Synergistic Treatment with Ozone Water and Morpholine Fatty Acid Salts Improves Postharvest Quality in Mandarin Oranges
Abstract
1. Introduction
2. Materials and Methods
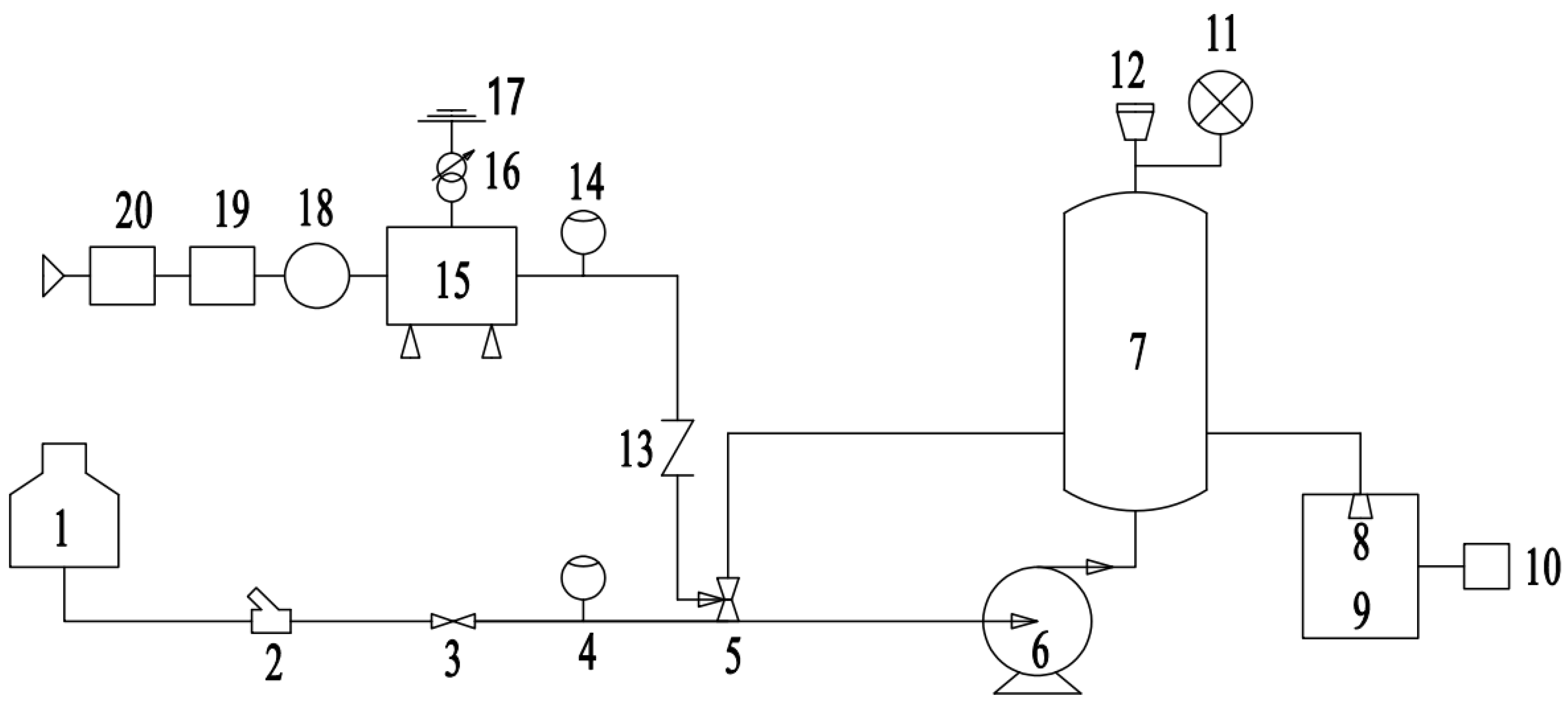
2.1. Ozone Water Generator Principle
2.2. Ozone Water Generator Structure
2.3. Experimental Design for Optimization of Ozone Water Treatment Conditions
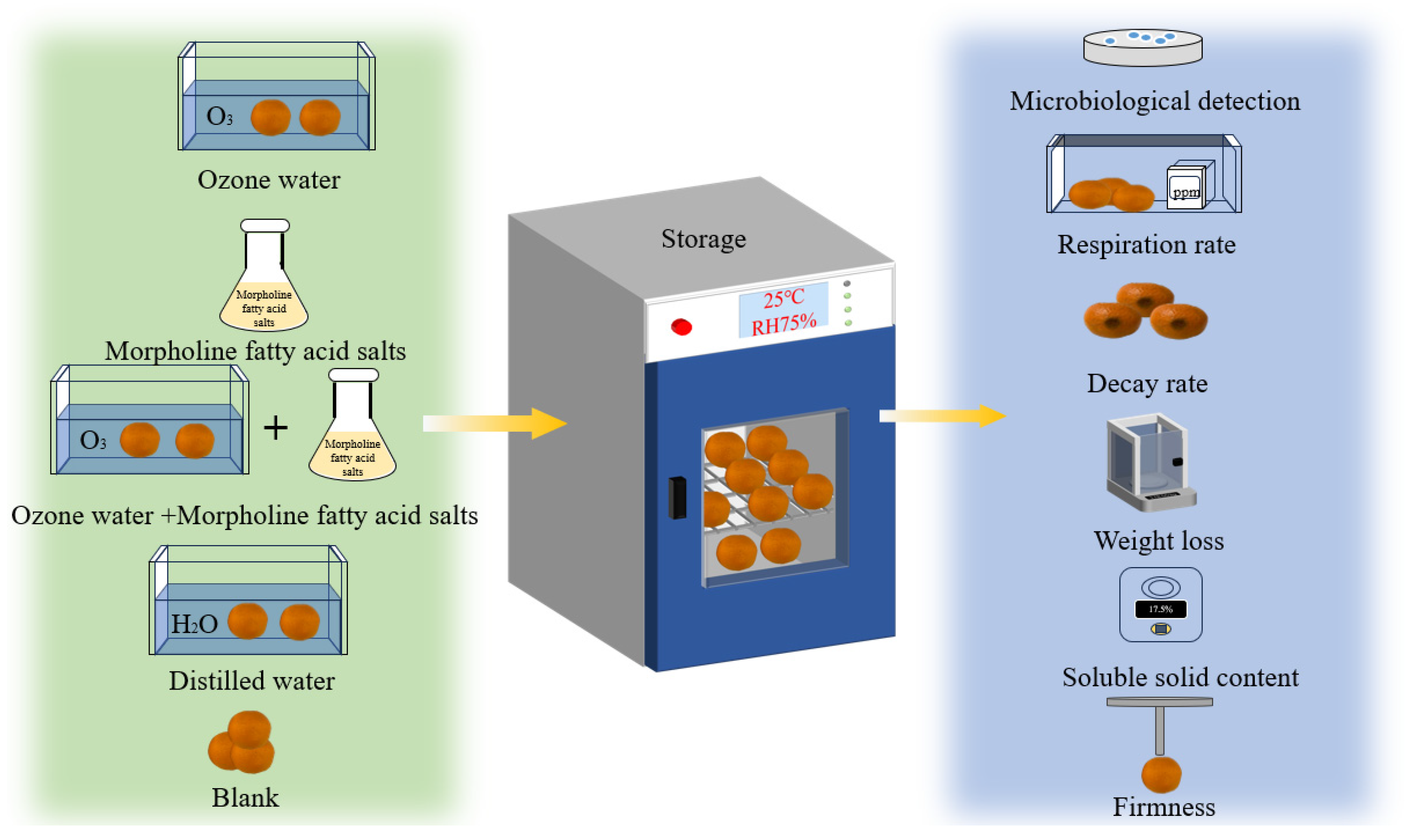
2.4. Different Pretreatment Methods and Detection
2.5. Physical and Chemical Properties of Mandarin Oranges During Storage
2.5.1. Surface Microbial Content Detection Method
2.5.2. Weight Loss Rate Detection Method
2.5.3. Soluble Solids Content Detection Method
2.5.4. Respiration Rate Detection Method
2.5.5. Decay Rate Detection Method
2.5.6. Firmness Detection Method
2.5.7. Morphological Properties Detection Method
2.6. Statistical Analysis
3. Results and Discussion
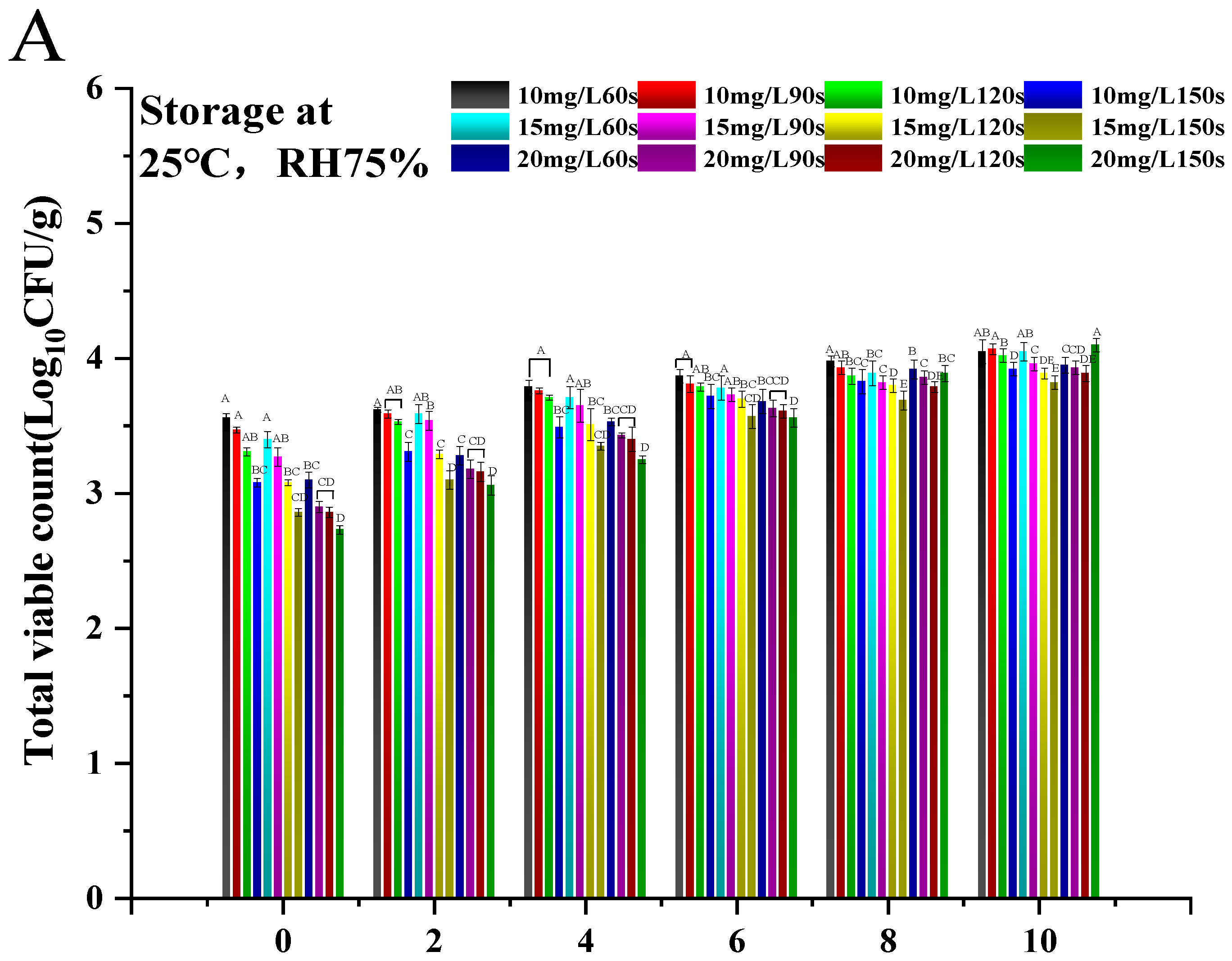
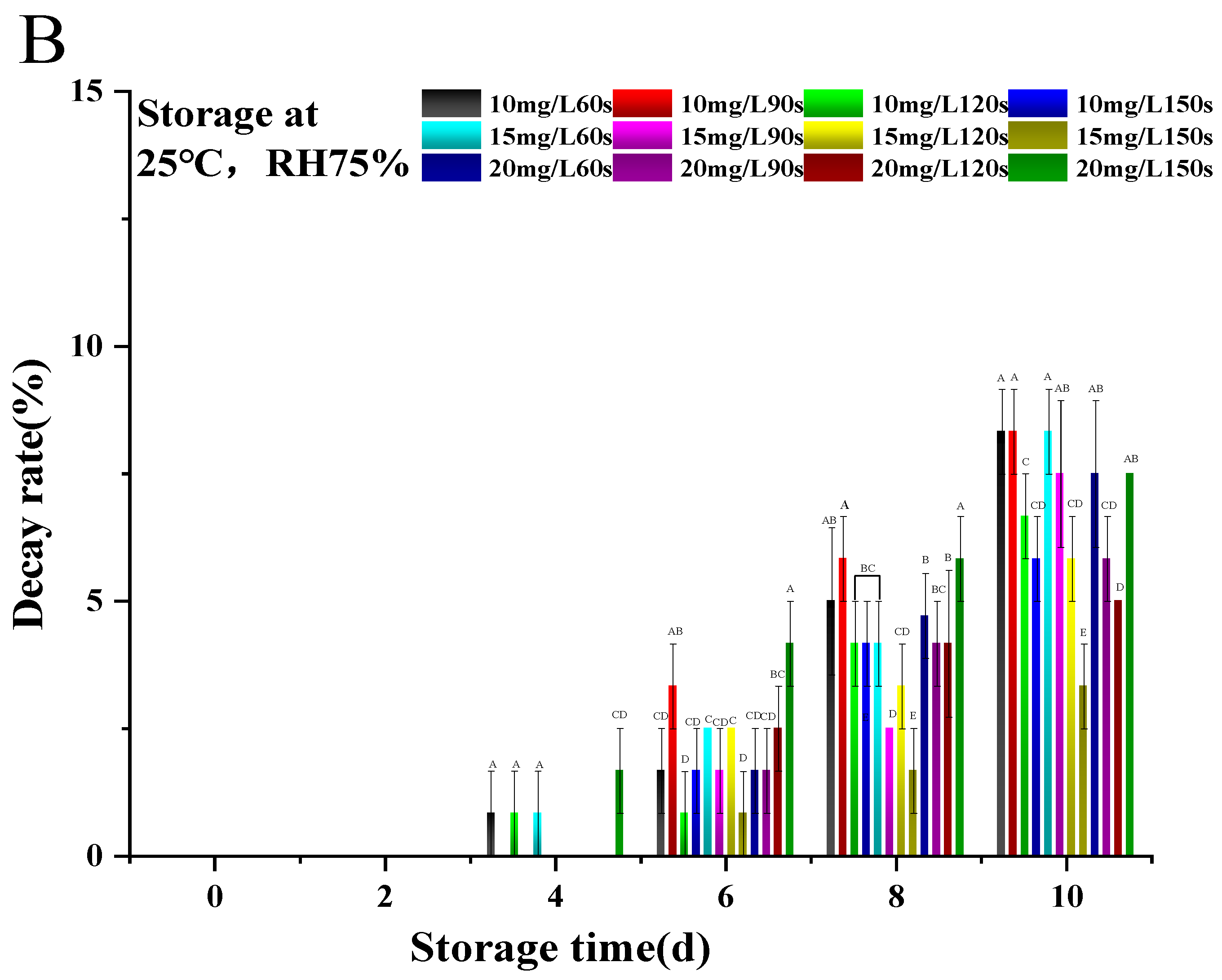
3.1. Optimization of Conditions for Ozone Water Treatment
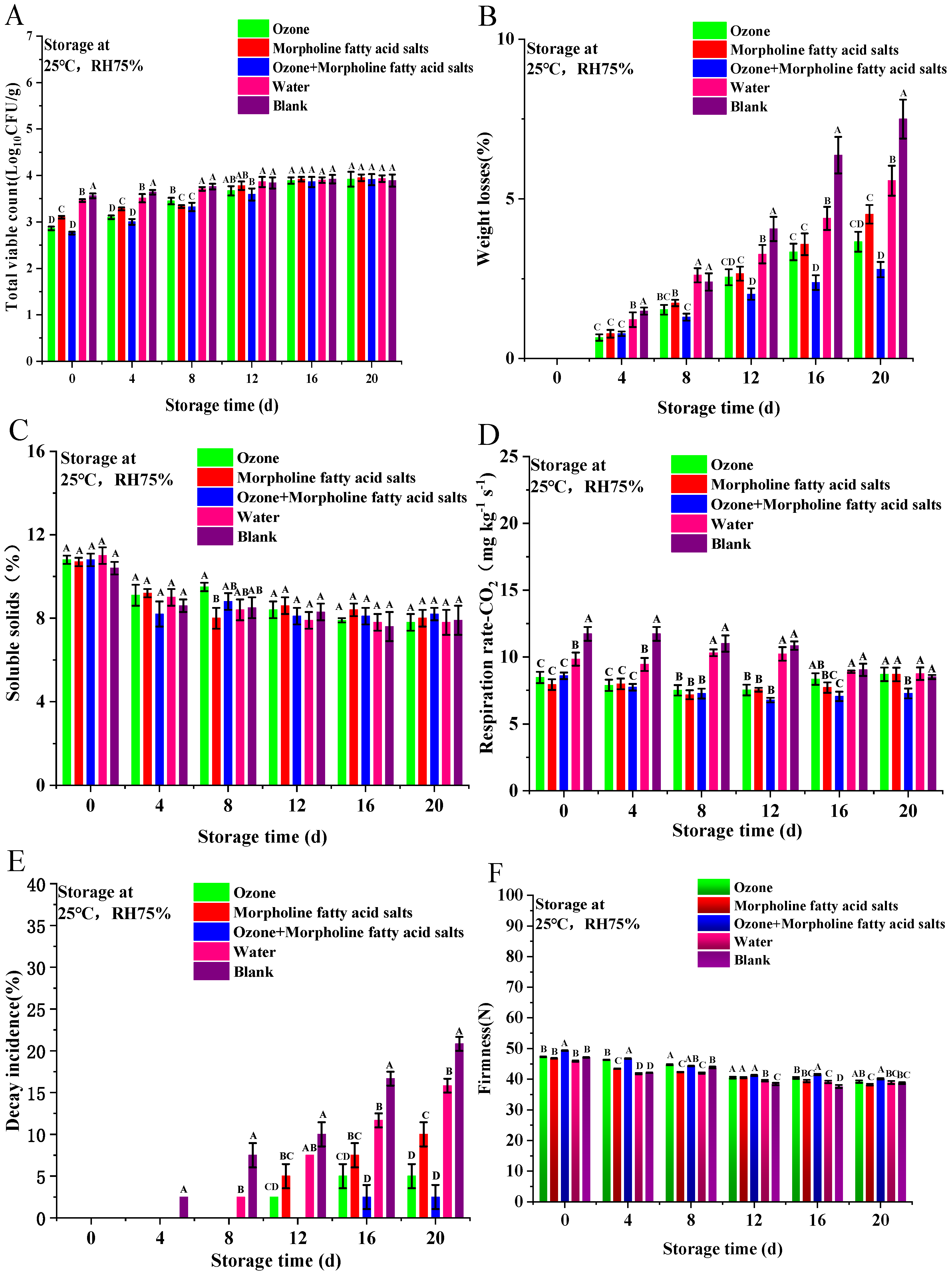
3.2. Analysis of Physicochemical Properties and Shelf-Life of Mandarin Oranges After Different Treatments
3.2.1. Surface Microbial Content
3.2.2. Weight Loss
3.2.3. Soluble Solids Content
3.2.4. Respiration Rate
3.2.5. Decay Rate
3.2.6. Firmness
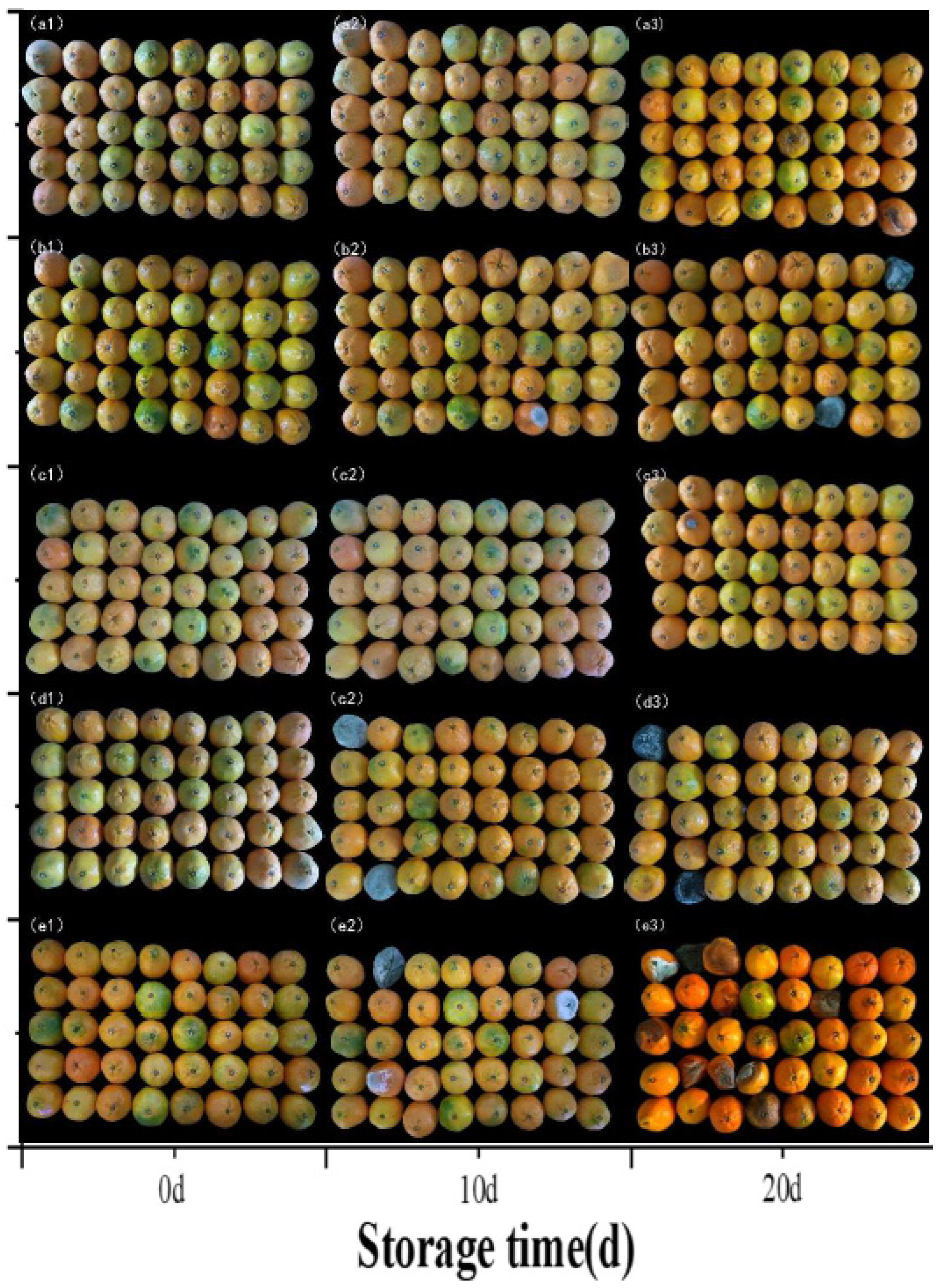
3.2.7. Morphological Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Askarne, L.; Talibi, I.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Serghini, M.A.; Ben Aoumar, A.A. In vitro and in vivo antifungal activity of several Moroccan plants against Penicillium italicum, the causal agent of citrus blue mold. Crop Prot. 2012, 40, 53–58. [Google Scholar] [CrossRef]
- Wuryatmo, E.; Able, A.J.; Ford, C.M.; Scott, E.S. Effect of volatile citral on the development of blue mould, green mould and sour rot on navel orange. Australas. Plant Pathol. 2014, 43, 403–411. [Google Scholar] [CrossRef]
- Dore, A.; Molinu, M.G.; Venditti, T.; D′Hallewin, G. Use of High-Intensity Ultrasound to Increase the Efficiency of Imazalil in Postharvest Storage of Citrus Fruits. Food Bioprocess Technol. 2013, 6, 3029–3037. [Google Scholar] [CrossRef]
- Watanabe, E.; Yoshimura, Y.; Yuasa, Y.; Nakazawa, H. Immunoaffinity column clean-up for the determination of imazalil in citrus fruits. Anal. Chim. Acta 2001, 433, 199–206. [Google Scholar] [CrossRef]
- Hao, W.; Zhong, G.; Hu, M.; Luo, J.; Weng, Q.; Rizwan-ul-Haq, M. Control of citrus postharvest green and blue mold and sour rot by tea saponin combined with imazalil and prochloraz. Postharvest Biol. Technol. 2010, 56, 39–43. [Google Scholar] [CrossRef]
- Garcia-Martin, J.F.; Olmo, M.; Maria Garcia, J. Effect of ozone treatment on postharvest disease and quality of different citrus varieties at laboratory and at industrial facility. Postharvest Biol. Technol. 2018, 137, 77–85. [Google Scholar] [CrossRef]
- Duan, X.; Jing, G.; Fan, F.; Tao, N. Control of postharvest green and blue molds of citrus fruit by application of sodium dehydroacetate. Postharvest Biol. Technol. 2016, 113, 17–19. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Hajeb, P.; Ehsani, R.J. Application of ozone for degradation of mycotoxins in food: A review. Comprehensive Rev. Food Sci. Food Saf. 2020, 19, 1777–1808. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Mansour, M.R. Influence of temperature and humidity on survival of Penicillium digitatum and Geotrichum citri-aurantii. Plant Dis. 2007, 91, 990–996. [Google Scholar] [CrossRef]
- Karaca, H. Use of Ozone in the Citrus Industry. Ozone-Sci. Eng. 2010, 32, 122–129. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, J.; Yin, C.; Li, G.; Jiang, Y.; Shan, Y. Effect of Ozone Treatment on Flavonoid Accumulation of Satsuma Mandarin (Citrus unshiu Marc.) during Ambient Storage. Biomolecules 2019, 9, 821. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.; Ganguli, A.; Ghosh, M. An effective combined treatment using malic acid and ozone inhibits Shigella spp. on sprouts. Food Control 2011, 22, 1032–1039. [Google Scholar] [CrossRef]
- Boonkorn, P.; Gemma, H.; Sugaya, S.; Setha, S.; Uthaibutra, J.; Whangchai, K. Impact of high-dose, short periods of ozone exposure on green mold and antioxidant enzyme activity of tangerine fruit. Postharvest Biol. Technol. 2012, 67, 25–28. [Google Scholar] [CrossRef]
- Vahisalu, T.; Puzõrjova, I.; Brosché, M.; Valk, E.; Lepiku, M.; Moldau, H.; Pechter, P.; Wang, Y.-S.; Lindgren, O.; Salojärvi, J.; et al. Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 2010, 62, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.K.; Lu, Z.X.; Yu, Z.F.; Gao, X. Preservation of fresh-cut celery by treatment of ozonated water. Food Control 2005, 16, 279–283. [Google Scholar] [CrossRef]
- Zhu, F. Effect of ozone treatment on the quality of grain products. Food Chem. 2018, 264, 358–366. [Google Scholar] [CrossRef]
- An, K.; Kim, I.; Lee, C.; Moon, J.-K.; Suh, H.-J.; Lee, J. Quantification of Morpholine in Peel and Pulp of Apples and Oranges by Gas Chromatography-Mass Spectrometry. Foods 2020, 9, 746. [Google Scholar] [CrossRef]
- Walker, M.J.; Gray, K.; Hopley, C.; Bell, D.; Colwell, P.; Maynard, P.; Burns, D.T. Forensically Robust Detection of the Presence of Morpholine in Apples-Proof of Principle. Food Anal. Methods 2012, 5, 874–880. [Google Scholar] [CrossRef]
- Duan, X.; OuYang, Q.; Tao, N. Effect of applying cinnamaldehyde incorporated in wax on green mould decay in citrus fruits. J. Sci. Food Agric. 2018, 98, 527–533. [Google Scholar] [CrossRef]
- Deng, F.; Gao, Z.; Sun, M.; Yu, S.; An, F.; Cai, J.; Lu, X.; Ou, W. Effect of waxing treatment on quality of fresh cassava tuber roots stored at ambient temperature. Food Ferment. Ind. 2023, 49, 187–193. [Google Scholar]
- Yang, J.; Zhang, R.; Chen, L.; Li, Q.; Zhang, Z.; Sun, X. Research Progress on Pollutant degradation by Micro-Nano Bubbles Composite Advanced Oxidation Technology. Technol. Water Treat. 2022, 48, 25–29. [Google Scholar]
- Zhang, R.; Cheng, Z.; Liang, Y.; Hu, X.; Shen, T.; Li, Y.; Han, Z.; Zhang, X.; Zou, X. A Novel Strategy for Accelerating Pumpable Ice Slurry Production with Ozone Micro-Nano Bubbles and Extending the Shelf Life of Larimichthys polyactis. Foods 2023, 12, 2206. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, Y.; Fan, W.; Guo, T.; Huo, M.; Yang, W.; Zhu, S.; An, W. Intensifying ozonation treatment of municipal secondary effluent using a combination of microbubbles and ultraviolet irradiation. Environ. Sci. Pollut. Res. 2019, 26, 21915–21924. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, X.; Gao, H.; Zhang, Z.; Zhu, H.; Duan, X.; Qu, H.; Yun, Z.; Jiang, Y. Comparative profiling of primary metabolites and volatile compounds in Satsuma mandarin peel after ozone treatment. Postharvest Biol. Technol. 2019, 153, 1–12. [Google Scholar] [CrossRef]
- Strano, M.C.; Timpanaro, N.; Allegra, M.; Foti, P.; Pangallo, S.; Romeo, F.V. Effect of ozonated water combined with sodium bicarbonate on microbial load and shelf life of cold stored clementine (Citrus clementina Hort. ex Tan.). Sci. Hortic. 2021, 276, 109775. [Google Scholar] [CrossRef]
- Zinato Rodrigues, A.A.; Lopes Ribeiro de Queiroz, M.E.; Neves, A.A.; de Oliveira, A.F.; Figueiredo Prates, L.H.; de Freitas, J.F.; Heleno, F.F.; D′Antonino Faroni, L.R. Use of ozone and detergent for removal of pesticides and improving storage quality of tomato. Food Res. Int. 2019, 125, 108626. [Google Scholar] [CrossRef] [PubMed]
- Brodowska, A.J.; Nowak, A.; Smigielski, K. Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview. Crit. Rev. Food Sci. Nutr. 2018, 58, 2176–2201. [Google Scholar] [CrossRef]
- Fan, F.; Tao, N.; Jia, L.; He, X. Use of citral incorporated in postharvest wax of citrus fruit as a botanical fungicide against Penicillium digitatum. Postharvest Biol. Technol. 2014, 90, 52–55. [Google Scholar] [CrossRef]
- Ahima, J.; Zhang, H.; Apaliya, M.T.; Yang, Q.; Jiang, Z. The mechanism involved in enhancing the biological control efficacy of Rhodotorula mucilaginosa with salicylic acid to postharvest green mold decay of oranges. J. Food Meas. Charact. 2020, 14, 3146–3155. [Google Scholar] [CrossRef]
- Mustapha, A.T.; Zhou, C. Novel assisted/unassisted ultrasound treatment: Effect on respiration rate, ethylene production, enzymes activity, volatile composition, and odor of cherry tomato. Lwt-Food Sci. Technol. 2021, 149, 111779. [Google Scholar] [CrossRef]
- Youssef, K.; Ligorio, A.; Nigro, F.; Ippolito, A. Activity of salts incorporated in wax in controlling postharvest diseases of citrus fruit. Postharvest Biol. Technol. 2012, 65, 39–43. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Xu, B.; Guo, Z. Fresh-cut orange preservation based on nano-zinc oxide combined with pressurized argon treatment. Lwt-Food Sci. Technol. 2021, 135, 110036. [Google Scholar] [CrossRef]
- Nayak, S.L.; Sethi, S.; Sharma, R.R.; Sharma, R.M.; Singh, S.; Singh, D. Aqueous ozone controls decay and maintains quality attributes of strawberry (Fragaria x ananassa Duch.). J. Food Sci. Technol.-Mysore 2020, 57, 319–326. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Zhai, X.; Li, X. Combination of precooling with ozone fumigation or low fluctuation of temperature for the quality modifications of postharvest sweet cherries. J. Food Process. Preserv. 2021, 45, e15504. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Wang, R.; Shan, Y.; Zhang, Q.; Ding, S. Progress in the Knowledge of the Cuticle of Citrus Fruits and Its Effect on Postharvest Fruit Quality during Storage. Food Sci. 2020, 41, 234–244. [Google Scholar]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Improving the shelf-life stability of apple and strawberry fruits applying chitosan-incorporated olive oil processing residues coating. Food Packag. Shelf Life 2016, 9, 10–19. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Magwaza, L.S.; Mbili, N.; Mditshwa, A.d. Carboxyl methylcellulose (CMC) containing moringa plant extracts as new postharvest organic edible coating for Avocado (Persea americana Mill.) fruit. Sci. Hortic. 2017, 226, 201–207. [Google Scholar] [CrossRef]
- Palou, L.; Smilanick, J.L.; Crisosto, C.H.; Mansour, M. Effect of gaseous ozone exposure on the development of green and blue molds on cold stored citrus fruit. Plant Dis. 2001, 85, 632–638. [Google Scholar] [CrossRef]


| No. | Pretreatment Method | Description |
|---|---|---|
| 1 | Ozone water 1 | Continuous immersion of 10 mg/L ozone water for 60 s |
| 2 | Ozone water 2 | Continuous immersion of 10 mg/L ozone water for 90 s |
| 3 | Ozone water 3 | Continuous immersion of 10 mg/L ozone water for 120 s |
| 4 | Ozone water 4 | Continuous immersion of 10 mg/L ozone water for 150 s |
| 5 | Ozone water 5 | Continuous immersion of 15 mg/L ozone water for 60 s |
| 6 | Ozone water 6 | Continuous immersion of 15 mg/L ozone water for 90 s |
| 7 | Ozone water 7 | Continuous immersion of 15 mg/L ozone water for 120 s |
| 8 | Ozone water 8 | Continuous immersion of 15 mg/L ozone water for 150 s |
| 9 | Ozone water 9 | Continuous immersion of 20 mg/L ozone water for 60 s |
| 10 | Ozone water 10 | Continuous immersion of 20 mg/L ozone water for 90 s |
| 11 | Ozone water 11 | Continuous immersion of 20 mg/L ozone water for 120 s |
| 12 | Ozone water 12 | Continuous immersion of 20 mg/L ozone water for 150 s |
| No. | Pretreatment Method | Description |
|---|---|---|
| 1 | Ozone water | Continuous immersion of 15 mg/L ozone water (23.2 L) for 150 s |
| 2 | Morpholine fatty acid salts | Apply morpholine fatty acid salts (20 g/kg Jianling Laboratory) thinly and evenly on the surface of the mandarin oranges |
| 3 | Ozone water + morpholine fatty acid salts | Continuous shower with 15 mg/L of ozone water for 150 s and then apply morpholine fatty acid salts to the surface of mandarin oranges in a thin and even layer |
| 4 | Distilled water | Continuous immersion in distilled water for 150 s |
| 5 | Blank | No treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Ma, L.; Xu, Q.; Tian, X.; Sun, L.; Cai, J. Synergistic Treatment with Ozone Water and Morpholine Fatty Acid Salts Improves Postharvest Quality in Mandarin Oranges. Foods 2025, 14, 1346. https://doi.org/10.3390/foods14081346
Liang Y, Ma L, Xu Q, Tian X, Sun L, Cai J. Synergistic Treatment with Ozone Water and Morpholine Fatty Acid Salts Improves Postharvest Quality in Mandarin Oranges. Foods. 2025; 14(8):1346. https://doi.org/10.3390/foods14081346
Chicago/Turabian StyleLiang, Yingbin, Lixin Ma, Qian Xu, Xiaoyu Tian, Li Sun, and Jianrong Cai. 2025. "Synergistic Treatment with Ozone Water and Morpholine Fatty Acid Salts Improves Postharvest Quality in Mandarin Oranges" Foods 14, no. 8: 1346. https://doi.org/10.3390/foods14081346
APA StyleLiang, Y., Ma, L., Xu, Q., Tian, X., Sun, L., & Cai, J. (2025). Synergistic Treatment with Ozone Water and Morpholine Fatty Acid Salts Improves Postharvest Quality in Mandarin Oranges. Foods, 14(8), 1346. https://doi.org/10.3390/foods14081346






