117–125. [Google Scholar] [CrossRef]
- Kacar, B.; Inal, A. Plant Analysis; Nobel Publishers: Ankara, Turkey, 2008; p. 892. [Google Scholar]
- Llusia, J.; Llorens, L.; Bernal, M.; Verdaguer, D.; Penuelas, J. Effects of UV radiation and water limitation on the volatile terpene emission rates, photosynthesis rates, and stomatal conductance in four Mediterranean species. Acta Physiol. Plant. 2012, 34, 757–769. [Google Scholar] [CrossRef]
- Correa, M.D.S.S.; Saavedra, M.E.R.R.; Parra, E.A.E.; Ontiveros, E.N.; Flores, J.D.C.B.; Montiel, J.G.O.; González, A.M.E. Ultraviolet Radiation and Its Effects on Plants. In Abiotic Stress in Plants—Adaptations to Climate Change; IntechOpen: London, UK, 2023. [Google Scholar]
- Shipley, B.; Vu, T.T. Dry matter content as a measure of dry matter concentration in plants and their parts. New Phytol. 2002, 153, 359–364. [Google Scholar] [CrossRef]
- Heuberger, H.; Praeger, U.; Georgi, M.; Schirrmacher, G.; Grasmann, J.; Schnitzler, W.H. Precision stressing by UV-B radiation to improve quality of spinach under protected cultivation. Acta Hortic. 2004, 659, 201–206. [Google Scholar] [CrossRef]
- Mohammed, A.; Akladious, S. Protective role of a methanolic extract of spinach (Spinacia oleracea L.) against adverse effects of UV-C irradiation on fenugreek (Trigonella foenum-graecum L.) seedlings. Gesunde Pflanz. 2017, 69, 243–252. [Google Scholar] [CrossRef]
- Zargar, T.B.; Basal, O.; Veres, S. Improving quality parameters of spinach by adjusting light spectra under moderate water deprivation conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13325. [Google Scholar] [CrossRef]
- Kasım, M.U.; Kasım, R. Effects of the different wavelength ultraviolet radiation on postharvest quality of fresh-cut spinach. Yuzuncu Yil Univ. J. Agric. Sci. 2016, 26, 348–359. [Google Scholar]
- Meitankeisangbam, B.; Singh, T.B.; Devi, Y.M.; Samjetsabam Chanulembi Devi, S.C. Electrical conductivity as a quantitative indicator of membrane integrity and viability in aged rapeseed and mustard seeds. Int. J. Innov. Sci. Res. Technol. 2024, 9, 100–103. [Google Scholar]
- Kobashigawa, C.; Tamaya, K.; Shimomachi, T. Effect of UV-C treatment on plant growth and nutrient contents. Acta Hortic. 2009, 907, 237–242. [Google Scholar] [CrossRef]
- Kakade, A.; More, P.; Jadhav, S.; Bhosle, V. Shelf life extension of fresh-cut spinach. Int. J. Agric. Environ. Biotechnol. 2015, 8, 609–614. [Google Scholar] [CrossRef]
- Esua, O.J.; Chin, N.L.; Yusof, Y.A.; Sukor, R. A review on individual and combination technologies of UV-C radiation and ultrasound in postharvest handling of fruits and vegetables. Processes 2020, 8, 1433. [Google Scholar] [CrossRef]
- Hikosaka, S.; Harada, M.; Yoshida, H.; Goto, E. Effects of preharvest uv irradiation combined with postharvest storage temperature and low light irradiation during storage on the functional compounds of Komatsuna (Brassica rapa var. perviridis). Acta Hortic. 2023, 1404, 409–416. [Google Scholar] [CrossRef]
- Escalona, V.H.; Aguayo, E.; Martínez-Hernández, G.B.; Artés, F. UV-C doses to reduce pathogen and spoilage bacterial growth in vitro and in baby spinach. Postharvest Biol. Technol. 2010, 56, 223–231. [Google Scholar] [CrossRef]
- Saenmuang, S.; Al-Haq, M.I.; Samarakoon, H.C.; Makino, Y.; Kawagoe, Y.; Oshita, S. Evaluation of models for spinach respiratory metabolism under low oxygen atmospheres. Food Bioprocess Technol. 2012, 5, 1950–1962. [Google Scholar] [CrossRef]
- Meitha, K.; Pramesti, Y.; Signorelli, S.; Kriswantoro, J.A. Postharvest chitosan application maintains the quality of spinach through suppression of bacterial growth and elicitation. Hortic. Environ. Biotechnol. 2022, 63, 217–227. [Google Scholar] [CrossRef]
- Duarte-Sierra, A.; Hasan, S.M.M.; Angers, P.; Arul, J. UV-B radiation hormesis in broccoli florets: Glucosinolates and hydroxy-cinnamates are enhanced by UV-B in florets during storage. Postharvest Biol. Technol. 2020, 168, 111278. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Tawfic, G.A.; Shehata, S.A.; Hamid, O.A.A.; Ahmed, A.E.R.A.; El-Mogy, M.M. The effect of seed priming with UV and Gamma rays on the growth, production, and storage ability of cauliflower heads. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13264. [Google Scholar] [CrossRef]
- Aamir, M.; Ovissipour, M.; Rasco, B.; Tang, J.; Sablani, S. Seasonality of the thermal kinetics of color changes in whole spinach (Spinacia oleracea) leaves under pasteurization conditions. Int. J. Food Prop. 2014, 17, 2012–2024. [Google Scholar] [CrossRef]
- Nguyen, T.-P.-D.; Ngoc-Thang, V.; Quang-Thach, N.; Thanh-Huyen, T.; Phi-Bang, T. Growth and quality of hydroponically cultivated spinach (Spinacia oleracea L.) affected by the light intensity of red and blue LEDs. Sains Malays. 2022, 51, 473–483. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Engel, R.G. Isolation of chlorophyll and carotenoid pigments from spinach. Introd. Org. Lab. Tech. Microscale Approach 1999, 1, 1–7. [Google Scholar]
- Kumar, A.; Mishra, A.A.; Kumar, V. Effect of pretreatment and packaging materials on quality of spinach powder during storage. Int. J. Adv. Agric. Sci. Technol. 2019, 6, 71–81. [Google Scholar]
- Lopez, L.D.; Pinto, E.P.; Börger, B.R.; Kaipers, K.F.C.; Lucchetta, L.; Tonial, I.B. Interferência do sistema de cultivo, radiação UV-C e método de secagem na qualidade da farinha de subprodutos de UVA. Científica 2017, 45, 347–354. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Olle, M. Light Spectrum and Its Role in Calcium Nutrition. Available online: https://www.agritecture.com/blog/margit-olles-discovery-light-spectrum-and-its-role-in-calcium-nutrition (accessed on 13 March 2025).
- Da Ros, L.M.; Mansfield, S.D. Biotechnological mechanism for improving plant remobilization of phosphorus during leaf senescence. Plant Biotechnol. J. 2020, 18, 470–478. [Google Scholar] [CrossRef]
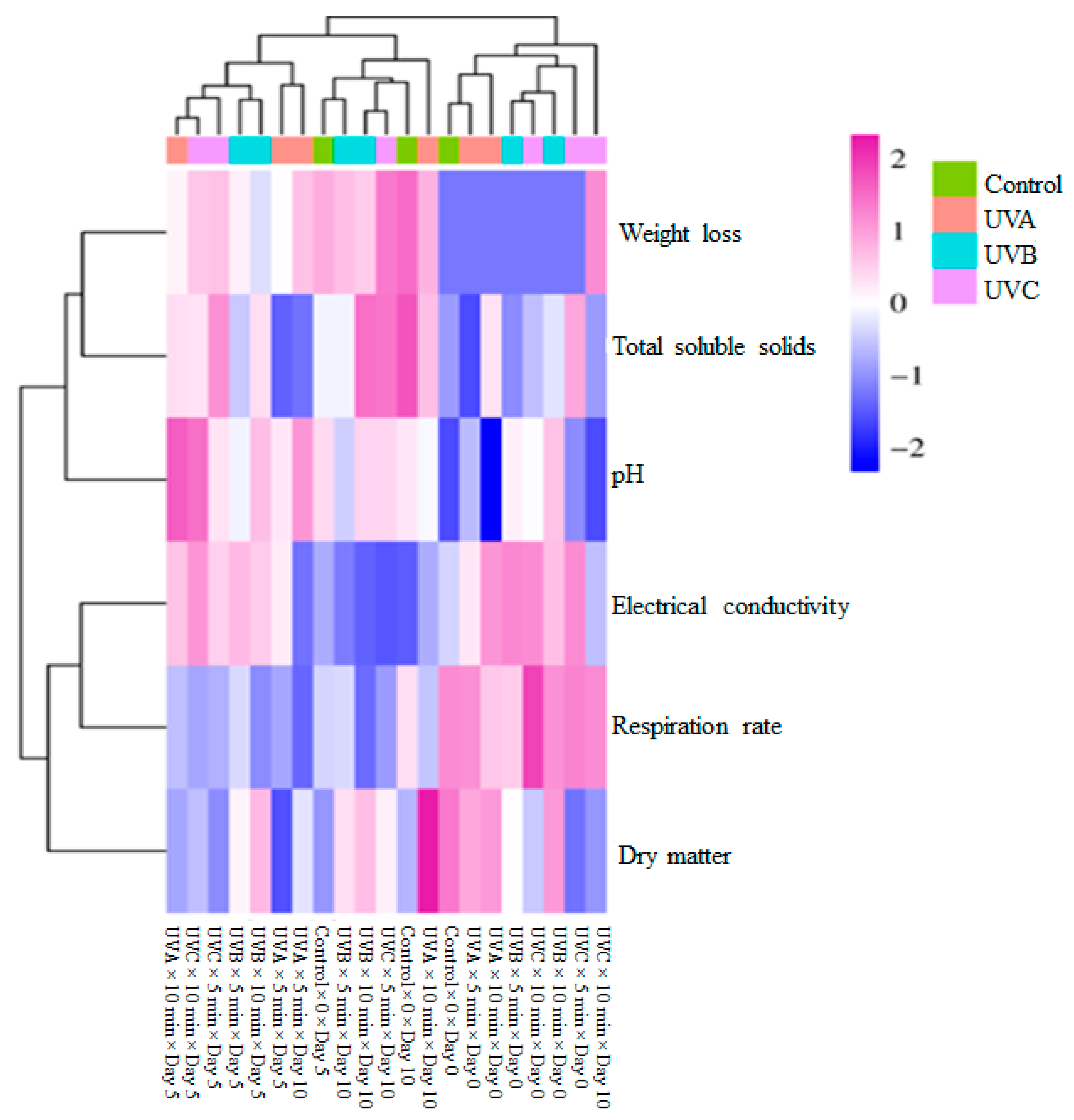
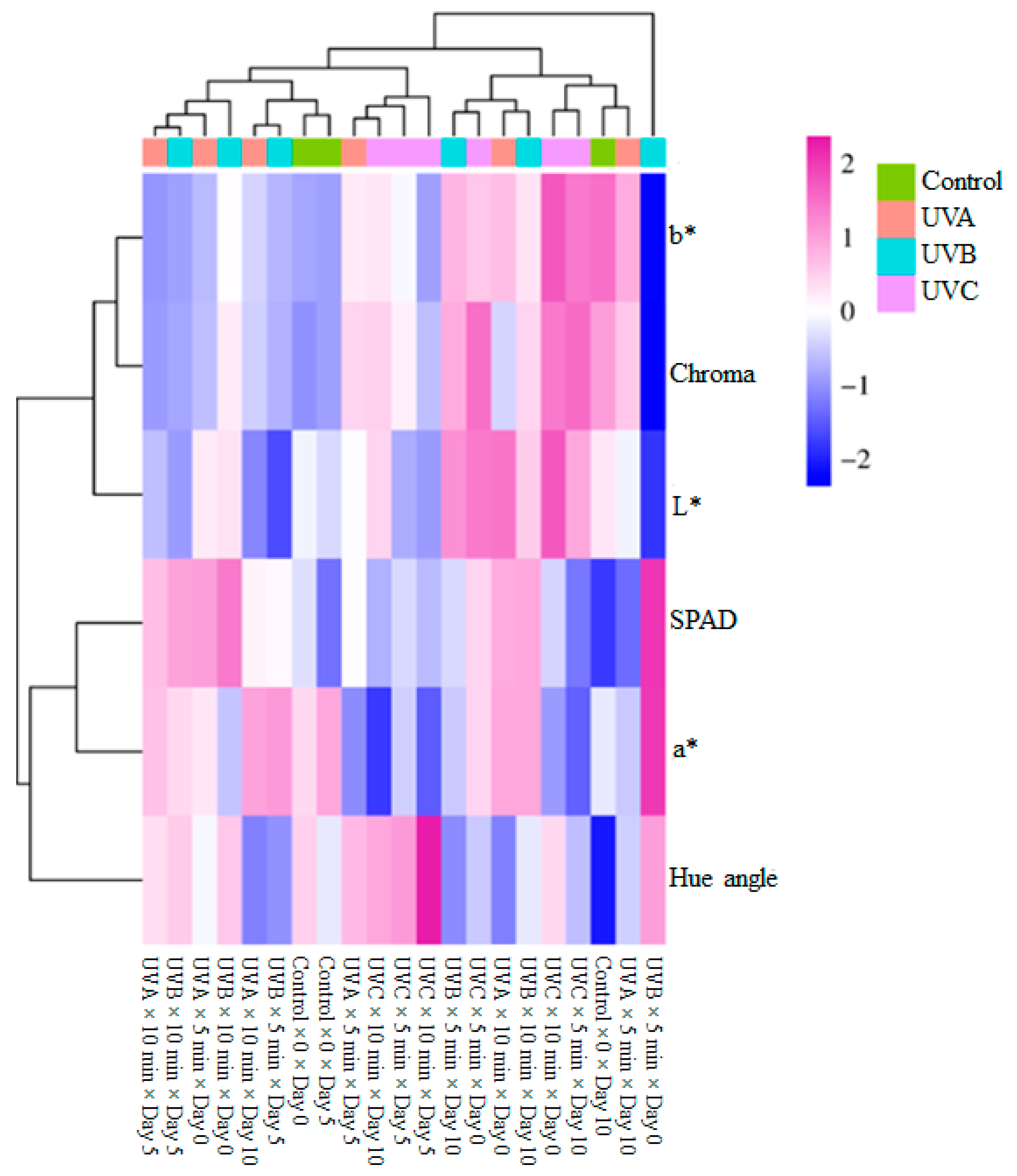
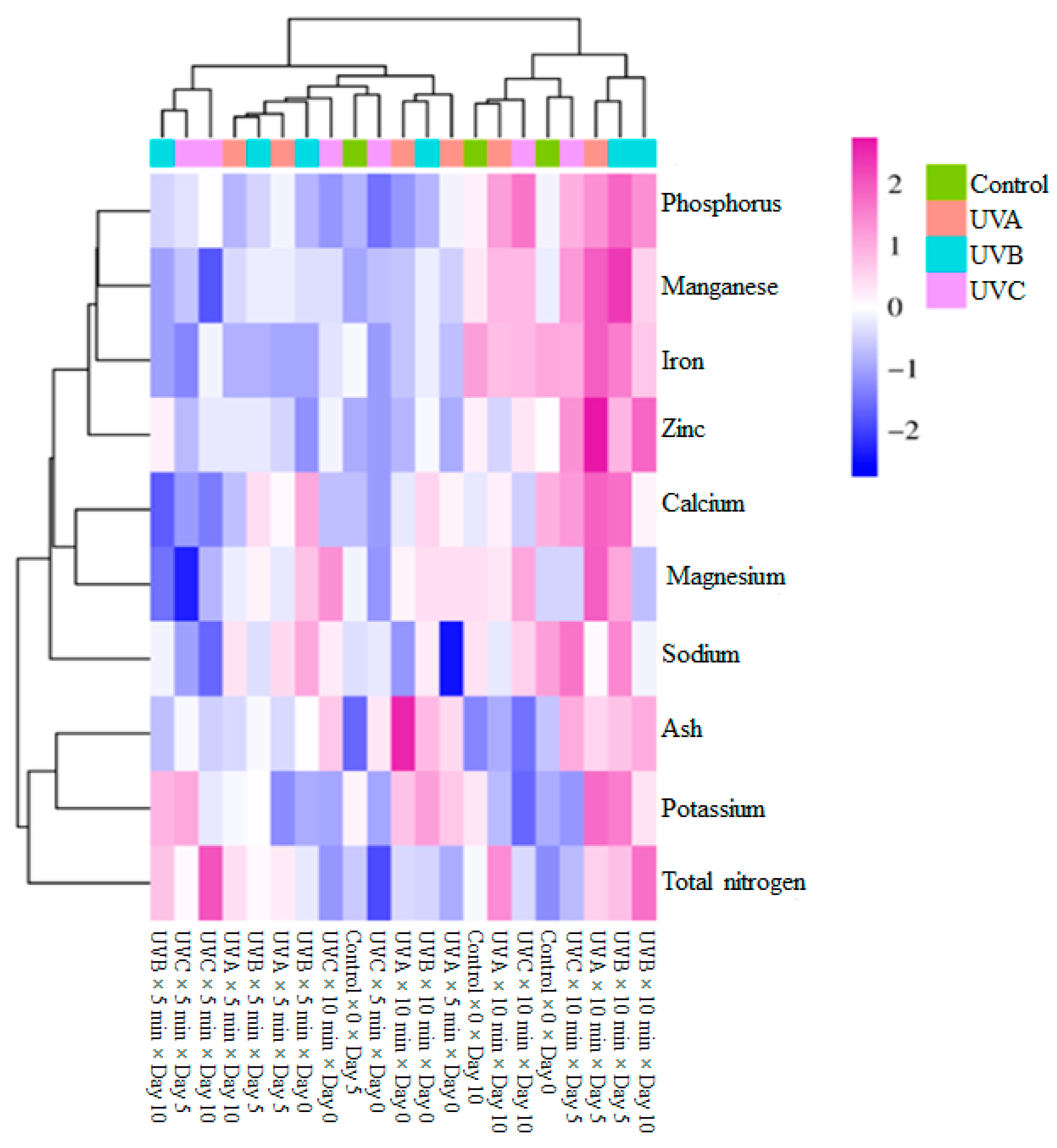
| Ultraviolet Light Sources (ULS) | WL (%) | Dry Matter (%) | TSSs (%) | EC (µS cm−1) | pH | RR (mg CO2 kg−1 h−1) |
|---|---|---|---|---|---|---|
| Control | 0.82 ± 0.15 a | 9.10 ± 0.99 ab | 8.57 ± 0.92 ab | 59.0 ± 23.2 b | 5.49 ± 0.22 a | 47.6 ± 7.96 a |
| UVA | 0.55 ± 0.12 bc | 9.39 ± 1.26 ab | 8.00 ± 0.77 b | 99.5 ± 42.8 ab | 5.54 ± 0.32 a | 40.6 ± 9.83 a |
| UVB | 0.52 ± 0.15 c | 9.56 ± 0.83 a | 8.38 ± 0.65 ab | 102 ± 48.7 ab | 5.60 ± 0.30 a | 40.9 ± 9.92 a |
| UVC | 0.75 ± 0.12 ab | 8.62 ± 0.65 b | 8.98 ± 0.59 a | 112 ± 50.1 a | 5.59 ± 0.23 a | 42.6 ± 13.7 a |
| Ultraviolet irradiation time (UIT) | ||||||
| 0 | 0.82 ± 0.15 a | 9.10 ± 0.99 a | 8.57 ± 0.92 a | 59.0 ± 23.2 b | 5.49 ± 0.22 a | 47.6 ± 7.96 a |
| 5 min | 0.62 ± 0.15 a | 8.95 ± 0.92 a | 8.17 ± 0.67 a | 99.1 ± 48.7 ab | 5.49 ± 0.26 a | 41.6 ± 10.1 a |
| 10 min | 0.59 ± 0.18 a | 9.44 ± 1.07 a | 8.73 ± 0.54 a | 110 ± 44.8 a | 5.61 ± 0.30 a | 41.2 ± 12.4 a |
| Storage period (SP) | ||||||
| Day 0 | - | 9.47 ± 1.02 a | 8.03 ± 0.64 b | 132 ± 31.7 a | 5.41 ± 0.30 b | 55.5 ± 5.21 a |
| Day 5 | 0.52 ± 0.14 b | 8.71 ± 0.95 b | 8.39 ± 0.60 ab | 117 ± 30.2 a | 5.68 ± 0.22 a | 36.2 ± 3.20 b |
| Day 10 | 0.75 ± 013 a | 9.36 ± 0.93 ab | 8.97 ± 0.82 a | 45.4 ± 19.2 b | 5.60 ± 0.23 a | 35.1 ± 6.69 b |
| Level of significance | ||||||
| ULS | ** | * | * | * | ns | ns |
| UIT | ns | ns | ns | * | ns | ns |
| SP | ** | * | ** | ** | ** | ** |
| Ultraviolet Light Sources (ULS) | SPAD | L* | a* | b* | Chroma | Hue Angle |
|---|---|---|---|---|---|---|
| Control | 29.1 ± 3.49 c | 49.1 ± 2.32 a | −11.5 ± 1.61 a | 24.0 ± 3.08 a | 26.1 ± 1.00 a | 116 ± 2.53 a |
| UVA | 34.5 ± 4.14 ab | 49.1 ± 2.16 a | −11.7 ± 1.53 a | 24.1 ± 2.56 a | 26.3 ± 3.04 a | 116 ± 2.04 a |
| UVB | 37.0 ± 4.33 a | 48.5 ± 2.76 a | −11.5 ± 1.60 a | 23.1 ± 3.40 a | 25.8 ± 3.70 a | 117 ± 1.63 a |
| UVC | 31.6 ± 3.41 bc | 49.9 ± 2.11 a | −13.1 ± 0.97 b | 25.4 ± 3.10 a | 28.6 ± 3.33 a | 117 ± 2.10 a |
| Ultraviolet irradiation time (UIT) | ||||||
| 0 | 29.1 ± 3.49 b | 49.1 ± 2.32 a | −11.5 ± 1.61 a | 24.0 ± 3.08 a | 26.1 ± 1.00 a | 116 ± 2.53 a |
| 5 min | 33.7 ± 4.89 a | 49.1 ± 2.59 a | −12.2 ± 1.79 a | 24.2 ± 3.39 a | 27.2 ± 3.93 a | 117 ± 1.73 a |
| 10 min | 35.0 ± 4.04 a | 49.3 ± 2.19 a | −12.0 ± 1.28 a | 24.2 ± 2.92 a | 26.6± 3.13 a | 117 ± 2.16 a |
| Storage period (SP) | ||||||
| Day 0 | 36.5 ± 4.49 a | 49.9 ± 2.61 a | −11.9 ± 1.82 a | 23.9 ± 3.79 ab | 26.4 ± 4.48 ab | 117 ± 1.85 a |
| Day 5 | 33.3 ± 3.76 b | 48.0 ± 2.13 b | −11.8 ± 1.65 a | 22.7 ± 2.12 b | 25.6 ± 2.53 b | 117 ± 2.06 a |
| Day 10 | 31.0 ± 4.33 b | 49.6 ± 1.94 ab | −12.3 ± 1.13 a | 25.8 ± 2.42 a | 28.3 ± 2.49 a | 116 ± 2.03 a |
| Level of significance | ||||||
| ULS | ** | ns | ** | ns | ns | ns |
| UIT | ** | ns | ns | ns | ns | ns |
| SP | ** | * | ns | ** | * | ns |
| Ultraviolet Light Sources (ULS) | Ash (%) | Nitrogen (%) | Potassium (mg 100 g−1) | Calcium (mg 100 g−1) | Phosphorus (mg 100 g−1) | Magnesium (mg 100 g−1) |
|---|---|---|---|---|---|---|
| Control | 1.59 ± 0.13 b | 2.67 ± 0.18 a | 3580 ± 401 ab | 1365 ± 179 a | 353 ± 33.2 a | 254 ± 76.5 a |
| UVA | 1.91 ± 0.36 a | 2.96 ± 0.26 a | 3831 ± 708 ab | 1418 ± 201 a | 372 ± 65.9 a | 269 ± 36.7 a |
| UVB | 1.91 ± 0.17 a | 3.03 ± 0.28 a | 4040 ± 592 a | 1445 ± 263 a | 375 ± 71.8 a | 273 ± 42.8 a |
| UVC | 1.84 ± 0.26 ab | 2.77 ± 0.44 a | 3260 ± 618 b | 1224 ± 217 a | 365 ± 75.8 a | 234 ± 48.7 a |
| Ultraviolet irradiation time (UIT) | ||||||
| 0 | 1.59 ± 0.13 b | 2.67 ± 0.18 a | 3580 ± 401 a | 1365 ± 179 ab | 353 ± 33.2 ab | 254 ± 76.5 ab |
| 5 min | 1.81 ± 0.12 ab | 2.91 ± 0.37 a | 3644 ± 571 a | 1253 ± 217 b | 338 ± 37.9 b | 239 ± 38.5 b |
| 10 min | 1.96 ± 0.35 a | 2.93 ± 0.34 a | 3777 ± 833 a | 1473 ± 221 a | 404 ± 78.9 a | 278 ± 43.7 a |
| Storage period (SP) | ||||||
| Day 0 | 1.98 ± 0.30 a | 2.58 ± 0.20 c | 3584 ± 641 a | 1387 ± 188 ab | 320 ± 37.6 b | 255 ± 28.3 a |
| Day 5 | 1.85 ± 0.27 ab | 2.91 ± 0.20 b | 3904 ± 786 a | 1482 ± 264 a | 390 ± 66.2 a | 276 ± 47.7 a |
| Day 10 | 1.71 ± 0.19 b | 3.16 ± 0.31 a | 3587 ± 559 a | 1221 ± 170 b | 396 ± 63.0 a | 243 ± 46.5 a |
| Level of significance | ||||||
| ULS | * | ns | * | ns | ns | ns |
| UIT | ** | ns | ns | * | ** | * |
| SP | ** | ** | ns | ** | ** | ns |
| Ultraviolet light sources (ULS) | Sodium (mg 100 g−1) | Iron (mg 100 g−1) | Zinc (mg 100 g−1) | Manganese (mg 100 g−1) | ||
| Control | 137 ± 31.4 a | 63.4 ± 13.7 a | 4.14 ± 1.25 a | 5.42 ± 0.67 a | ||
| UVA | 101 ± 44.4 a | 46.7 ± 11.1 b | 4.62 ± 3.14 a | 5.94 ± 1.13 a | ||
| UVB | 135 ± 32.3 a | 45.5 ± 10.2 b | 5.21 ± 2.34 a | 5.95 ± 1.29 a | ||
| UVC | 118 ± 47.3 a | 45.1 ± 10.9 b | 4.46 ± 1.94 a | 5.49 ± 1.21 a | ||
| Ultraviolet irradiation time (UIT) | ||||||
| 0 | 137 ± 31.4 a | 63.4 ± 13.7 a | 4.14 ± 1.25 ab | 5.42 ± 0.67 b | ||
| 5 min | 103 ± 45.6 b | 29.8 ± 6.75 b | 3.37 ± 1.09 b | 5.01 ± 0.60 b | ||
| 10 min | 133 ± 35.1 a | 61.7 ± 18.1 a | 6.16 ± 2.70 a | 6.58 ± 1.13 a | ||
| Storage period (SP) | ||||||
| Day 0 | 114 ± 21.2 a | 39.6 ± 8.41 b | 3.26 ± 1.21 b | 5.27 ± 0.35 b | ||
| Day 5 | 132 ± 40.9 a | 52.5 ± 11.7 a | 5.58 ± 3.06 a | 6.32 ± 1.46 a | ||
| Day 10 | 115 ± 31.3 a | 52.7 ± 12.6 a | 5.18 ± 1.74 ab | 5.64 ± 1.12 ab | ||
| Level of significance | ||||||
| ULS | ns | ** | ns | ns | ||
| UIT | * | ** | ** | ** | ||
| SP | ns | ** | * | * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kibar, H.; Kibar, B. Comparison of Ultraviolet A, B and C Treatments in Preserving the Quality and Nutritional Integrity of Fresh-Cut Spinach. Foods 2025, 14, 1374. https://doi.org/10.3390/foods14081374
Kibar H, Kibar B. Comparison of Ultraviolet A, B and C Treatments in Preserving the Quality and Nutritional Integrity of Fresh-Cut Spinach. Foods. 2025; 14(8):1374. https://doi.org/10.3390/foods14081374
Chicago/Turabian StyleKibar, Hakan, and Beyhan Kibar. 2025. "Comparison of Ultraviolet A, B and C Treatments in Preserving the Quality and Nutritional Integrity of Fresh-Cut Spinach" Foods 14, no. 8: 1374. https://doi.org/10.3390/foods14081374
APA StyleKibar, H., & Kibar, B. (2025). Comparison of Ultraviolet A, B and C Treatments in Preserving the Quality and Nutritional Integrity of Fresh-Cut Spinach. Foods, 14(8), 1374. https://doi.org/10.3390/foods14081374





