Phytochemical Profiling, Antioxidant Activity, Food Preservation, and Insecticidal Properties of Origanum syriacum and Cymbopogon winterianus Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Chemicals and Reagents
2.3. Powder Preparation
2.4. Extract Preparation
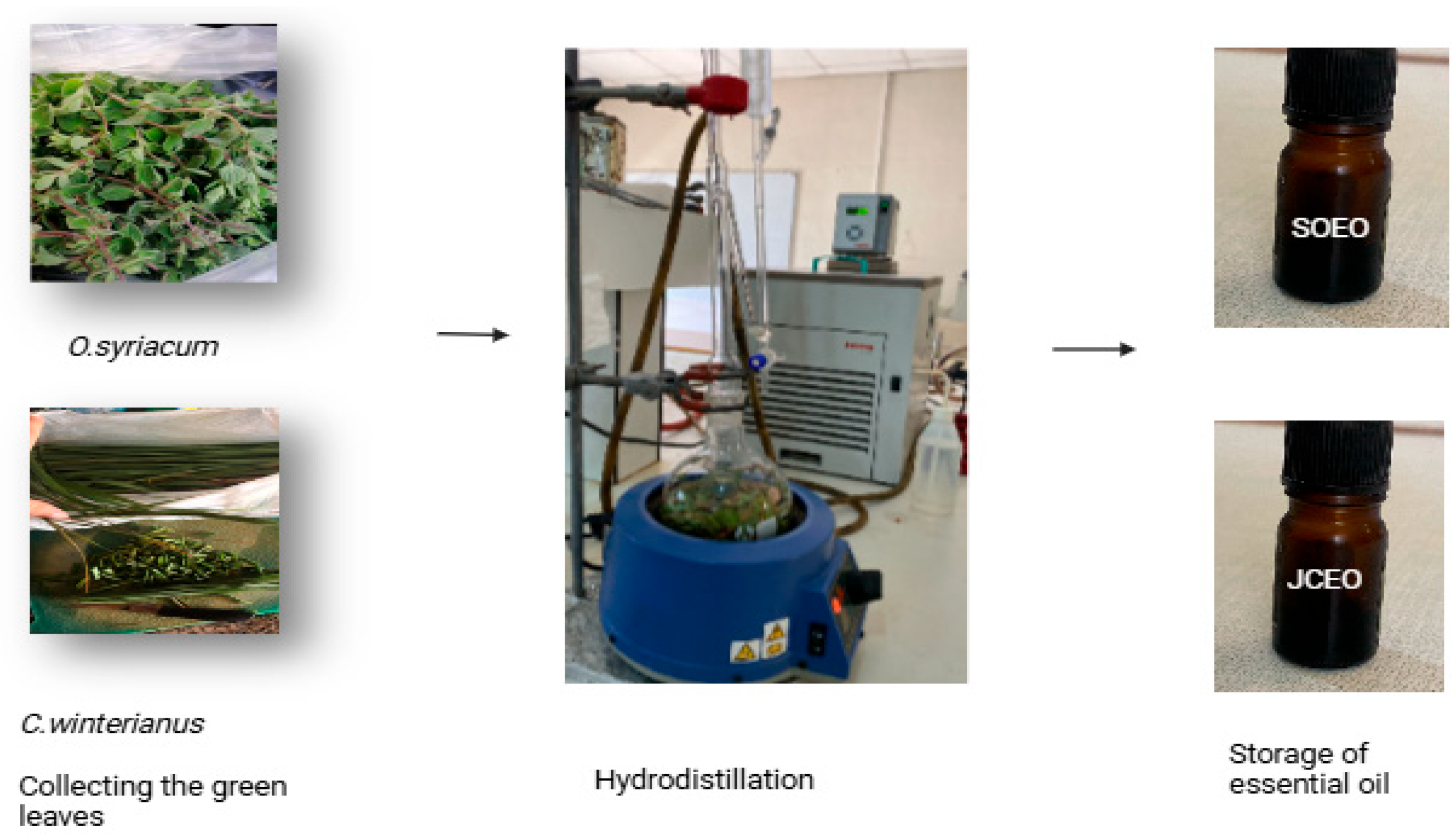
2.5. Essential Oil Extraction
2.5.1. Relative Density of the Essential Oil
2.5.2. Refractive Index of the Essential Oil
2.5.3. Chromatographic Analysis of the Essential Oil
2.6. Qualitative Analysis–Phytochemical Screening
2.7. Quantitative Analysis
2.7.1. Total Phenolic Content (TPC)
2.7.2. Total Flavonoid Content (TFC)
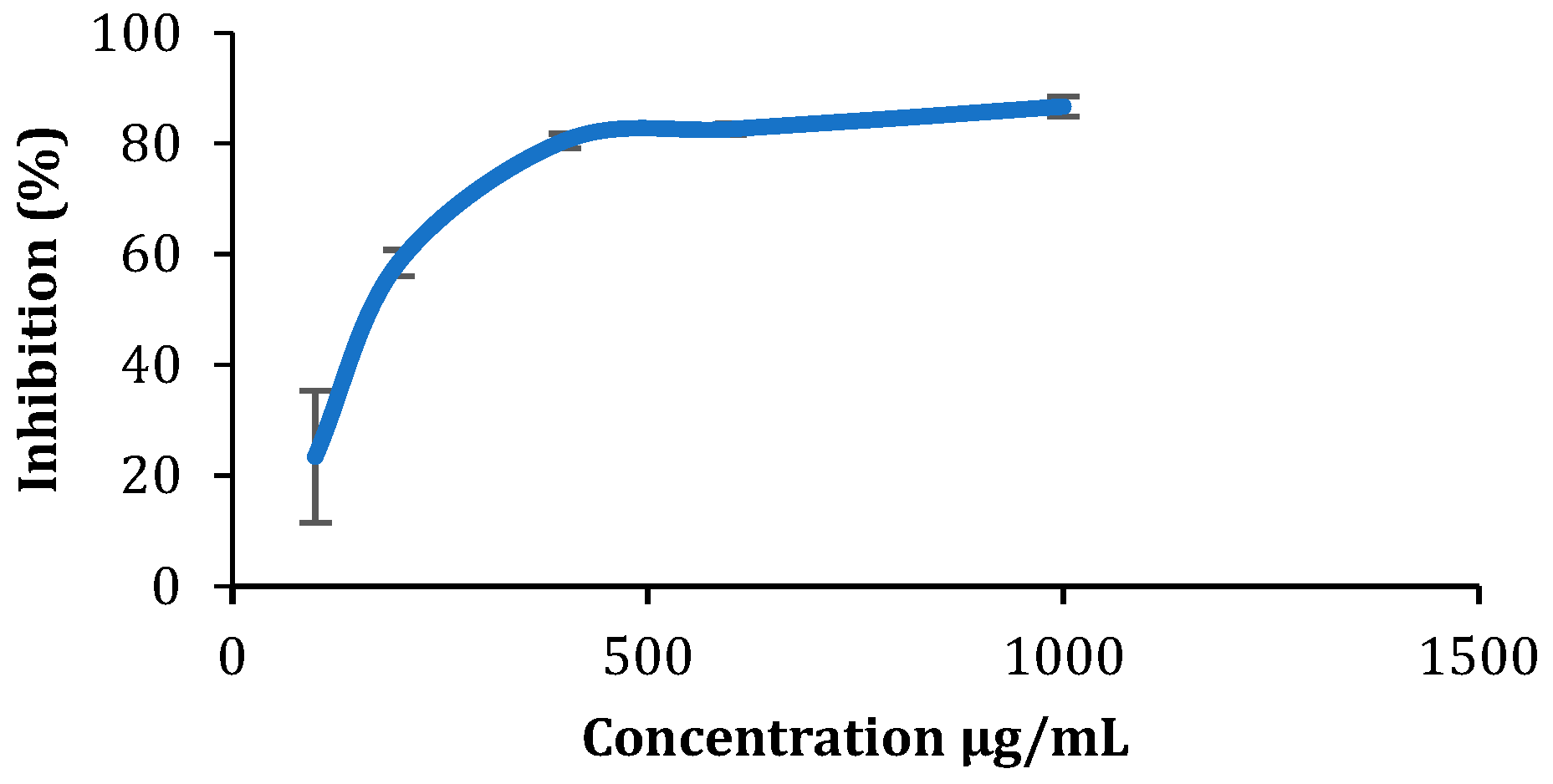
2.8. Antioxidant Activity: DPPH Assay
2.9. Food Preservation
2.9.1. Flatbread Preparation Method
2.9.2. Flatbread Moisture Content
2.9.3. Sensory Evaluation
2.10. Insecticidal Activity
2.10.1. Preparation of Dishes
2.10.2. Preparation of Solutions
3. Results and Discussion
3.1. Essential Oil Yield and Characteristics
3.2. Essential Oil Chemical Composition
3.3. Qualitative Analysis–Phytochemical Screening
3.4. Quantitative Study
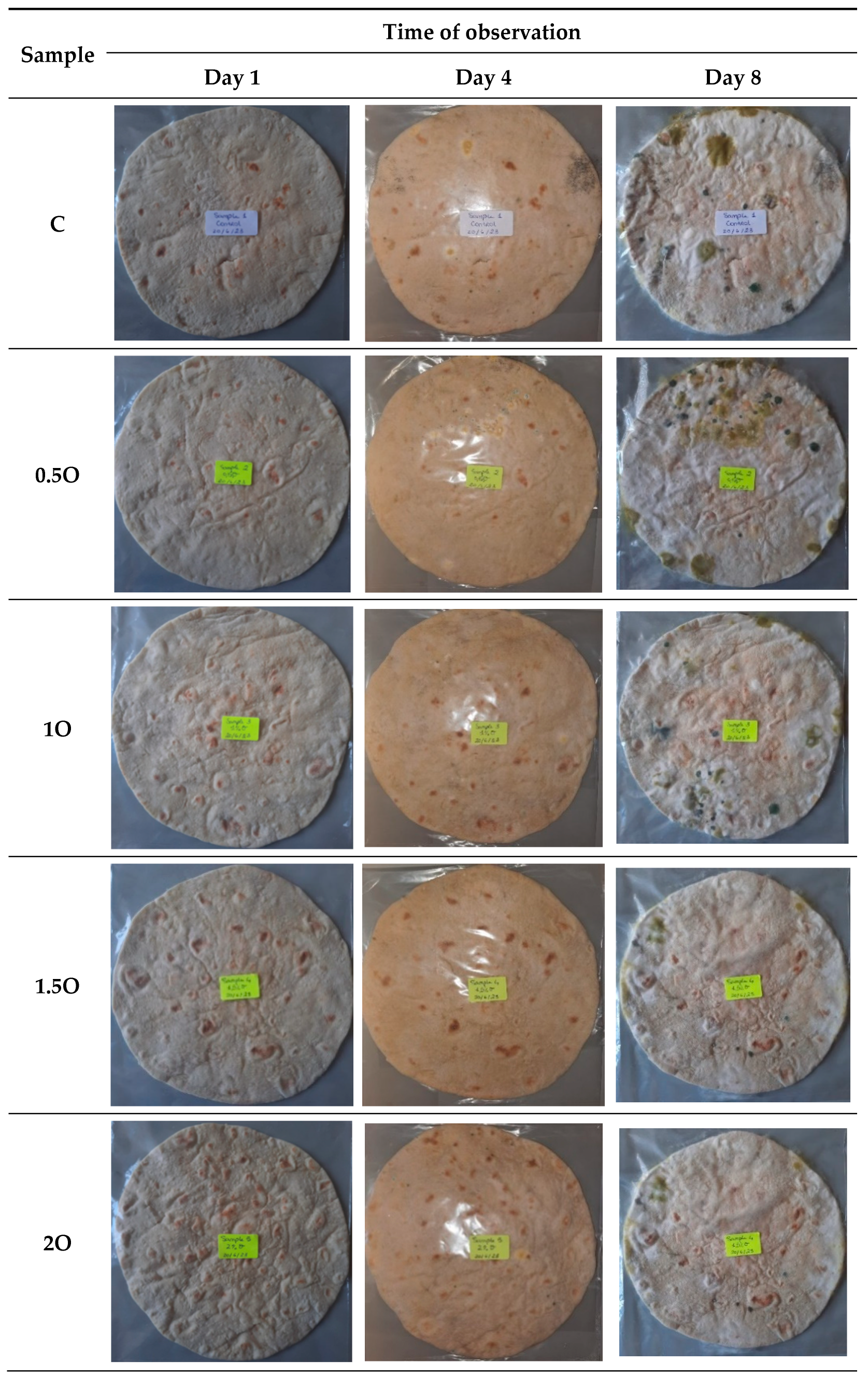
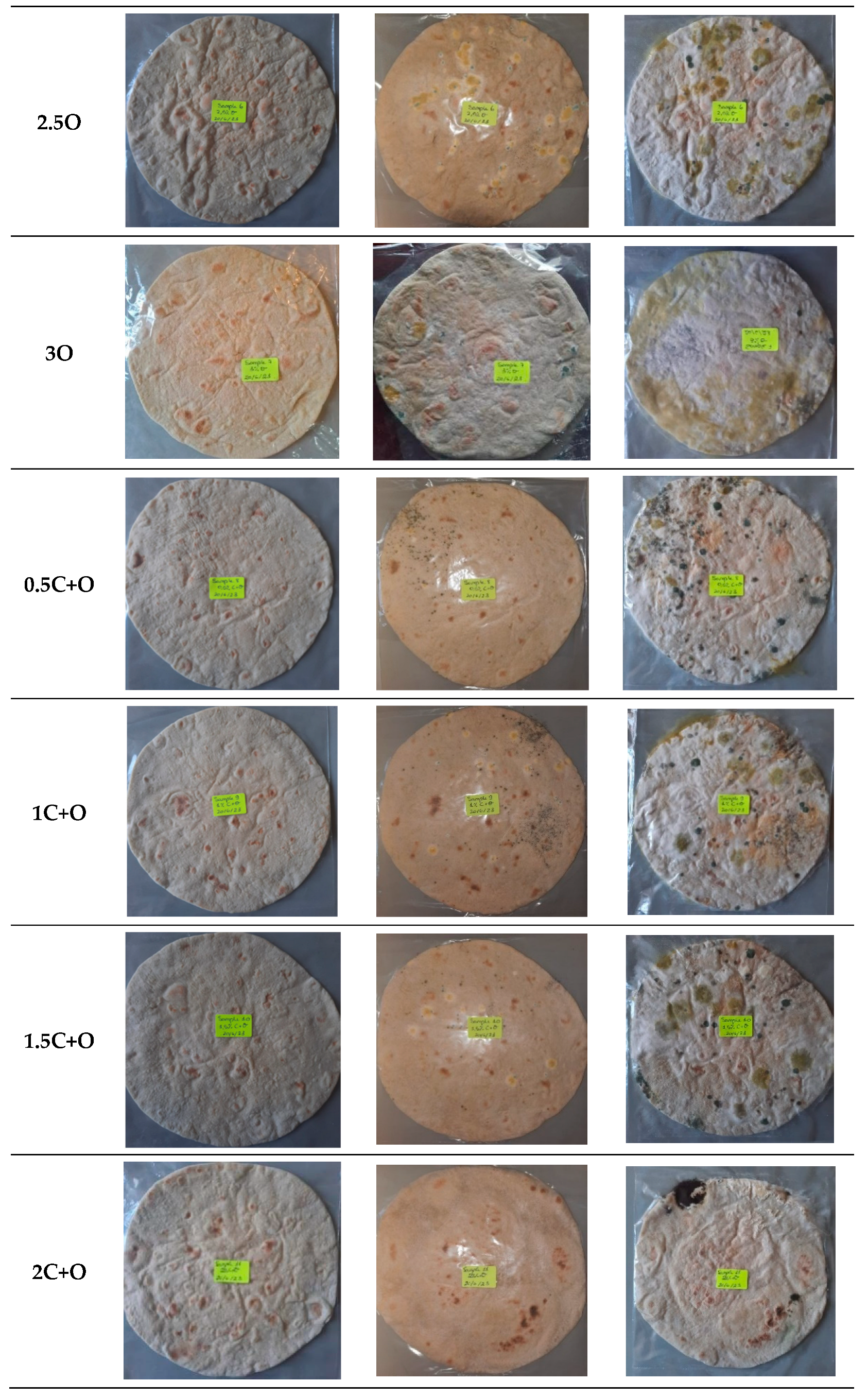
3.5. Oregano and Java Citronella Powders as Food Preservatives in Flatbread
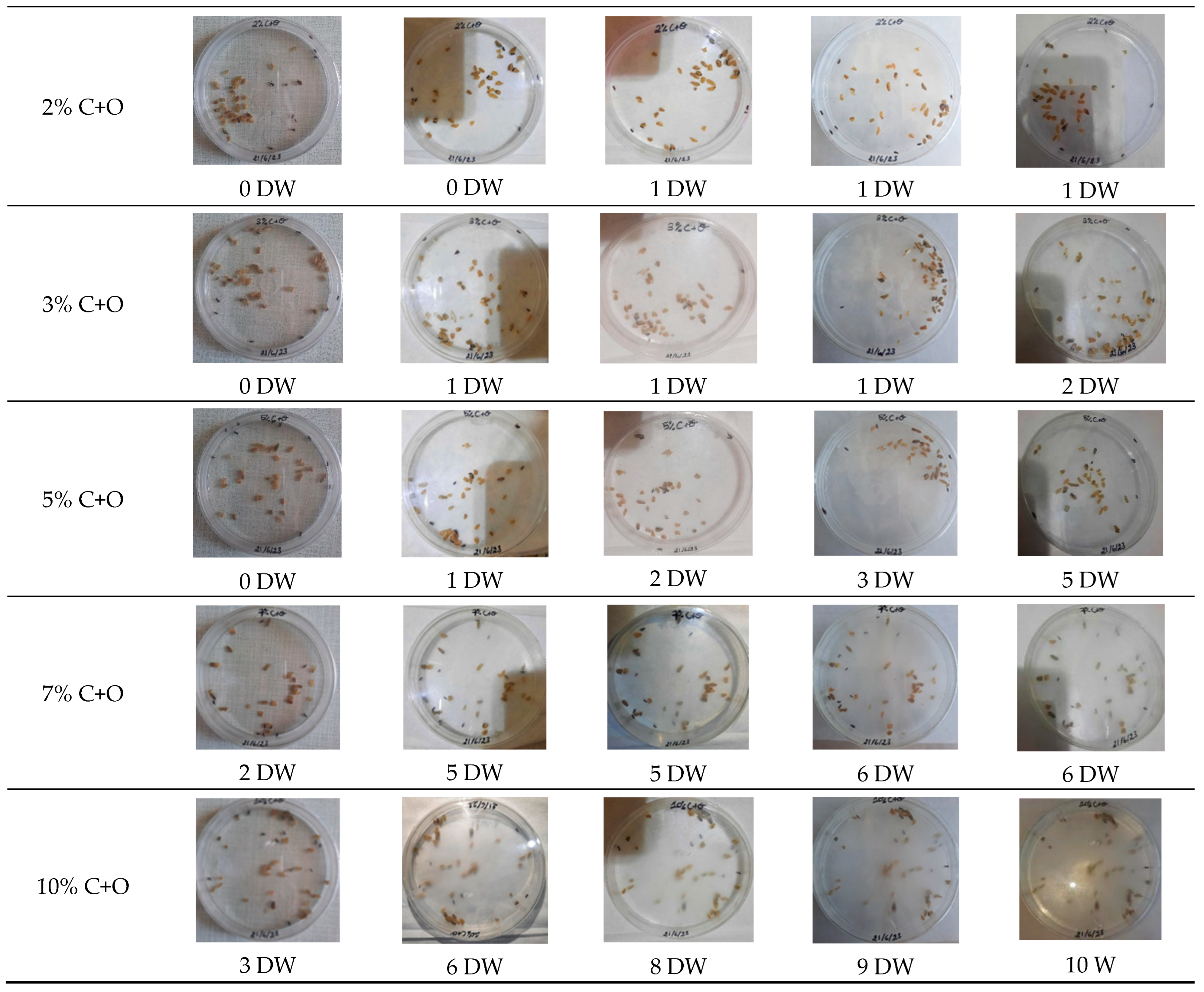
3.5.1. Visual Analysis over Time
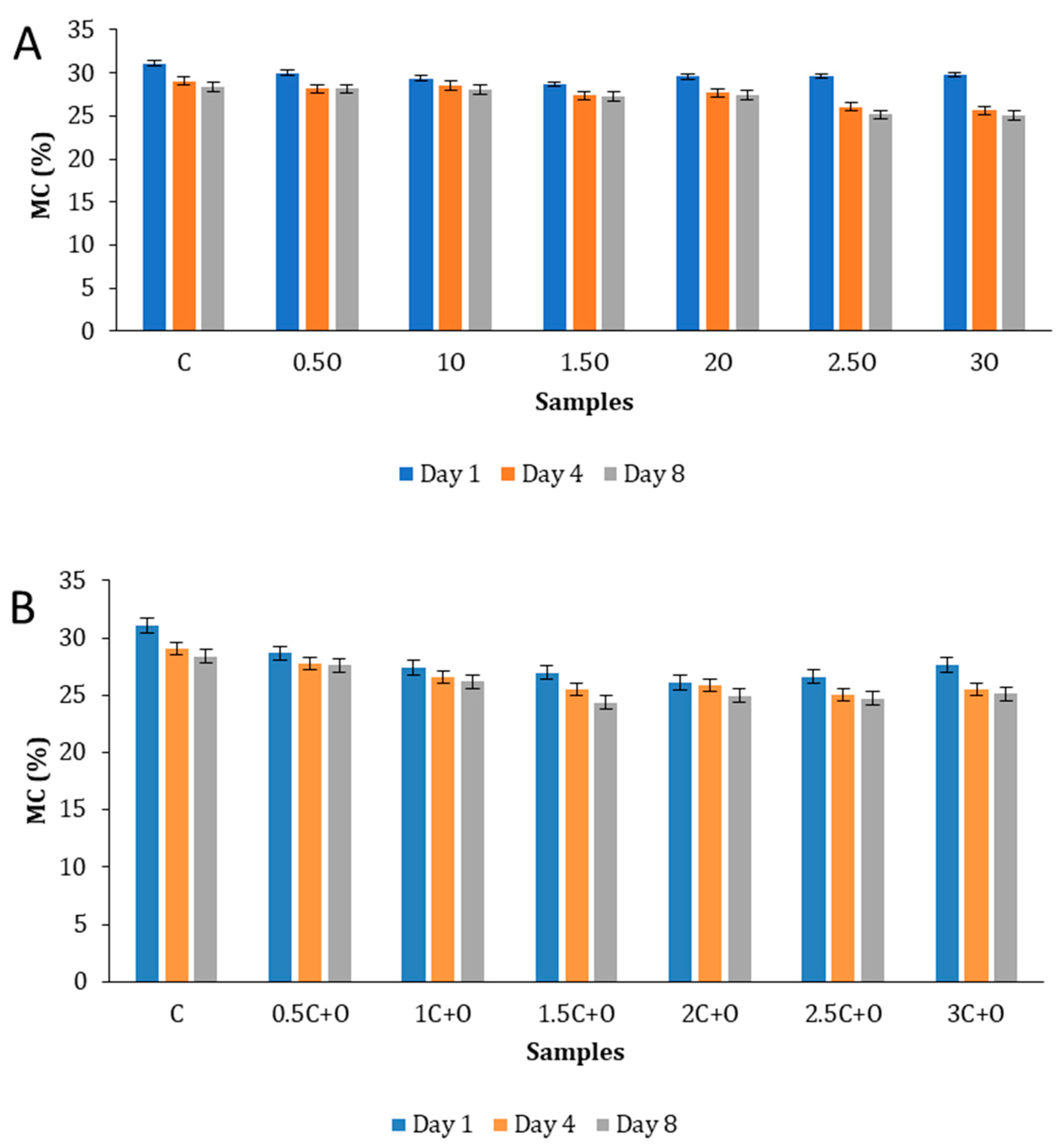
3.5.2. Flatbread Moisture Content
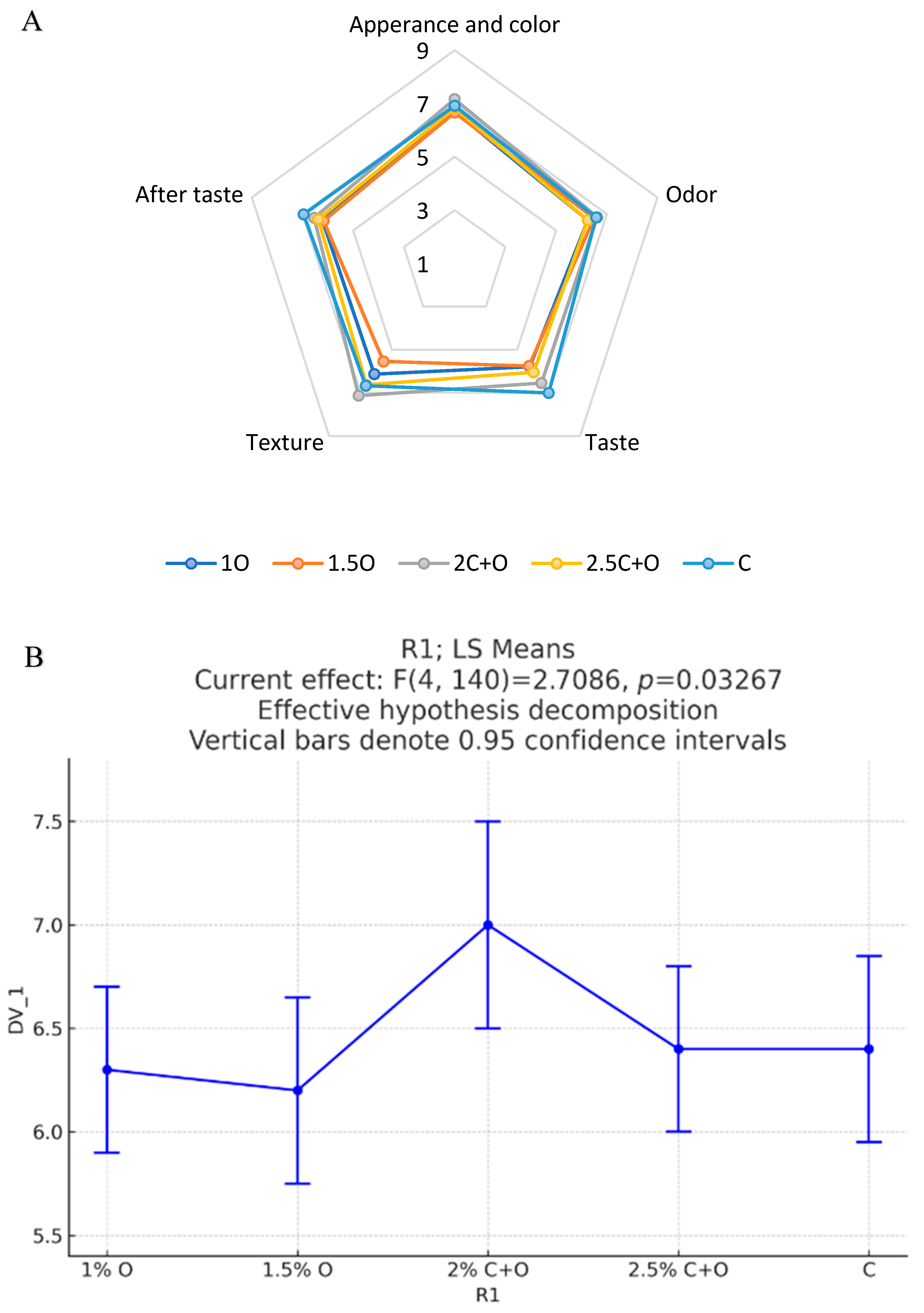
3.5.3. Flatbread Sensory Analysis
3.6. Essential Oils for Pest Control
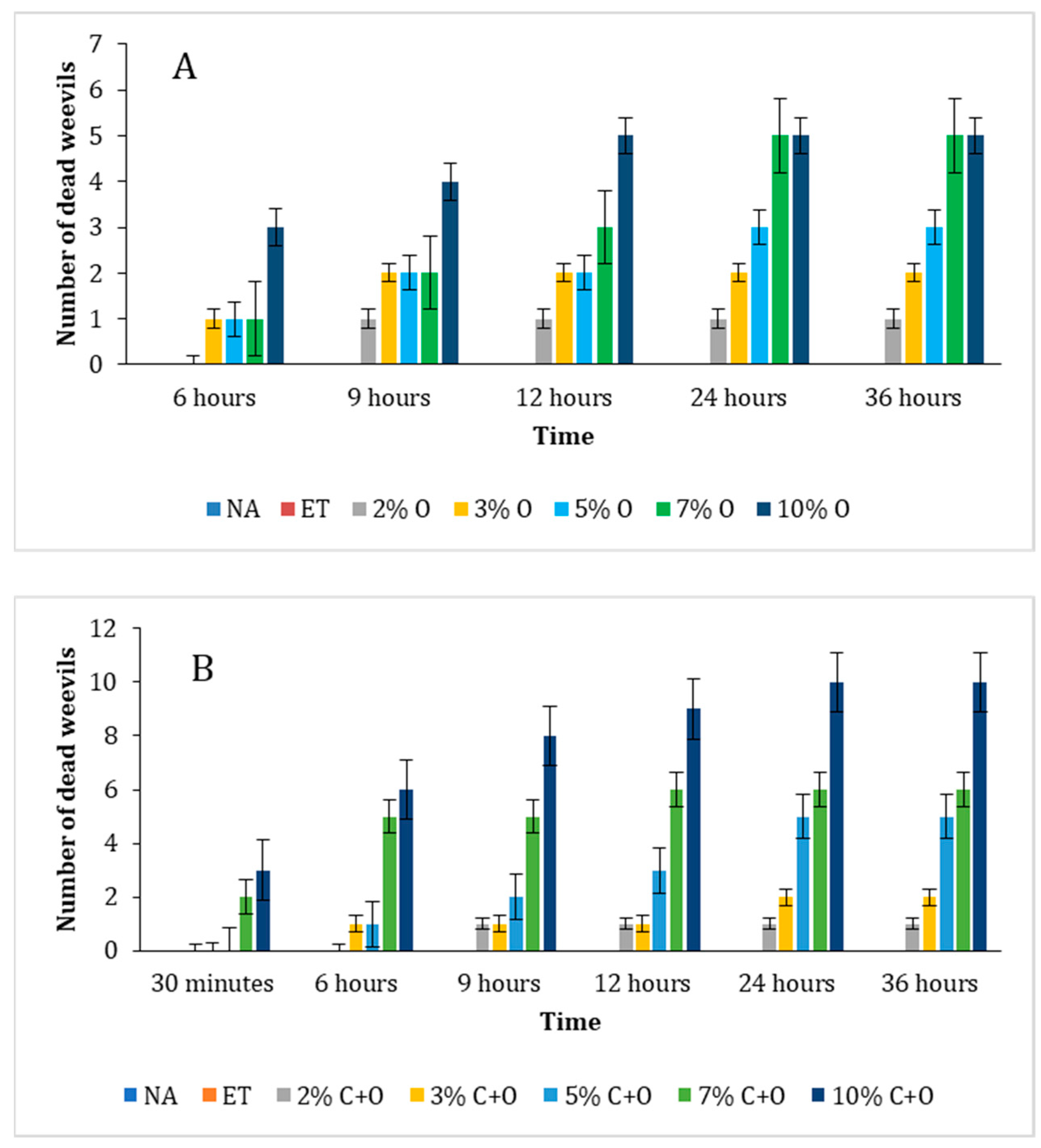
3.6.1. Visual Assessment over Time
3.6.2. Determination of Lethal Time 50 (LT50)
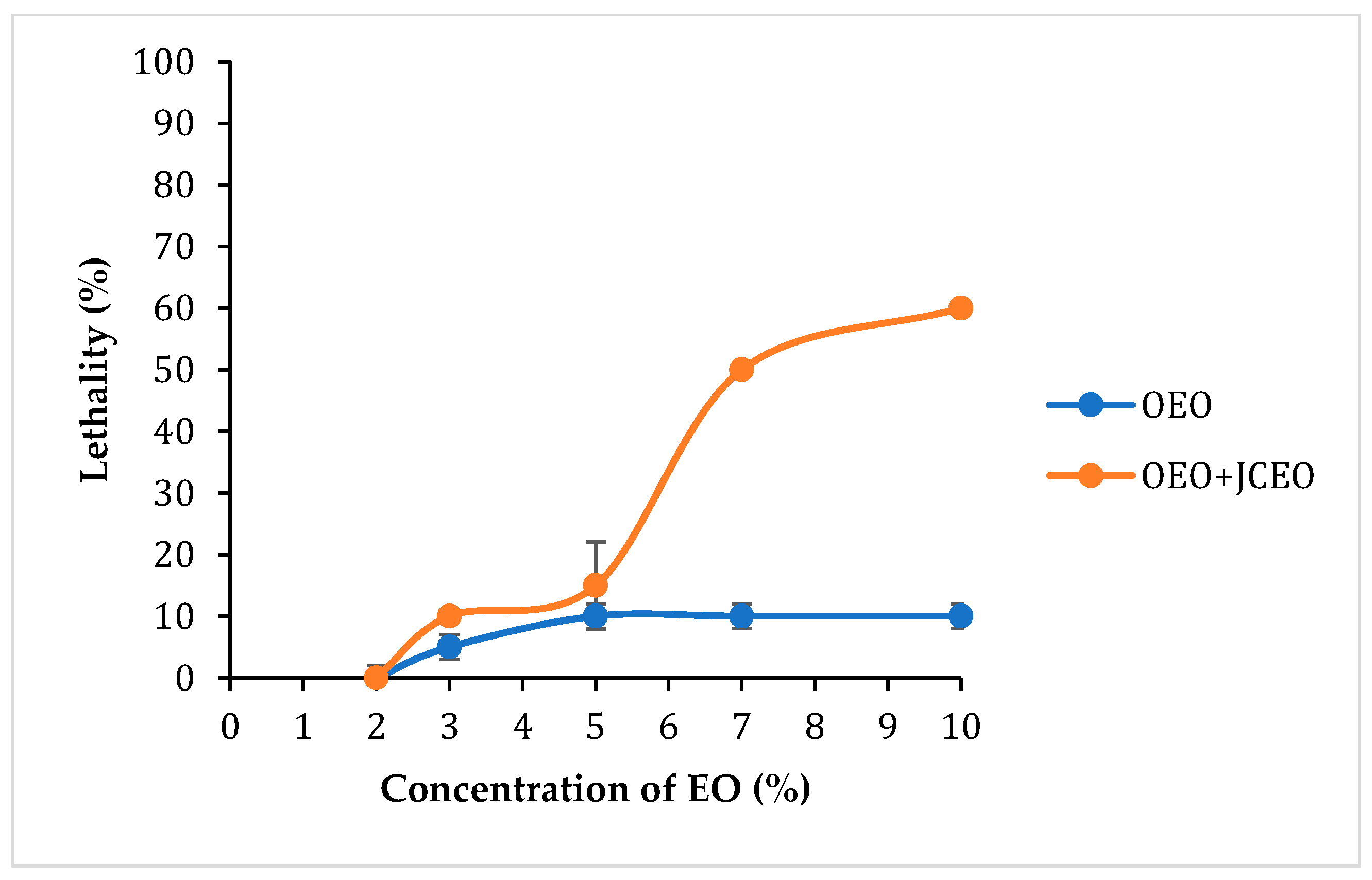
3.6.3. Determination of Lethal Dose 50 (LD50)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escudero-Gilete, M.L.; Meléndez-Martínez, A.J.; Heredia, F.J.; Vicario, I.M. Optimization of Olive-Fruit Paste Production Using a Methodological Proposal Based on a Sensory and Objective Color Analysis. Grasas Aceites 2009, 60, 396–404. [Google Scholar] [CrossRef]
- Ortega-Ramirez, L.A.; Rodriguez-Garcia, I.; Leyva, J.M.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Siddiqui, M.W.; Ayala-Zavala, J.F. Potential of Medicinal Plants as Antimicrobial and Antioxidant Agents in Food Industry: A Hypothesis. J. Food Sci. 2014, 79, R129–R137. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G. How Are Medicinal Plants Useful When Added to Foods? Medicines 2020, 7, 58. [Google Scholar] [CrossRef]
- Alwafa, R.A.; Mudalal, S.; Mauriello, G. Origanum syriacum L. (Za’atar), from Raw to Go: A Review. Plants 2021, 10, 1001. [Google Scholar] [CrossRef]
- Mesmar, J.; Abdallah, R.; Badran, A.; Maresca, M.; Baydoun, E. Origanum syriacum Phytochemistry and Pharmacological Properties: A Comprehensive Review. Molecules 2022, 27, 4272. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Franz, C.; Novak, J. Composition of Essential Oil Compounds from Different Syrian Populations of Origanum syriacum L. (Lamiaceae). J. Agric. Food Chem. 2009, 57, 1362–1365. [Google Scholar] [CrossRef]
- El-Alam, I.; Zgheib, R.; Iriti, M.; El Beyrouthy, M.; Hattouny, P.; Verdin, A.; Fontaine, J.; Chahine, R.; Lounès-Hadj Sahraoui, A.; Makhlouf, H. Origanum syriacum Essential Oil Chemical Polymorphism According to Soil Type. Foods 2019, 8, 900. [Google Scholar] [CrossRef]
- Alma, M.H.; Mavi, A.; Yildirim, A.; Digrak, M.; Hirata, T. Screening Chemical Composition and in Vitro Antioxidant and Antimicrobial Activities of the Essential Oils from Origanum syriacum L. Growing in Turkey. Biol. Pharm. Bull. 2003, 26, 1725–1729. [Google Scholar] [CrossRef]
- Rammal, M.; Khreiss, S.; Badran, A.; Mezher, A.; Bechelany, M.; Haidar, C.; Khalil, M.; Baydoun, E.; El-Dakdouki, M. Antibacterial and Antifungal Activities of Cimbopogon winterianus and Origanum syriacum Extracts and Essential Oils against Uropathogenic Bacteria and Foodborne Fungal Isolates. Foods 2024, 13, 1684. [Google Scholar] [CrossRef]
- Tanu; Prakash, A.; Adholeya, A. Effect of Different Organic Manures/Composts on the Herbage and Essential Oil Yield of Cymbopogon winterianus and Their Influence on the Native AM Population in a Marginal Alfisol. Bioresour. Technol. 2004, 92, 311–319. [Google Scholar] [CrossRef]
- Rodrigues, K.A.D.F.; Dias, C.N.; do Amaral, F.M.M.; Moraes, D.F.C.; Mouchrek Filho, V.E.; Andrade, E.H.A.; Maia, J.G.S. Molluscicidal and Larvicidal Activities and Essential Oil Composition of Cymbopogon winterianus. Pharm. Biol. 2013, 51, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Verma, S.K.; Tandon, S.; Padalia, R.C.; Darokar, M.P. Chemical Composition and Antimicrobial Activity of Java Citronella (Cymbopogon winterianus Jowitt Ex Bor) Essential Oil Extracted by Different Methods. J. Essent. Oil Res. 2020, 32, 449–455. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B.; Manivel, P.; Kumar, J. Valorization of Java Citronella (Cymbopogon wnterianus Jowitt) Distillation Waste as a Potential Source of Phenolics/Antioxidant: Influence of Extraction Solvents. J. Food Sci. Technol. 2021, 58, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Awulachew, M.T. Bread Deterioration and a Way to Use Healthful Methods. CyTA-J. Food 2024, 22, 2424848. [Google Scholar] [CrossRef]
- Dolea, D.; Rizo, A.; Fuentes, A.; Barat, J.M.; Fernández-Segovia, I. Effect of Thyme and Oregano Essential Oils on the Shelf life of Salmon and Seaweed Burgers. Food Sci. Technol. Int. 2018, 24, 394–403. [Google Scholar] [CrossRef]
- Olmedo, R.H.; Valeria Nepote, V.; Grosso, N.R. Preservation of Sensory and Chemical Properties in Flavoured Cheese Prepared with Cream Cheese Base Using Oregano and Rosemary Essential Oils. LWT-Food Sci. Technol. 2013, 53, 409–417. [Google Scholar] [CrossRef]
- Ozaki, M.M.; Dos Santos, M.; Ribeiro, W.O.; de Azambuja Ferreira, N.C.; Picone, C.S.F.; Domínguez, R.; Lorenzo, J.M.; Pollonio, M.A.R. Radish Powder and Oregano Essential Oil as Nitrite Substitutes in Fermented Cooked Sausages. Food Res. Int. 2021, 140, 109855. [Google Scholar] [CrossRef]
- ISO 279:1998; Essential Oils—Determination of Relative Density at 20 °C—Reference Method. International Organization for Standardization: Geneva, Switzerland, 1998. Available online: https://www.iso.org/standard/25308.html (accessed on 25 March 2025).
- Zenebe, H.; Afework, M.; Gopalakrishnan, V.K.; Krishna Chaithanya, K.; Nagaraju, B. Chemical Composition and Physico-chemical Properties of Essential Oil from Myrtus communis. Int. J. Pharm. Clin. Res. 2017, 9, 439–443. [Google Scholar]
- Adediwura, F.-J.; Ayotunde, A. Phytochemical and Pharmacognostic Studies of Telosma africanum (N.E.Br) Colville Leaf and Stem. Int. J. Pharm. Sci. Res. 2012, 3, 1860–1862. [Google Scholar]
- Ansari, P.; Azam, S.; Reyad-ul-ferdous, M.; Hossain, A.; Azad, T.; Goswami, S.; Mondal, K.K. Potential Investigation of Anti-Inflammatory Activity and Phytochemical Investigations of Ethanolic Extract of Glycosmis Pentaphylla Leaves. Am. J. Biomed. Res. 2015, 3, 6–8. [Google Scholar]
- Kumar, V.; Anjana; Poonam; Sharma, S. Latest Overview of Proteases: A Review. Asian J. Adv. Basic Sci. 2019, 7, 20–28. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Devi, T.; Bono, A.; Sarbatly, R. Studies on Phytochemical Constituents of Six Malaysian Medicinal Plants. J. Med. Plants Res. 2009, 3, 67–72. [Google Scholar]
- Aiyegoro, O.A.; Okoh, A.I. Preliminary Phytochemical Screening and in Vitro Antioxidant Activities of the Aqueous Extract of Helichrysum Longifolium DC. BMC Complement. Altern. Med. 2010, 10, 21. [Google Scholar] [CrossRef]
- Rammal, M.; Badran, A.; Haidar, C.; Sabbah, A.; Bechelany, M.; Awada, M.; Hassan, K.H.; El-Dakdouki, M.; Raad, M.T. Cymbopogon winterianus (Java Citronella Plant): A Multi-Faceted Approach for Food Preservation, Insecticidal Effects, and Bread Application. Foods 2024, 13, 803. [Google Scholar] [CrossRef]
- Khandelwal, K.R. Practical Pharmacognosy: Techniques and Experiments. In Nirali Prakashan; ScienceOpen Inc.: Berlin, Germany, 2008. [Google Scholar]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.-C.; Bailleul, F.; Trotin, F. Phenolic Compounds and Antioxidant Activities of Buckwheat (Fagopyrum esculentum Moench) Hulls and Flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Mir, M.A.; Sawhney, S.S.; Jassal, M.M.S. Qualitative and Quantitative Analysis of Phytochemicals of Taraxacum officinale. Wudpecker J. Pharm. Pharmocology 2013, 2, 1–5. [Google Scholar]
- Rammal, H.; Bouayed, J.; Hijazi, A.; Ezzedine, M.; Rachid Soulimani, R. Scavenger Capacity of Momordica charantia for Reactive Oxygen Species. J. Nat. Prod. 2012, 5, 54–59. [Google Scholar]
- Sayed Ahmad, B.; Talou, T.; Straumite, E.; Sabovics, M.; Kruma, Z.; Saad, Z.; Hijazi, A.; Merah, O. Protein Bread Fortification with Cumin and Caraway Seeds and By-Product Flour. Foods 2018, 7, 28. [Google Scholar] [CrossRef]
- Sarah, M.; Ardiansyah, D.; Misran, E.; Madinah, I. Extraction of Citronella Oil from Lemongrass (Cymbopogon winterianus) by Sequential Ultrasonic and Microwave-Assisted Hydro-Distillation. Alex. Eng. J. 2023, 70, 569–583. [Google Scholar] [CrossRef]
- Farhat, M.; Tóth, J.; Héthelyi, B.É.; Szarka, S.; Czigle, S. Analysis of the Essential Oil Compounds of Origanum syriacum L. Acta Fac. Pharm. Univ. Comen. 2012, 59, 6–14. [Google Scholar] [CrossRef][Green Version]
- Fleisher, A.; Fleisher, Z. Chemical Composition of Origanum syriacum L. Essential Oil: Aromatic Plants of the Holy Land and the Sinai, Part V. J. Essent. Oil Res. 1991, 3, 121–123. [Google Scholar] [CrossRef]
- Das, S.; Prakash, B. Effect of Environmental Factors on Essential Oil Biosynthesis, Chemical Stability, and Yields. In Plant Essential Oils; Prakash, B., Dubey, N.K., Freitas Brilhante de São José, J., Eds.; Springer: Singapore, 2024; pp. 225–247. [Google Scholar]
- Nwozo, O.S.; Effiong, E.M.; Aja, P.M.; Awuchi, C.G. Antioxidant, Phytochemical, and Therapeutic Properties of Medicinal Plants: A Review. Int. J. Food Prop. 2023, 26, 359–388. [Google Scholar] [CrossRef]
- El-Moneim, A.; Afify, M.R.; Esawy, S.; Abdel-Salam, M. Antioxidant content and cytotoxicity of Origanum syriacum L. Adv. Food Sci. 2014, 13, 36. [Google Scholar]
- Majzoobi, M.; Ghavi, F.S.; Farahnaky, A.; Jamalian, J.; Mesbahi, G. Effect of Tomato Pomace Powder on The Physicochemical properties of Flat Bread (Barbari Bread): Effect of Tomato Pomace on Properties of Flat Bread. J. Food Process. Preserv. 2011, 35, 247–256. [Google Scholar] [CrossRef]
- OuYang, Q.; Liu, Y.; Oketch, O.R.; Zhang, M.; Shao, X.; Tao, N. Citronellal Exerts Its Antifungal Activity by Targeting Ergosterol Biosynthesis in Penicillium digitatum. J. Fungi 2021, 7, 432. [Google Scholar] [CrossRef]
- Vaičiulytė, V.; Ložienė, K.; Taraškevičius, R. Impact of Edaphic and Climatic Factors on Thymus pulegioides Essential Oil Composition and Potential Prevalence of Chemotypes. Plants 2022, 11, 2536. [Google Scholar] [CrossRef]
- Martins, G.A.; Bicas, J.L. Antifungal Activity of Essential Oils of Tea Tree, Oregano, Thyme, and Cinnamon, and their Components. Braz. J. Food Technol. 2024, 27, e2023071. [Google Scholar] [CrossRef]
- Quail, K.J. Flatbreads of the World. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Putri, D.P.; Yulianti, L.E.; Afifah, N. Accelerated Shelf Life Testing of Mocatilla Chip Using Critical Moisture Content Approach and Models of Sorption Isotherms. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012023. [Google Scholar] [CrossRef]
- Dhillon, G.K.; Ahluwalia, P. Effect of Oregano Herb on Dough Rheology and Bread Quality. Int J. Food Sci. Nutr. Diet. 2013, 2, 40–44. [Google Scholar]
- Oliveira, J.A.C.; Fernandes, L.A.; Figueiredo, K.G.; Corrêa, E.J.A.; Lima, L.H.F.; Alves, D.S.; Bertolucci, S.K.V.; Carvalho, G.A. Essential Oils on Biological Characteristics and Potential Molecular Targets in Spodoptera frugiperda. Plants 2024, 13, 180. [Google Scholar] [CrossRef]
- Aryal, S.; Poudel, A.; Kapil Kafle, K.; Aryal, L.K. Insecticidal toxicity of essential oil of Nepalese Acorus calamus (Acorales:Acoraceae) against Sitophilus zeamais (Coleoptera:Curculionidae). Heliyon 2023, 9, e22130. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, D.; Sharma, P.; Pandey, A.; Dhakal, K.; Baral, R.P.; Adhikari, A. Chemical Characterization, Antioxidant and Antibacterial Activity of Essential Oil of Cymbopogon winterianus Jowitt (Citronella) from Western Nepal. Curr. Biotechnol. 2022, 11, 86–91. [Google Scholar] [CrossRef]
- López, V.; Pavela, R.; Gómez-Rincón, C.; Les, F.; Bartolucci, F.; Galiffa, V.; Petrelli, R.; Cappellacci, L.; Maggi, F.; Canale, A.; et al. Efficacy of Origanum syriacum Essential Oil against the Mosquito Vector Culexquinquefasciatus and the Gastrointestinal Parasite Anisakis simplex, with Insights on Acetylcholinesterase Inhibition. Molecules 2019, 24, 2563. [Google Scholar] [CrossRef]
- Lee, B.-H.; Choi, W.-S.; Lee, S.-E.; Park, B.-S. Fumigant Toxicity of Essential Oils and Their Constituent Compounds towards the Rice Weevil, Sitophilusoryzae (L.). Crop Prot. 2001, 20, 317–320. [Google Scholar] [CrossRef]
- Devi, M.A.; Nameirakpam, B.; Devi, T.B.; Mayanglambam, S.; Singh, K.D.; Sougrakpam, S.; Shadia, S.; Tongbram, M.; Singh, S.D.; Sahoo, D.; et al. Chemical Compositions and Insecticidal Efficacies of Four Aromatic Essential Oils on Rice Weevil Sitophilus oryzae L. Int. J. Trop. Insect Sci. 2020, 40, 549–559. [Google Scholar] [CrossRef]
- Guettal, S.; Tine, S.; Hamaidia, K.; Tine-Djebbar, F.; Soltani, N. Effect of Citrus limonum Essential Oil against Granary Weevil, Sitophilusgranarius and Its Chemical Composition, Biological Activities and Energy Reserves. Int. J. Trop. Insect Sci. 2021, 41, 1531–1541. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Bartolucci, F.; Canale, A.; Maggi, F. Origanum syriacum Subsp. Syriacum: From an Ingredient of Lebanese ‘Manoushe’ to a Source of Effective and Eco-Friendly Botanical Insecticides. Ind. Crops Prod. 2019, 134, 26–32. [Google Scholar] [CrossRef]



| Metabolites | Extract | Reagent | Color | Reference |
|---|---|---|---|---|
| Reducing sugar | 0.5 mL | 1 mL water + 5–8 drops Fehling’s (A+B) + boiling (5 min) | Brick-red precipitate | [20] |
| Anthraquinones | 1 mL | 1 mL HCl (10%) + boiling (5 min) | Precipitate | [21] |
| Proteins and amino acids | 1 mL | 1 mL ninhydrin (0.25%) + boiling (5 min) | Blue color | [22] |
| Phlobatannins | 1 mL | 1 mL HCl (1%) + boiling (5 min) + cooling | Red precipitate | [23] |
| Tannins | 1 mL | 1 mL ferric chloride (FeCl3 1%) | Blue color | [24] |
| Resins | 1 mL | 0.5 mL acetone + small amount of water + agitation | Turbidity | [25] |
| Terpenoids | 1 mL | 2 mL chloroform + 1 mL concentrated sulfuric acid | Reddish brown color on the surface | [20] |
| Flavonoids | 1 mL | 5 mL potassium hydroxide (KOH 50%) | Yellow color | [26] |
| Quinones | 1 mL | 1 mL HCl concentrated | Precipitate or yellow color | [21] |
| Sterols and steroids | 1 mL | 2 mL chloroform + 1 mL concentrated sulfuric acid | Red color of the upper layer + greenish yellow fluorescence in the acid layer | [22,27] |
| Diterpenes | 1 mL | Few drops of copper acetate | Green color | [24] |
| Anthocyanins | 1 mL | 1 mL NaOH (10%) | Blue color | [28] |
| Flavanones | 1 mL | concentrated | Purple red color | [28] |
| Cardiac glycosides | 2 mL | 5%) + 1 mL concentrated sulfuric acid | Purple ring + Brown ring + Green ring | [22,28] |
| Saponins | 2 mL | Vigorous shaking (5 min on vortex) | Layer of foam | [20] |
| Phenols | 5 mL | (1%) + ) (1%) | Greenish blue color | [24] |
| Fixed oils and fatty acids | Small amount of extract | On filter paper | Oil spot | [20] |
| Sample Code | Syrian Oregano Powder (%) | Java Citronella Powder (%) |
|---|---|---|
| C | - | - |
| 0.5O | 0.5 | - |
| 1O | 1 | - |
| 1.5O | 1.5 | - |
| 2O | 2 | - |
| 2.5O | 2.5 | - |
| 3O | 3 | - |
| 0.5C+O | 0.25 | 0.25 |
| 1C+O | 0.5 | 0.5 |
| 1.5C+O | 0.75 | 0.75 |
| 2C+O | 1 | 1 |
| 2.5C+O | 1.25 | 1.25 |
| 3C+O | 1.5 | 1.5 |
| Sample Code | SOEO (µL) | JCEO (µL) | Ethanol (µL) |
|---|---|---|---|
| NA | - | - | - |
| ET | - | - | 1000 |
| 2% O | 20 | - | 980 |
| 3% O | 30 | - | 970 |
| 5% O | 50 | - | 950 |
| 7% O | 70 | - | 930 |
| 10% O | 100 | - | 900 |
| 2% C+O | 10 | 10 | 980 |
| 3% C+O | 15 | 15 | 970 |
| 5% C+O | 25 | 25 | 950 |
| 7% C+O | 35 | 35 | 930 |
| 10% C+O | 50 | 50 | 900 |
| Peak | Compound Name | Retention Time (min) | Area Percentage (%) |
|---|---|---|---|
| 1 | α-thujene | 4.73 | 1.65 |
| 2 | 4-carene | 5.37 | 2.49 |
| 3 | p-cymene | 5.64 | 5.31 |
| 4 | Cyclohexene | 5.69 | 0.56 |
| 5 | γ-Terpinene | 6.69 | 9.77 |
| 6 | cis-β-terpineol | 6.82 | 0.46 |
| 7 | Thymol | 15.47 | 0.47 |
| 8 | Carvacrol | 17.75 | 79.30 |
| Metabolites | Aqueous Extract | Ethanolic Extract |
|---|---|---|
| Reducing sugars | ++ | - |
| Anthraquinones | + | + |
| Proteins and amino acids | - | ++ |
| Phlobatannins | + | + |
| Tannins | ++ | ++ |
| Resins | ++ | ++ |
| Terpenoids | ++ | ++ |
| Flavonoids | +++ | +++ |
| Quinones | ++ | ++ |
| Sterols and steroids | - | ++ |
| Diterpenes | - | ++ |
| Anthocyanins | - | - |
| Flavanones | ++ | ++ |
| Cardiac glycosides | ++ | ++ |
| Saponins | ++ | ++ |
| Phenols | ++ | ++ |
| Fixed oils and fatty acids | ++ | ++ |
| Essential Oil Concentration | LT50 |
|---|---|
| 2% O | NA |
| 3% O | NA |
| 5% O | NA |
| 7% O | 24 h |
| 10% O | 12 h |
| 2% C+O | NA |
| 3% C+O | NA |
| 5% C+O | 24 h |
| 7% C+O | 6 h |
| 10% C+O | Between 30 min and 6 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rammal, M.; Kara, M.; Badran, A.; Haidar, C.; Zahreddine, H.; Bassal, H.; Bechelany, M.; El-Dakdouki, M.H.; Hijazi, A. Phytochemical Profiling, Antioxidant Activity, Food Preservation, and Insecticidal Properties of Origanum syriacum and Cymbopogon winterianus Extracts. Foods 2025, 14, 1347. https://doi.org/10.3390/foods14081347
Rammal M, Kara M, Badran A, Haidar C, Zahreddine H, Bassal H, Bechelany M, El-Dakdouki MH, Hijazi A. Phytochemical Profiling, Antioxidant Activity, Food Preservation, and Insecticidal Properties of Origanum syriacum and Cymbopogon winterianus Extracts. Foods. 2025; 14(8):1347. https://doi.org/10.3390/foods14081347
Chicago/Turabian StyleRammal, Marwa, Maya Kara, Adnan Badran, Chaden Haidar, Hawraa Zahreddine, Hussein Bassal, Mikhael Bechelany, Mohammad H. El-Dakdouki, and Akram Hijazi. 2025. "Phytochemical Profiling, Antioxidant Activity, Food Preservation, and Insecticidal Properties of Origanum syriacum and Cymbopogon winterianus Extracts" Foods 14, no. 8: 1347. https://doi.org/10.3390/foods14081347
APA StyleRammal, M., Kara, M., Badran, A., Haidar, C., Zahreddine, H., Bassal, H., Bechelany, M., El-Dakdouki, M. H., & Hijazi, A. (2025). Phytochemical Profiling, Antioxidant Activity, Food Preservation, and Insecticidal Properties of Origanum syriacum and Cymbopogon winterianus Extracts. Foods, 14(8), 1347. https://doi.org/10.3390/foods14081347







