New Insights into a Conceptual Bionic Colonic Bioreactor: A Model, ‘Probiotics in Human Colon’, Showing How Probiotics Alleviate Constipation from a Bioprocess Engineering Perspective
Abstract
1. Introduction
2. Symptoms and Causes of Constipation
2.1. Definitions of Constipation
2.2. Colon Movement and Constipation
2.3. Physical Property of Human Feces
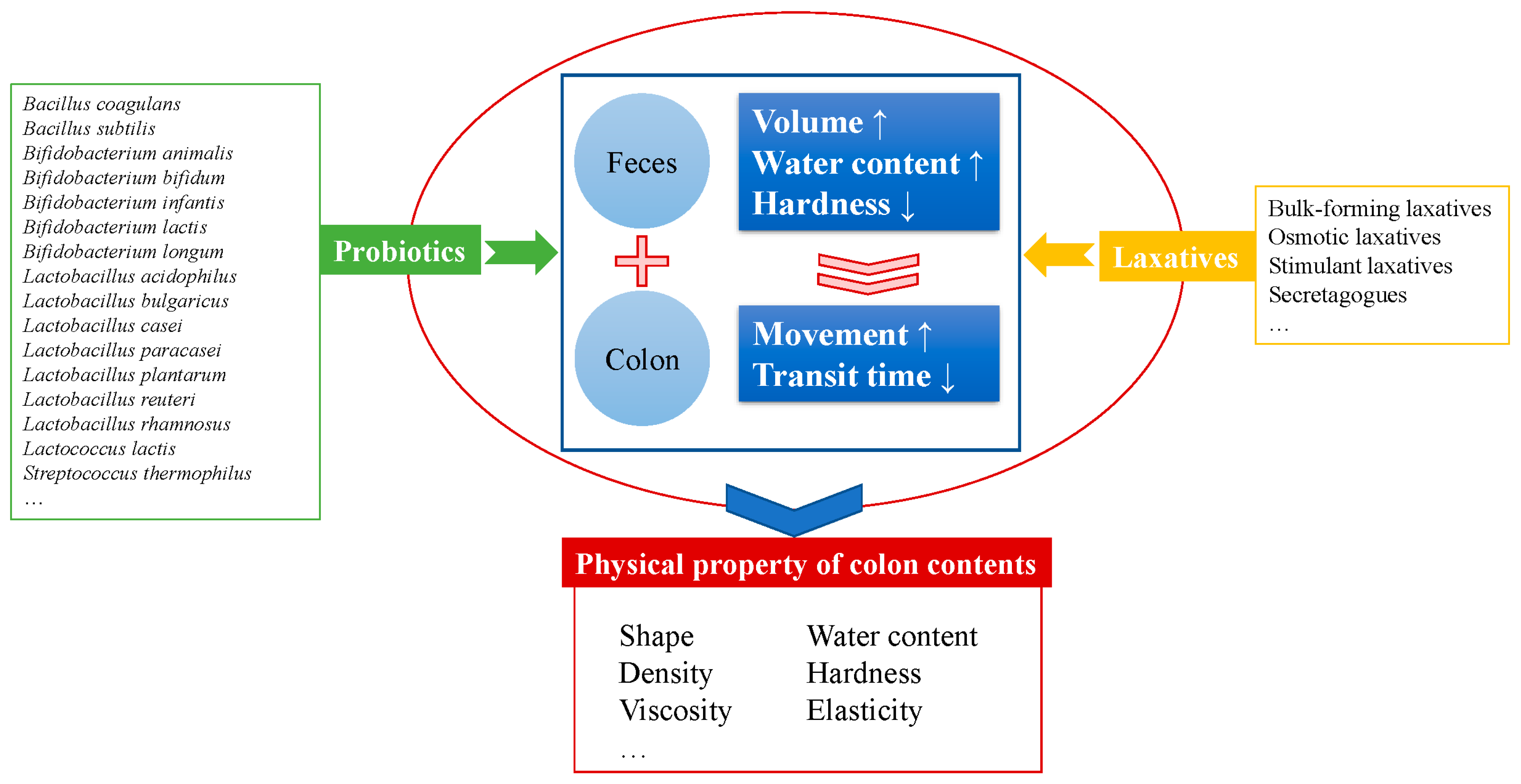
3. Effective Probiotic Strains on Alleviating Constipation
3.1. Probiotic Strains
| Strains | Subjects | Doses | Main Findings | References |
|---|---|---|---|---|
| Bifidobacterium longum S3 | Mice | 0.2 mL of bacterial suspension (5 × 109 CFU mL−1) for 4 weeks. | B. longum S3 exerted a constipation-alleviating effect primarily by improving the gut microbiota, repairing intestinal barrier damage and reducing inflammation, as well as inhibiting oxidative stress levels and decreasing expression of water channel proteins. | [42] |
| Lacticaseibacillus paracasei NCU-04 | Mice | 1 × 108 CFU/d or 1 × 109 CFU/d for 14 days. | L. paracasei NCU-04 significantly enhanced gut immobility, reduced colon inflammation, and increased levels of colonic motilin, 5-HT and c-kit. Notably, L. paracasei NCU-04 effectively upregulated the expression of 5-HT and its receptor in the brains of constipated mice. | [37] |
| Bifidobacterium longum BB536 | Humans (elderly) | 2 g/package (5 × 1010 CFU or more), 1 sachet daily for 4 weeks. | The randomized controlled trial showed B. longum BB536 improved defecation and some upper abdominal symptoms in elderly patients with chronic constipation. Some of the improved symptoms were maintained even 4 weeks after stopping the probiotics. | [34] |
| Bifidobacterium animalis subsp. lactis HN019 + Lacticaseibacillus rhamnosus HN001 + fructooligosaccharide | Humans (adults) | HN019: 7.7 × 109 CFU/sachet; HN001: 1.9 × 109 CFU/sachet; Fructooligosaccharide: 0.48 g/sachet. 2 sachet every day for 4 weeks. | The results suggested the potential roles of enteric neurohormone and 5-HT in the probiotic-derived optimizations in gut microbial genera, related to bowel movement in human. | [47] |
| Lactiplantibacillus plantarum P9 | Humans (adults) | 2 g per sachet, 1 × 1011 CFU/sachet/day for 28 days. | The constipation alleviation effect of L. plantarum P9 was fine tuning of certain functional intestinal microbiota, bacteriophages, and microbial metabolites. | [35] |
| Lactobacillus plantarum Lp3a | Humans | 1.0 × 1010 CFU per bag, 2 bags per day for 7 days. | L. plantarum Lp3a can alleviate functional constipation symptoms by enhancing intestinal motility, which is putatively associated with methane metabolism and bile acid synthesis. 16s rRNA sequencing analysis revealed no significant difference in microbiome composition, which suggests that the observed effect of L. plantarum Lp3a on constipation is not directly mediated by microbial diversity. | [36] |
| Mice | 10–30 times of the human daily dose for 15 days. | |||
| Fermented black garlic by Lactobacillus plantarum X7022 | Mice | 20 mL/kg bodyweight, once a day for 14 days. | The defecation time was significantly decreased, and the defecation number and the defecation weight were increased. Fermented black garlic by L. plantarum X7022 played roles in promoting small intestinal peristalsis on constipated mice. | [31] |
| Lactococcus lactis subsp. Lactis HFY14 | Mice | 1 × 109 CFU/kg for 4 weeks. | Lactococcus lactis subsp. Lactis HFY14 inhibits constipation by regulating mRNA expression of the VIP-cAMP-PKA-AQP3 signaling pathway. | [38] |
| Mixture of probiotics and prebiotics Probiotics: Lactobacillus plantarum, Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium lactis, Streptococcus thermophilus; Prebiotics: Lactitol, Kamut steamed powder, Microbiome X. | Rats | LACTO 5X: 31 mg/kg, Synbiotics: 120 mg/kg, once daily for 21 days. | The use of multi-strain probiotics alone showed a constipation-alleviating effect, but probiotics used with prebiotics showed better effects in alleviating constipation than using probiotics alone. | [46] |
| Combination laxative: konjac glucomannan + Lactobacillus paracasei X11 | Mice | L. paracasei X11: 1.0 × 108 CFU/mL at 0.6 mL/d, konjac glucomannan: 10 mg/mL, for 17 days. | Konjac glucomannan and L. paracasei X11 effectively promoted the metabolism of SCFA and the secretion of 5-HT in mice. Up-regulated mRNA and protein levels of 5-HT receptor 4 and serotonin transporter via the 5-HT pathway effectively alleviated constipation. | [32] |
| Combination laxative: konjac glucomannan and Bifidobacterium animalis F1-7 | Mice | 5 mL of konjac glucomannan (0.015 g/15 mL) + 5 mL of B. animalis F1-7 (2 × 108 CFU/mL), 10 mg/kg per day for 21 days. | Konjac glucomannan + B. animalis F1-7 mixture effectively improved intestinal motility and alleviated constipation through humoral transport-related pathways. The mixture effectively promoted defecation in mice, increased the fecal water content, shortened the defecation time and improved the gastrointestinal transit rate. Secretion of 5-HT and SCFA were also promoted. | [33] |
| Bifidobacterium animalis subsp. lactis MN-Gup | Mice | 2 × 109 cfu/kg, once per day for 14 days. | B. animalis subsp. lactis MN-Gup significantly decreased the first black stool defecation time, and significantly increased black fecal wet weight, black fecal number and the gastrointestinal transit rate, thereby alleviating constipation. | [39] |
| Humans | 1010 CFU per day for 4 weeks. | Concentration of acetate significantly increased. MN-Gup can alleviate constipation related to increased acetate-producing Bifidobacterium, Ruminoccaceae_UCG-002 and Ruminoccaceae_UCG-005. | ||
| Bifidobacterium bifidum G9-1 | Rats | 1.0 × 1010 CFU, three times a day for 4 days. | B. bifidum G9-1 improved dysbiosis and prevented a decrease in butyric acid concentration in the gut, increased serum serotonin, and suppressed an increase in dopamine and a decrease in acetylcholine in serum, while increased the expression level of tryptophan hydroxylase 1, a 5-HT-synthetizing enzyme. | [40] |
| Lactobacillus reuteri DSM17938 | Humans (children with anorexia nervosa) | 108 CFU, twice daily for 3 months. | L. reuteri DSM17938 is more effective than placebo for improving bowel movements and weight normalization in anorexia nervosa pediatric patients with constipation. The number of patients alleviated from constipation was higher in the L. reuteri than in the placebo group (87% vs. 63%), but the difference was not statistically significant. | [48] |
| Bacillus coagulans Unique IS2 | Humans (adults) | 2 × 109 CFU, once daily for 4 weeks. | In the probiotic treated group, significant increase was observed in number of bowel movements, symptoms of incomplete evacuation, painful defecation and abdominal pain associated with constipation were alleviated. 98% subjects in the probiotic group achieved normal stool consistency as compared to placebo (74%). | [43] |
| Lactobacillus rhamnosus CCFM1068; Lactobacillus rhamnosus FFJND15-L2; Lactobacillus rhamnosus FHeNJZ7-1; Lactobacillus rhamnosus FTJDJ11-1; Lactobacillus rhamnosus FZJHZ11-7. | Mice | 0.2 mL of bacterial suspension (5 × 109 CFU/mL in 3% sucrose solution) daily for 18 days. | Five strains of L. rhamnosus can alleviate constipation-related symptoms via different pathways independent of SCFAs regulation. The effects were associated with gastrointestinal regulatory peptides, neurotransmitters, neurotrophic factors, and gut microbiota. | [41] |
| Fermented milk containing Lactobacillus casei Zhang and Bifidobacterium animalis ssp. lactis V9 | Humans (adults) | 200 g/d of fermented milk for 4 weeks. | After intervention, the anti-inflammatory cytokine IL-10 increased and the proinflammatory cytokine C-reactive protein and lipopolysaccharides decreased. B. animalis was correlated with an increase in defecation frequency. Acylcarnitine, located on the significantly altered carnitine shuttle pathway, had a significantly positive correlation with defecation frequency. | [30] |
| Chocolate containing Streptococcus thermophilus MG510 and Lactobacillus plantarum LRCC5193 | Humans (adults) | S. thermophilus: 3.0 × 108 CFU/g, L. plantarum: 1.0 × 108 CFU/g, once daily for 4 weeks. | The relative abundance of L. plantarum was significantly greater in the probiotic group than in the placebo group, the relative abundance of S. thermophilus, Bifidobacterium spp., Bacteroidetes and Firmicutes did not change significantly in either group during the study period. The levels of serum cytokines (IL-10/IL-12 ratio and TNF-α) did not differ significantly between the two groups. | [45] |
| Fermented yogurt containing Lactococcus lactis, Lactobacillus plantarum and Lactobacillus casei | Mice | 106 CFU/mL, 4 mL per day for 5 days. | A new formulation of yogurt could significantly improve defecation time and intestinal health. Yogurt intake could also increase probiotic numbers and change the intestinal bacterial community composition. | [27] |
| Pasteurized yogurt fermented with two strains of Lactobacillus bulgaricus and two strains of Streptococcus thermophilus | Humans (adults) | No living bacteria (<5 CFU/mL) | Pasteurized yogurt, with inactive lactic acid bacteria, was found to be effective in improving defecation frequency and constipation symptoms. The numbers of fecal bifidobacteria and lactobacilli, and the SCFA concentrations increased. | [26] |
| Bacillus subtilis CBD2; Bacillus subtilis KMKW4 | Mice | 1 × 107 CFU per day for 7 days. | B. subtilis CBD2 and KMKW4 strains positively affect the intestinal function by suppressing the growth of pathogenic microflora and the activity of harmful enzymes as well as ameliorating constipation. | [44] |
3.2. Probiotics Versus Pharmacologic Approaches
3.3. Probiotics Versus Food
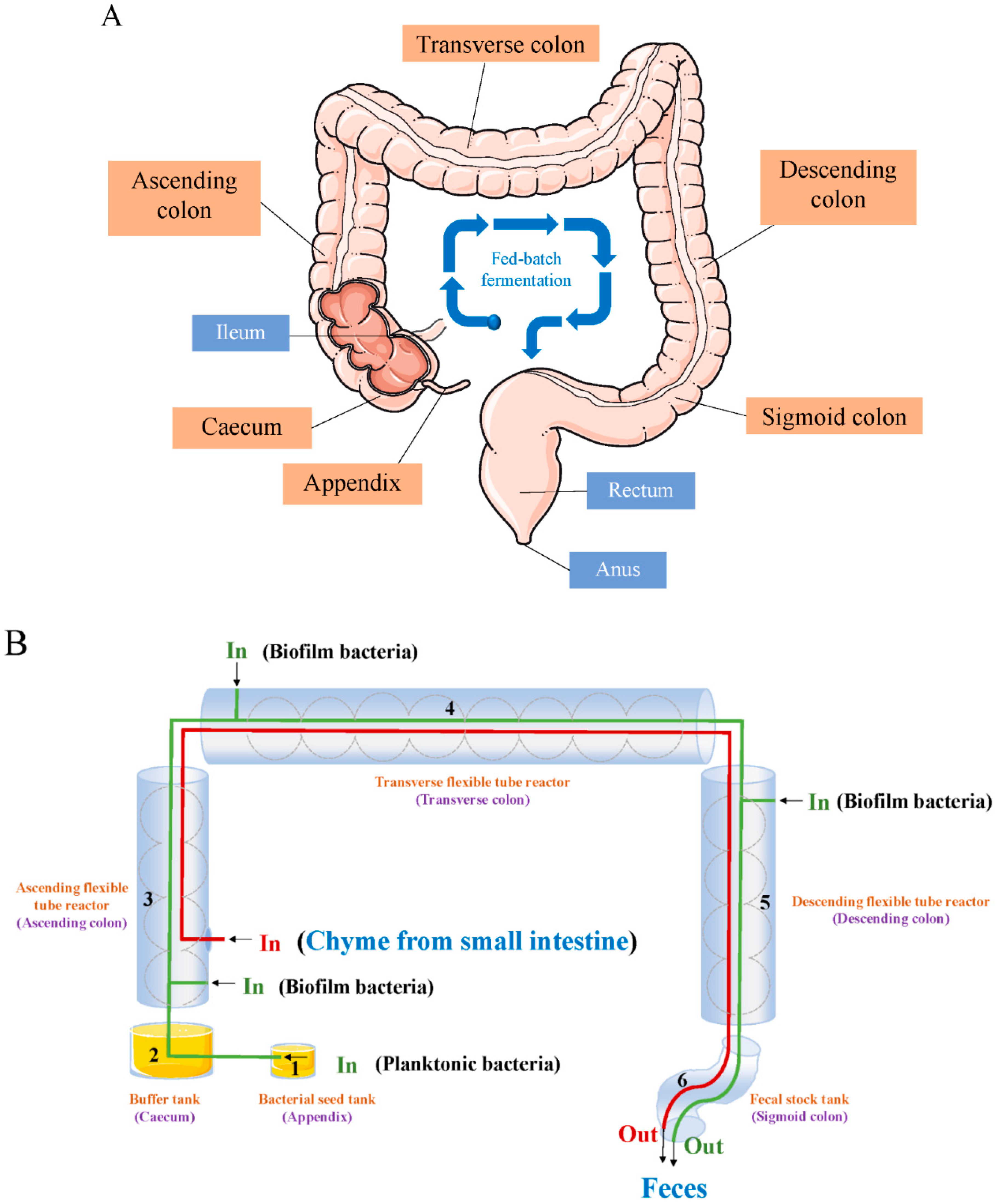
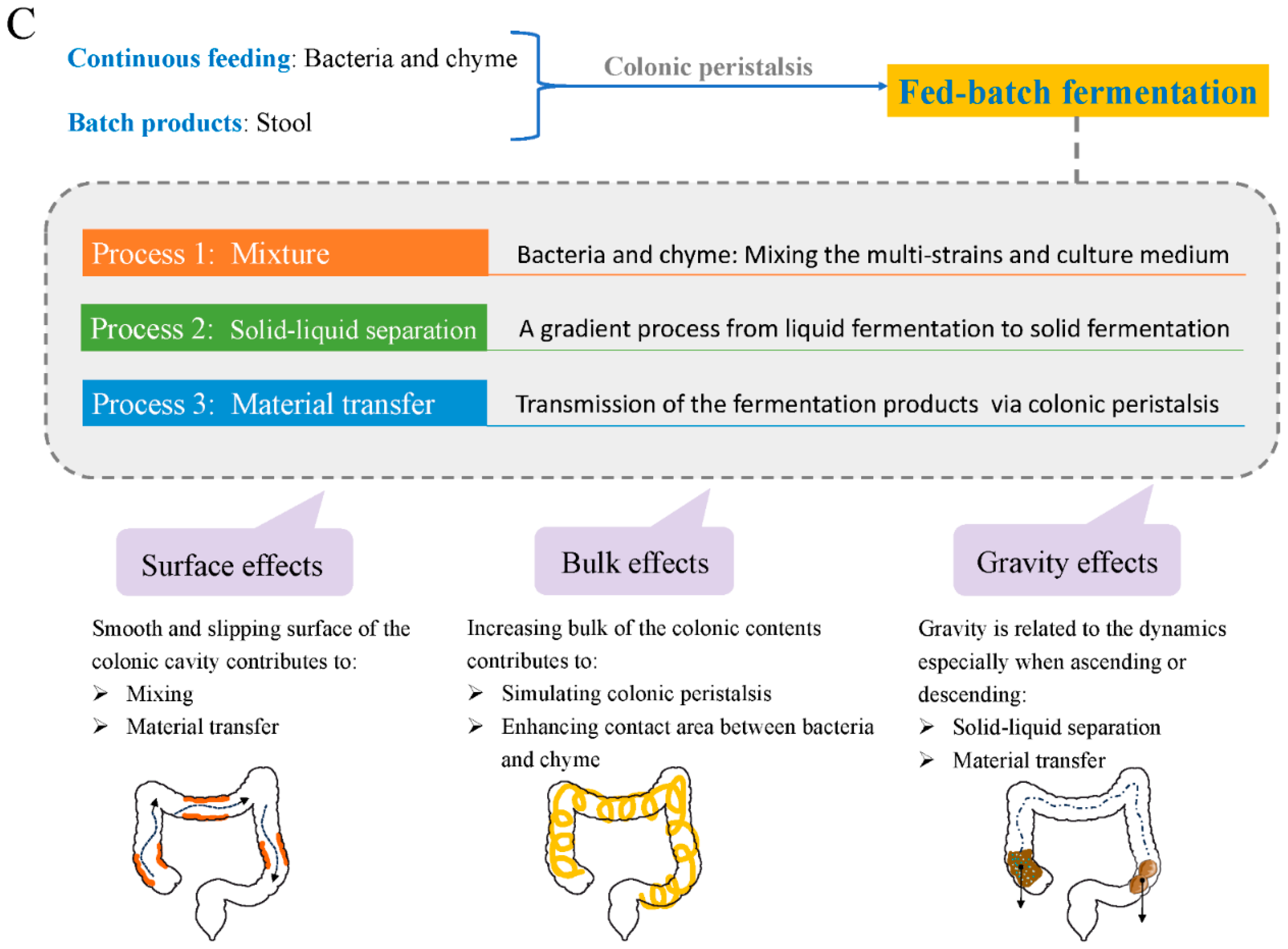
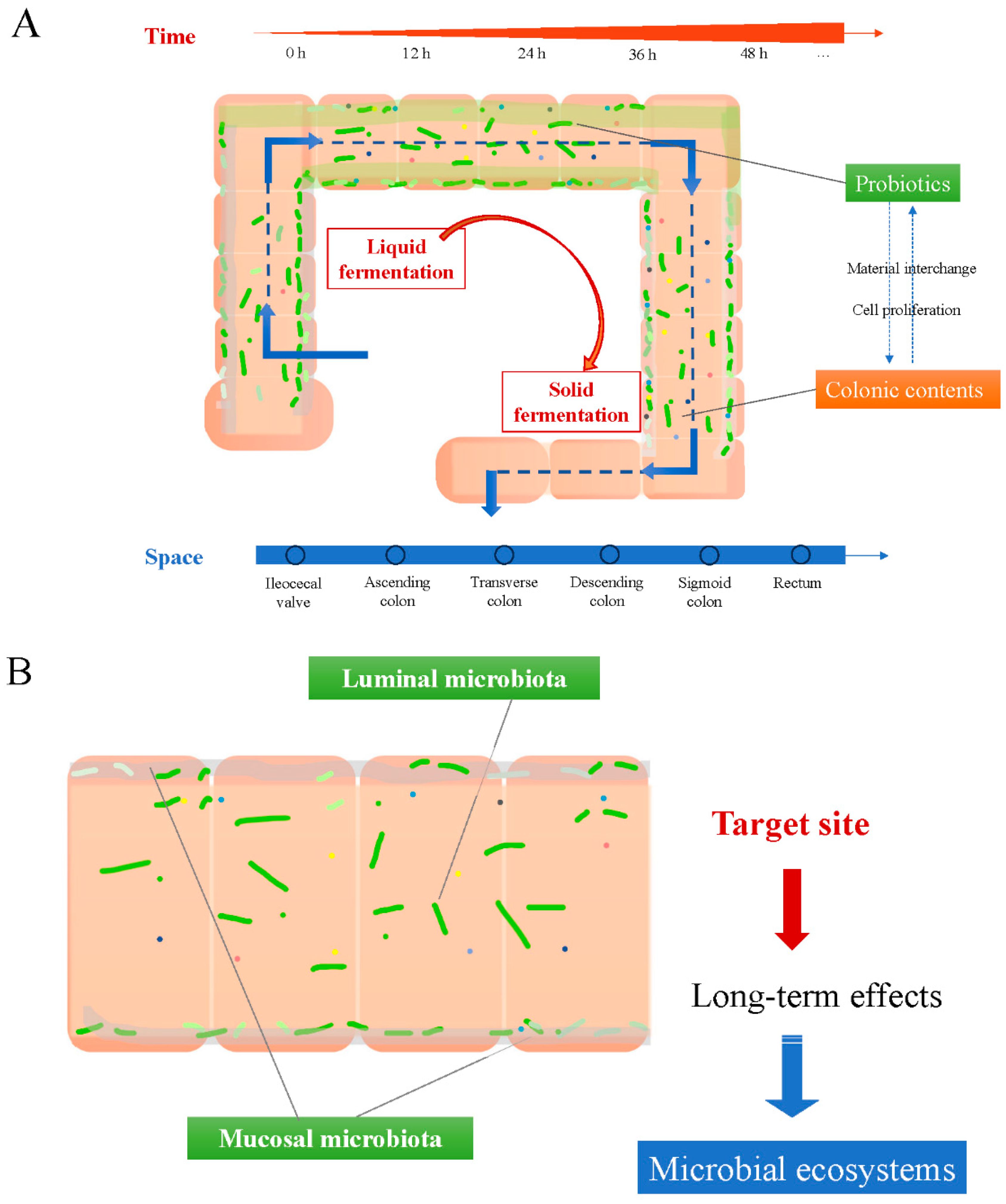
4. A Model of ‘Probiotics in Human Colon’ from an Engineering Perspective
4.1. Illustration of the ‘Probiotics in Human Colon’ Model
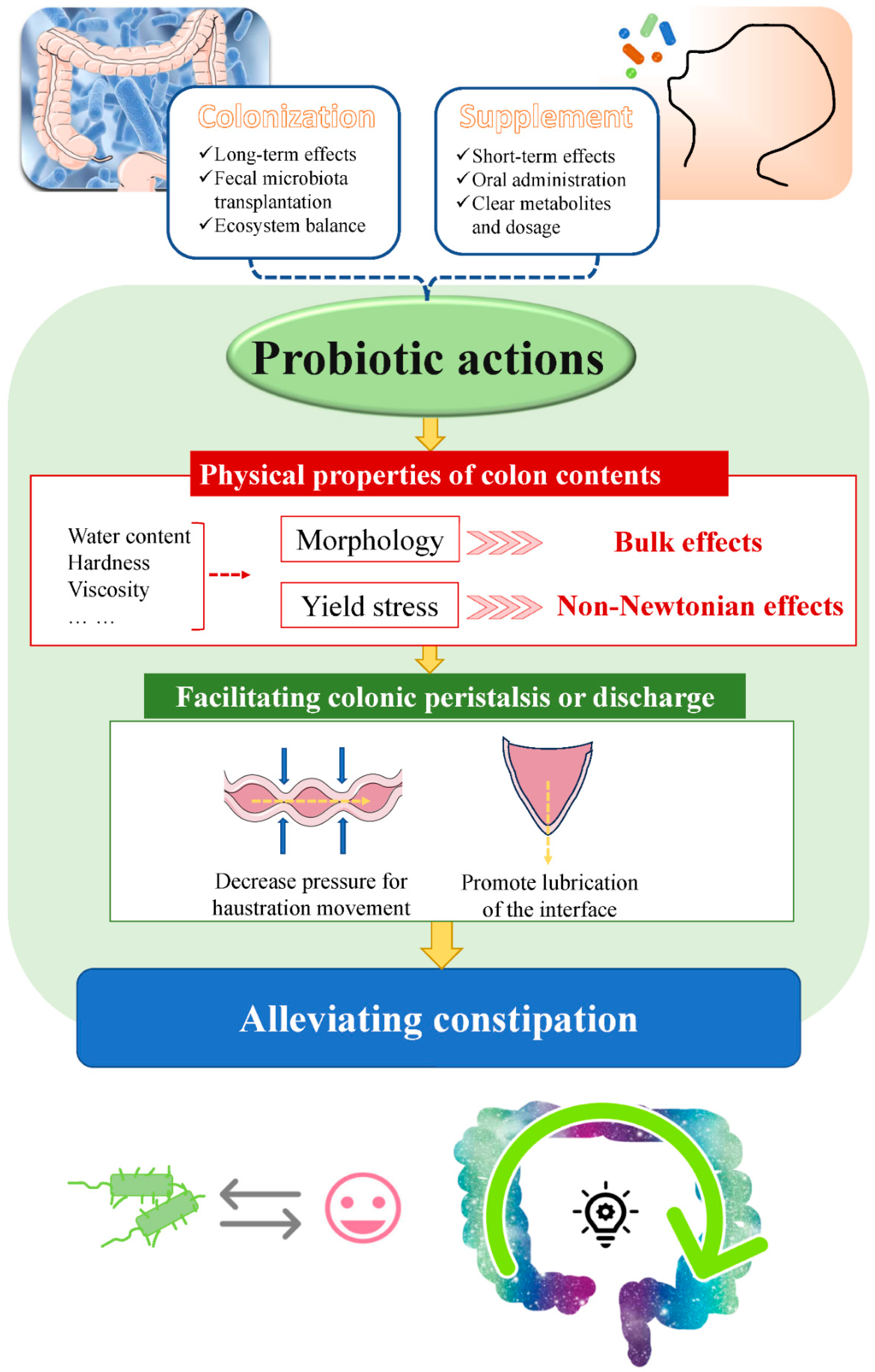
4.2. Long-Term Effects from Colonized Probiotics on the Colonic Mucosa
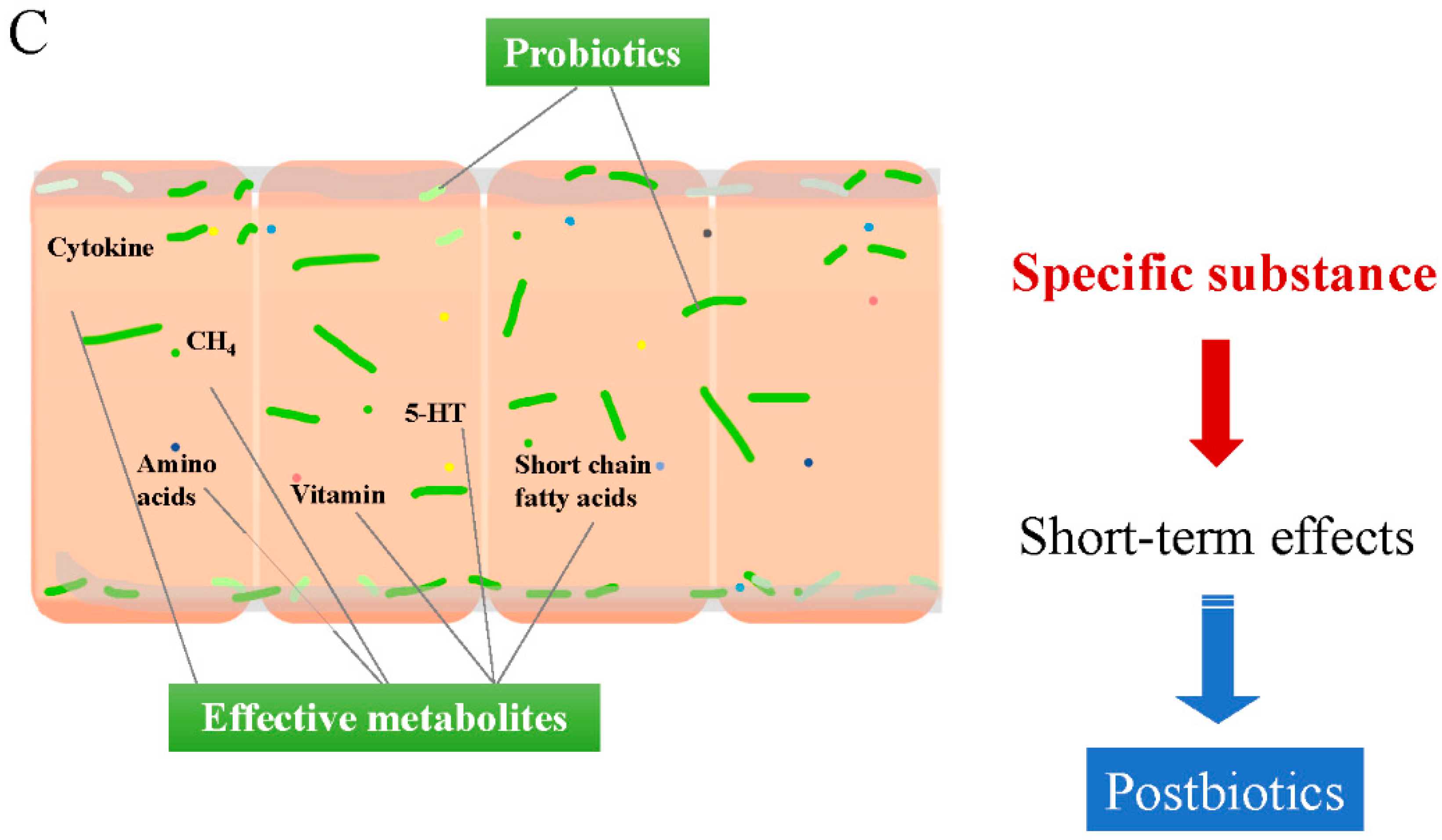
4.3. Short-Term Effects from Supplemented Probiotics Inside the Colonic Cavity
5. New Insights into the Mechanisms of Alleviating Constipation by Probiotics Related to the Physical Properties of Colon Contents
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Barberio, B.; Judge, C.; Savarino, E.V.; Ford, A.C. Global prevalence of functional constipation according to the Rome criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 638–648. [Google Scholar] [CrossRef]
- Nellesen, D.; Yee, K.; Chawla, A.; Lewis, B.E.; Carson, R.T. A Systematic Review of the Economic and Humanistic Burden of Illness in Irritable Bowel Syndrome and Chronic Constipation. J. Manag. Care Pharm. 2013, 19, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Klaschik, E.; Nauck, F.; Ostgathe, C. Constipation—Modern laxative therapy. Support. Care Cancer 2003, 11, 679–685. [Google Scholar] [CrossRef]
- Johanson, J.F.; Kralstein, J. Chronic constipation: A survey of the patient perspective. Aliment. Pharmacol. Ther. 2007, 25, 599–608. [Google Scholar] [CrossRef]
- Dimidi, E.; Zdanaviciene, A.; Christodoulides, S.; Taheri, S.; Louis, P.; Duncan, P.I.; Emami, N.; Crabbe, R.; De Castro, C.A.; McLean, P.; et al. Randomised clinical trial: Bifidobacterium lactis NCC2818 probiotic vs placebo, and impact on gut transit time, symptoms, and gut microbiology in chronic constipation. Aliment. Pharmacol. Ther. 2019, 49, 251–264. [Google Scholar] [CrossRef]
- Kaminski, M.; Skonieczna-Zydecka, K.; Loniewski, I.; Koulaouzidis, A.; Marlicz, W. Are probiotics useful in the treatment of chronic idiopathic constipation in adults? A review of existing systematic reviews, meta-analyses, and recommendations. Prz. Gastroenterol. 2020, 15, 103–118. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Javier Ruiz-Ojeda, F.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Schmulson, M.J.; Drossman, D.A. What Is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, C.; Grant, R.; Scott, S.M.; Whelan, K. Perceptions of Constipation Among the General Public and People With Constipation Differ Strikingly From Those of General and Specialist Doctors and the Rome IV Criteria. Am. J. Gastroenterol. 2019, 114, 1116–1129. [Google Scholar] [CrossRef]
- Vollebregt, P.F.; Wiklendt, L.; Dinning, P.G.; Knowles, C.H.; Scott, S.M. Coexistent faecal incontinence and constipation: A cross-sectional study of 4027 adults undergoing specialist assessment. Eclinicalmedicine 2020, 27, 100572. [Google Scholar] [CrossRef] [PubMed]
- Hernalsteens, S.; Huang, S.; Cong, H.H.; Chen, X.D. The final fate of food: On the establishment of in vitro colon models. Food Res. Int. 2021, 150, 110743. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, P.T.; Vollebregt, P.F.; Knowles, C.H.; Lunniss, P.J.; Dinning, P.G.; Scott, S.M. Understanding the physiology of human defaecation and disorders of continence and evacuation. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 751–769. [Google Scholar]
- Southwell, B.R. Colon lengthening slows transit: Is this the mechanism underlying redundant colon or slow transit constipation? J. Physiol. 2010, 588, 3343. [Google Scholar] [CrossRef]
- Brookes, S.J.; Dinning, P.G.; Gladman, M.A. Neuroanatomy and physiology of colorectal function and defaecation: From basic science to human clinical studies. Neurogastroenterol. Motil. 2009, 21, 9–19. [Google Scholar] [CrossRef]
- Bampton, P.A.; Dinning, P.G.; Kennedy, M.L.; Lubowski, D.Z.; deCarle, D.; Cook, I.J. Spatial and temporal organization of pressure patterns throughout the unprepared colon during spontaneous defecation. Am. J. Gastroenterol. 2000, 95, 1027–1035. [Google Scholar] [CrossRef]
- Penn, R.; Ward, B.J.; Strande, L.; Maurer, M. Review of synthetic human faeces and faecal sludge for sanitation and wastewater research. Water Res. 2018, 132, 222–240. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Sun, N.; Tierney, R.; Li, H.; Corsetti, M.; Williams, L.; Wong, P.K.; Wong, T.S. Viscoelastic solid-repellent coatings for extreme water saving and global sanitation. Nat. Sustain. 2019, 2, 1097–1105. [Google Scholar] [CrossRef]
- Infusino, E.; Caloiero, T. Municipal wastewater sludge rheology: Impact of temperature, solid content, and settling solids. Water Environ. Res. 2021, 93, 2971–2981. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef]
- Woolley, S.M.; Buckley, C.A.; Pocock, J.; Foutch, G.L. Rheological modelling of fresh human faeces. J. Water Sanit. Hyg. Dev. 2014, 4, 484–489. [Google Scholar] [CrossRef]
- Woolley, S.M.; Cottingham, R.S.; Pocock, J.; Buckley, C.A. Shear rheological properties of fresh human faeces with different moisture content. Water SA 2014, 40, 273–276. [Google Scholar] [CrossRef]
- Choi, S.C.; Kim, B.J.; Rhee, P.-L.; Chang, D.K.; Son, H.J.; Kim, J.J.; Rhee, J.C.; Kim, S.I.; Han, Y.S.; Sim, K.H.; et al. Probiotic Fermented Milk Containing Dietary Fiber Has Additive Effects in IBS with Constipation Compared to Plain Probiotic Fermented Milk. Gut Liver 2011, 5, 22–28. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.-M.; Xu, Z.-Y.; Han, M.; Guo, B.-H. Efficacy of pasteurised yoghurt in improving chronic constipation: A randomised, double-blind, placebo-controlled trial. Int. Dairy J. 2015, 40, 1–5. [Google Scholar] [CrossRef]
- Liu, C.-J.; Tang, X.-D.; Yu, J.; Zhang, H.-Y.; Li, X.-R. Gut microbiota alterations from different Lactobacillus probiotic-fermented yoghurt treatments in slow-transit constipation. J. Funct. Foods 2017, 38, 110–118. [Google Scholar] [CrossRef]
- Maki, R.; Matsukawa, M.; Matsuduka, A.; Hashinaga, M.; Anai, H.; Yamaoka, Y.; Hanada, K.; Fujii, C. Therapeutic effect of lyophilized, Kefir-fermented milk on constipation among persons with mental and physical disabilities. Jpn. J. Nurs. Sci. 2018, 15, 218–225. [Google Scholar] [CrossRef]
- Nejati, F.; Junne, S.; Neubauer, P. A Big World in Small Grain: A Review of Natural Milk Kefir Starters. Microorganisms 2020, 8, 192. [Google Scholar] [CrossRef]
- Wang, J.; Bai, X.; Peng, C.; Yu, Z.; Li, B.; Zhang, W.; Sun, Z.; Zhang, H. Fermented milk containing Lactobacillus casei Zhang and Bifidobacterium animalis ssp. lactis V9 alleviated constipation symptoms through regulation of intestinal microbiota, inflammation, and metabolic pathways. J. Dairy Sci. 2020, 103, 11025–11038. [Google Scholar] [CrossRef]
- Ro, K.-S.; Chen, Y.; Du, L.; Wang, L.; Zhao, L.; Xie, J.; Wei, D. Improvement of S-allyl-L-cysteine content, probiotic properties and constipation prevention effect of black garlic by the lactic acid bacteria fermentation. Process Biochem. 2022, 115, 110–117. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Zhang, Z.; Liang, X.; Liu, T.; Yi, H.; Gong, P.; Wang, L.; Yang, W.; Zhang, X.; et al. Konjac glucomannan with probiotics acts as a combination laxative to relieve constipation in mice by increasing short-chain fatty acid metabolism and 5-hydroxytryptamine hormone release. Nutrition 2021, 84, 111112. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, Z.; Zhang, Z.; Liang, X.; Gong, P.; Yi, H.; Yang, L.; Liu, T.; Shi, H.; Zhang, L. Bifidobacterium animalis F1-7 in combination with konjac glucomannan improves constipation in mice via humoral transport. Food Funct. 2021, 12, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Asaoka, D.; Nojiri, S.; Yanagisawa, N.; Nishizaki, Y.; Osada, T.; Koido, S.; Nagahara, A.; Katsumata, N.; Odamaki, T.; et al. Usefulness of Bifidobacterium longum BB536 in Elderly Individuals With Chronic Constipation: A Randomized Controlled Trial. Am. J. Gastroenterol. 2023, 118, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yang, N.; Xie, Y.; Li, Y.; Xiao, Q.; Li, Q.; Jin, H.; Zheng, L.; Sun, Z.; Zuo, K.; et al. Effect of the probiotic strain, Lactiplantibacillus plantarum P9, on chronic constipation: A randomized, double-blind, placebo-controlled study. Pharmacol. Res. 2023, 191, 106755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Ma, K.; Wang, G.; Tang, M.; Wang, R.; Xia, Z.; Xu, Z.; Sun, M.; Bao, X.; et al. Lactobacillus plantarum Lp3a improves functional constipation: Evidence from a human randomized clinical trial and animal model. Ann. Transl. Med. 2022, 10, 316. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Cai, Y.; Yan, Z.; Wei, J.; Zhang, H.; Yue, F.; Chen, T. Lacticaseibacillus paracasei NCU-04 relieves constipation and the depressive-like behaviors induced by loperamide in mice through the microbiome-gut-brain axis. Curr. Res. Food Sci. 2024, 9, 100875. [Google Scholar] [CrossRef]
- Tan, Q.; Hu, J.; Zhou, Y.; Wan, Y.; Zhang, C.; Liu, X.; Long, X.; Tan, F.; Zhao, X. Inhibitory Effect of Lactococcus lactis subsp. lactis HFY14 on Diphenoxylate-Induced Constipation in Mice by Regulating the VIP-cAMP-PKA-AQP3 Signaling Pathway. Drug Des. Dev. Ther. 2021, 15, 1971–1980. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.; Li, G.; Zhang, M.; Niu, T.; Kang, X.; Zhao, H.; Chen, J.; Sun, E.; Li, Y. Effect of Bifidobacterium animalis subsp. lactis MN-Gup on constipation and the composition of gut microbiota. Benef. Microbes 2021, 12, 31–42. [Google Scholar] [CrossRef]
- Makizaki, Y.; Uemoto, T.; Yokota, H.; Yamamoto, M.; Tanaka, Y.; Ohno, H. Improvement of loperamide-induced slow transit constipation by Bifidobacterium bifidum G9-1 is mediated by the correction of butyrate production and neurotransmitter profile due to improvement in dysbiosis. PLoS ONE 2021, 16, e0267927. [Google Scholar] [CrossRef]
- Wang, G.; Yang, S.; Sun, S.; Si, Q.; Wang, L.; Zhang, Q.; Wu, G.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus rhamnosus Strains Relieve Loperamide-Induced Constipation via Different Pathways Independent of Short-Chain Fatty Acids. Front. Cell. Infect. Microbiol. 2020, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, H.; Cheng, T.; Wang, L.; Wang, G.; Zhang, H.; Chen, W. Bifidobacterium longum S3 alleviates loperamide-induced constipation by modulating intestinal acetic acid and stearic acid levels in mice. Food Funct. 2024, 15, 6118–6133. [Google Scholar] [CrossRef] [PubMed]
- Madempudi, R.S.; Neelamraju, J.; Ahire, J.J.; Gupta, S.K.; Shukla, V.K. Bacillus coagulans Unique IS2 in Constipation: A Double-Blind, Placebo-Controlled Study. Probiotics Antimicrob. Proteins 2020, 12, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Hong, J.-H.; Jeong, Y.S.; Jung, H.K. Evaluation of Two Bacillus subtilis Strains Isolated from Korean Fermented Food as Probiotics against Loperamide-induced Constipation in Mice. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 797–806. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Cha, J.M.; Oh, J.K.; Tan, P.L.; Kim, S.H.; Kwak, M.S.; Jeon, J.W.; Shin, H.P. Probiotics Ameliorate Stool Consistency in Patients with Chronic Constipation: A Randomized, Double-Blind, Placebo-Controlled Study. Dig. Dis. Sci. 2018, 63, 2754–2764. [Google Scholar] [CrossRef]
- Kim, M.G.; Jo, K.; Cho, K.; Park, S.S.; Suh, H.J.; Hong, K.-B. Prebiotics/Probiotics Mixture Induced Changes in Cecal Microbiome and Intestinal Morphology Alleviated the Loperamide-Induced Constipation in Rat. Food Sci. Anim. Resour. 2021, 41, 527–541. [Google Scholar] [CrossRef]
- Lai, H.; Li, Y.; He, Y.; Chen, F.; Mi, B.; Li, J.; Xie, J.; Ma, G.; Yang, J.; Xu, K.; et al. Effects of dietary fibers or probiotics on functional constipation symptoms and roles of gut microbiota: A double-blinded randomized placebo trial. Gut Microbes 2023, 15. [Google Scholar] [CrossRef]
- Zaja, O.; Fiolic, M.; Cuk, M.C.; Tiljak, M.K. The role of L.reuteri DSM17938 in nutritional recovery and treatment of constipation in children and adolescents with anorexia nervosa—A randomized, double blind, placebo controlled study. Clin. Nutr. ESPEN 2021, 46, 47–53. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Lacy, B.E. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020, 158, 1232–1249.E3. [Google Scholar] [CrossRef]
- Belorio, M.; Gomez, M. Psyllium: A useful functional ingredient in food systems. Crit. Rev. Food Sci. Nutr. 2022, 62, 527–538. [Google Scholar] [CrossRef]
- Pucciani, F.; Raggioli, M.; Ringressi, M.N. Usefulness of psyllium in rehabilitation of obstructed defecation. Tech. Coloproctol. 2011, 15, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Jalanka, J.; Major, G.; Murray, K.; Singh, G.; Nowak, A.; Kurtz, C.; Silos-Santiago, I.; Johnston, J.M.; de Vos, W.M.; Spiller, R. The Effect of Psyllium Husk on Intestinal Microbiota in Constipated Patients and Healthy Controls. Int. J. Mol. Sci. 2019, 20, 433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Kong, M.; Cheng, X.; Lin, A.; Liu, H. Modulation of gut microbiota and intestinal metabolites by lactulose improves loperamide-induced constipation in mice. Eur. J. Pharm. Sci. 2021, 158, 105676. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Fu, Z.; Yan, W.; Nie, K.; Ding, L.; Ma, D.; Huang, H.; Li, T.; Xie, J.; Fu, L. The different effects of Chinese Herb Solid Drink and lactulose on gut microbiota in rats with slow transit constipation induced by compound diphenoxylate. Food Res. Int. 2021, 143, 110273. [Google Scholar] [CrossRef]
- Koppen, I.J.N.; Broekaert, I.J.; Wilschanski, M.; Papadopoulou, A.; Ribes-Koninckx, C.; Thapar, N.; Gottrand, F.; Orel, R.; Lionetti, P.; Benninga, M.A. Role of Polyethylene Glycol in the Treatment of Functional Constipation in Children. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 361–363. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.-Y.; Xu, D.-Q.; Yue, S.-J.; Fu, R.-J.; Yang, J.; Xing, L.-M.; Tang, Y.-P. Action Mode of Gut Motility, Fluid and Electrolyte Transport in Chronic Constipation. Front. Pharmacol. 2021, 12, 630249. [Google Scholar] [CrossRef]
- Luthra, P.; Camilleri, M.; Burr, N.E.; Quigley, E.M.M.; Black, C.J.; Ford, A.C. Efficacy of drugs in chronic idiopathic constipation: A systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 831–844. [Google Scholar] [CrossRef]
- Werth, B.L.; Williams, K.A.; Fisher, M.J.; Pont, L.G. Use of over-the-counter laxatives by community-dwelling adults to treat and prevent constipation: A national cross-sectional study. Eur. J. Clin. Pharmacol. 2020, 76, 1003–1010. [Google Scholar] [CrossRef]
- Werth, B.L.; Christopher, S.-A. Laxative Use in the Community: A Literature Review. J. Clin. Med. 2021, 10, 143. [Google Scholar] [CrossRef]
- Khuituan, P.; K-da, S.; Bannob, K.; Hayeeawaema, F.; Peerakietkhajorn, S.; Tipbunjong, C.; Wichienchot, S.; Charoenphandhu, N. Prebiotic oligosaccharides from dragon fruits alter gut motility in mice. Biomed. Pharmacother. 2019, 114, 108821. [Google Scholar] [CrossRef]
- Chey, S.W.; Chey, W.D.; Jackson, K.; Eswaran, S. Exploratory Comparative Effectiveness Trial of Green Kiwifruit, Psyllium, or Prunes in US Patients With Chronic Constipation. Am. J. Gastroenterol. 2021, 116, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Katsirma, Z.; Dimidi, E.; Rodriguez-Mateos, A.; Whelan, K. Fruits and their impact on the gut microbiota, gut motility and constipation. Food Funct. 2021, 12, 8850–8866. [Google Scholar] [CrossRef]
- Liu, B.Y.; Wang, Z.Y.; Wang, H.R.; Hu, P.; Xu, D.; Wang, Q. Molecular profiling of bacterial species in the caecum of geese. Czech J. Anim. Sci. 2011, 56, 192–203. [Google Scholar] [CrossRef]
- McCallum, G.; Tropini, C. The gut microbiota and its biogeography. Nat. Rev. Microbiol. 2024, 22, 105–118. [Google Scholar] [CrossRef]
- Motta, J.-P.; Wallace, J.L.; Buret, A.G.; Deraison, C.; Vergnolle, N. Gastrointestinal biofilms in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 314–334. [Google Scholar] [CrossRef]
- Tytgat, H.L.P.; Nobrega, F.L.; van der Oost, J.; de Vos, W.M. Bowel Biofilms: Tipping Points between a Healthy and Compromised Gut? Trends Microbiol. 2019, 27, 17–25. [Google Scholar] [CrossRef]
- Collard, M.K.; Bardin, J.; Laurin, M.; Ogier-Denis, E. The cecal appendix is correlated with greater maximal longevity in mammals. J. Anat. 2021, 239, 1157–1169. [Google Scholar] [CrossRef]
- Agrawal, M.; Allin, K.H.; Mehandru, S.; Faith, J.; Jess, T.; Colombel, J.-F. The appendix and ulcerative colitis—An unsolved connection. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D. Chemical Reaction Engineering of Nutritional Phenomena in Human Body. Front. Chem. Eng. 2024, 6, 1480523. [Google Scholar] [CrossRef]
- Woodworth, M.H.; Carpentieri, C.; Sitchenko, K.L.; Kraft, C.S. Challenges in fecal donor selection and screening for fecal microbiota transplantation: A review. Gut Microbes 2017, 8, 225–237. [Google Scholar] [CrossRef]
- Gu, L.; Ding, C.; Tian, H.; Yang, B.; Zhang, X.; Hua, Y.; Zhu, Y.; Gong, J.; Zhu, W.; Li, J.; et al. Serial Frozen Fecal Microbiota Transplantation in the Treatment of Chronic Intestinal Pseudo-obstruction: A Preliminary Study. J. Neurogastroenterol. Motil. 2017, 23, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gu, L.; Zhang, M.; Zhang, S.; Wang, M.; Long, Y.; Zhang, X. The Fecal Microbiota Transplantation: A Remarkable Clinical Therapy for Slow Transit Constipation in Future. Front. Cell. Infect. Microbiol. 2021, 11, 732474. [Google Scholar] [CrossRef]
- Li, S.S.; Zhu, A.; Benes, V.; Costea, P.I.; Hercog, R.; Hildebrand, F.; Huerta-Cepas, J.; Nieuwdorp, M.; Salojarvi, J.; Voigt, A.Y.; et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science 2016, 352, 586–589. [Google Scholar] [CrossRef]
- Le Roy, T.; Debedat, J.; Marquet, F.; Da-Cunha, C.; Ichou, F.; Guerre-Millo, M.; Kapel, N.; Aron-Wisnewsky, J.; Clement, K. Comparative Evaluation of Microbiota Engraftment Following Fecal Microbiota Transfer in Mice Models: Age, Kinetic and Microbial Status Matter. Front. Microbiol. 2019, 9, 3289. [Google Scholar] [CrossRef] [PubMed]
- Bokoliya, S.C.; Dorsett, Y.; Panier, H.; Zhou, Y. Procedures for Fecal Microbiota Transplantation in Murine Microbiome Studies. Front. Cell. Infect. Microbiol. 2021, 11, 711055. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gutierrez, E.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Gut microbiota as a source of novel antimicrobials. Gut Microbes 2019, 10, 1–21. [Google Scholar] [CrossRef]
- Hasannejad Bibalan, M.; Eshaghi, M.; Rohani, M.; Pourshafie, M.R.; Talebi, M. Determination of Bacteriocin Genes and Antibacterial Activity of Lactobacillus Strains Isolated from Fecal of Healthy Individuals. Int. J. Mol. Cell. Med. 2017, 6, 50–55. [Google Scholar]
- Wu, G.; Zhao, N.; Zhang, C.; Lam, Y.Y.; Zhao, L. Guild-based analysis for understanding gut microbiome in human health and diseases. Genome Med. 2021, 13, 22. [Google Scholar] [CrossRef]
- Machado, D.; Maistrenko, O.M.; Andrejev, S.; Kim, Y.; Bork, P.; Patil, K.R.; Patil, K.R. Polarization of microbial communities between competitive and cooperative metabolism. Nat. Ecol. Evol. 2021, 5, 195–203. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Chen, J.; Tang, X.; Pan, M.; Yuan, W.; Wang, H. Efficacy of Probiotic Compounds in Relieving Constipation and Their Colonization in Gut Microbiota. Molecules 2022, 27, 666. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Javier Ruiz-Ojeda, F.; Maria Vilchez-Padial, L.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; De Grandi, R.; Pastorelli, L.; Vecchi, M.; Drago, L. A consumer’s guide for probiotics: 10 golden rules for a correct use. Dig. Liver Dis. 2017, 49, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Chugh, B.; Kamal-Eldin, A. Bioactive compounds produced by probiotics in food products. Curr. Opin. Food Sci. 2020, 32, 76–82. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Wang, L.; Cen, S.; Wang, G.; Lee, Y.-K.; Zhao, J.; Zhan, H.; Chen, W. Acetic acid and butyric acid released in large intestine play different roles in the alleviation of constipation. J. Funct. Foods 2020, 69, 103953. [Google Scholar] [CrossRef]
- Lee, K.-M.; Paik, C.-N.; Chung, W.C.; Yang, J.-M.; Choi, M.-G. Breath methane positivity is more common and higher in patients with objectively proven delayed transit constipation. Eur. J. Gastroenterol. Hepatol. 2013, 25, 726–732. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Kumar, S.; Mehrotra, M.; Lakshmi, C.; Misra, A. Frequency of small intestinal bacterial overgrowth in patients with irritable bowel syndrome and chronic non-specific diarrhea. J. Neurogastroenterol. Motil. 2010, 16, 40–46. [Google Scholar] [CrossRef]
- Ojetti, V.; Petruzziello, C.; Migneco, A.; Gnarra, M.; Gasbarrini, A.; Franceschi, F. Effect of Lactobacillus reuteri (DSM 17938) on methane production in patients affected by functional constipation: A retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1702–1708. [Google Scholar]
- Singh, P.; Duehren, S.; Katon, J.; Rangan, V.; Ballou, S.; Patel, R.; Iturrino, J.; Lembo, A.; Nee, J. Breath Methane Does Not Correlate With Constipation Severity or Bloating in Patients With Constipation. J. Clin. Gastroenterol. 2020, 54, 365–369. [Google Scholar] [CrossRef]
- Cirstea, M.S.; Yu, A.C.; Golz, E.; Sundvick, K.; Kliger, D.; Radisavljevic, N.; Foulger, L.H.; Mackenzie, M.; Huan, T.; Finlay, B.B.; et al. Microbiota Composition and Metabolism Are Associated With Gut Function in Parkinson’s Disease. Mov. Disord. 2020, 35, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Sturtzel, B.; Dietrich, A.; Wagner, K.H.; Gisinger, C.; Elmadfa, I. The status of vitamins B6, B12, folate, and of homocysteine in geriatric home residents receiving laxatives or dietary fiber. J. Nutr. Health Aging 2010, 14, 219–223. [Google Scholar] [CrossRef]
- Aguilar-Toala, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; Gonzalez-Cordova, A.F.; Vallejo-Cordoba, B.; Hernandez-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef]
- Barros, C.P.; Guimaraes, J.T.; Esmerino, E.A.; Duarte, M.C.K.H.; Silva, M.C.; Silva, R.; Ferreira, B.M.; Sant’Ana, A.S.; Freitas, M.Q.; Cruz, A.G. Paraprobiotics and postbiotics: Concepts and potential applications in dairy products. Curr. Opin. Food Sci. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Cuevas-Gonzalez, P.F.; Liceaga, A.M.; Aguilar-Toala, J.E. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Geibel, J.P. Secretion and absorption by colonic crypts. Annu. Rev. Physiol. 2005, 67, 471–490. [Google Scholar] [CrossRef]
- Negussie, A.B.; Dell, A.C.; Davis, B.A.; Geibel, J.P. Colonic Fluid and Electrolyte Transport 2022: An Update. Cells 2022, 11, 1712. [Google Scholar] [CrossRef]
- Lima, A.A.M.; Fonteles, M.C. From Escherichia coli heat-stable enterotoxin to mammalian endogenous guanylin hormones. Braz. J. Med. Biol. Res. 2014, 47, 179–191. [Google Scholar] [CrossRef]
- Aichbichler, B.W.; Wenzl, H.H.; Santa Ana, C.A.; Porter, J.L.; Schiller, L.R.; Fordtran, J.S. A comparison of stool characteristics from normal and constipated people. Dig. Dis. Sci. 1998, 43, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, P.T.; Lockton, S.; Irwin, J.; Lucas, A.L. The relationship between stool hardness and stool composition in breast-fed and formula-fed infants. J. Pediatr. Gastroenterol. Nutr. 1995, 20, 81–90. [Google Scholar] [PubMed]
- Lloyd, B.; Halter, R.J.; Kuchan, M.J.; Baggs, G.E.; Ryan, A.S.; Masor, M.L. Formula tolerance in postbreastfed and exclusively formula-fed infants. Pediatrics 1999, 103, e7. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Lien, E.L.; Capeding, M.R.Z.; Fitzgerald, M.; Ramanujam, K.; Yuhas, R.; Northington, R.; Lebumfacil, J.; Wang, L.; DeRusso, P.A. Effects of Term Infant Formulas Containing High sn-2 Palmitate With and Without Oligofructose on Stool Composition, Stool Characteristics, and Bifidogenicity. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 440–448. [Google Scholar] [CrossRef]
- Woo, H.-I.; Kwak, S.H.; Lee, Y.; Choi, J.H.; Cho, Y.M.; Om, A.-S. A Controlled, Randomized, Double-blind Trial to Evaluate the Effect of Vegetables and Whole Grain Powder That Is Rich in Dietary Fibers on Bowel Functions and Defecation in Constipated Young Adults. J. Cancer Prev. 2015, 20, 64–69. [Google Scholar] [CrossRef]
- Taylor, C.M.; Northstone, K.; Wernimont, S.M.; Emmett, P.M. Picky eating in preschool children: Associations with dietary fibre intakes and stool hardness. Appetite 2016, 100, 263–271. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Luo, M.; Wang, Z.; Zhu, L.; Wang, S.; Zhu, D.; Liu, H. The influence of gut microbiota on the rheological characterization of soy hull polysaccharide and mucin interactions. Rsc Adv. 2020, 10, 2830–2840. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, J.; Yan, Y.; Feng, L. Enhanced production of short-chain fatty acid by co-fermentation of waste activated sludge and kitchen waste under alkaline conditions and its application to microbial fuel cells. Appl. Energy 2013, 102, 1197–1204. [Google Scholar] [CrossRef]
- Jha, R.; Zijlstra, R.T. Physico-chemical properties of purified fiber affect their in vitro fermentation characteristics and are linked to in vivo characteristics in pigs. Can. J. Anim. Sci. 2018, 98, 394–398. [Google Scholar] [CrossRef]
- Tamargo, A.; Cueva, C.; Dolores Alvarez, M.; Herranz, B.; Bartolome, B.; Victoria Moreno-Arribas, M.; Laguna, L. Influence of viscosity on the growth of human gut microbiota. Food Hydrocoll. 2018, 77, 163–167. [Google Scholar] [CrossRef]
- de Loubens, C.; Dubreuil, A.; Lentle, R.G.; Magnin, A.; El Kissi, N.; Faucheron, J.L. Rheology of human faeces and pathophysiology of defaecation. Tech. Coloproctol. 2020, 24, 323–329. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Wu, P.; Chen, X.D. New Insights into a Conceptual Bionic Colonic Bioreactor: A Model, ‘Probiotics in Human Colon’, Showing How Probiotics Alleviate Constipation from a Bioprocess Engineering Perspective. Foods 2025, 14, 1335. https://doi.org/10.3390/foods14081335
Wang N, Wu P, Chen XD. New Insights into a Conceptual Bionic Colonic Bioreactor: A Model, ‘Probiotics in Human Colon’, Showing How Probiotics Alleviate Constipation from a Bioprocess Engineering Perspective. Foods. 2025; 14(8):1335. https://doi.org/10.3390/foods14081335
Chicago/Turabian StyleWang, Ni, Peng Wu, and Xiao Dong Chen. 2025. "New Insights into a Conceptual Bionic Colonic Bioreactor: A Model, ‘Probiotics in Human Colon’, Showing How Probiotics Alleviate Constipation from a Bioprocess Engineering Perspective" Foods 14, no. 8: 1335. https://doi.org/10.3390/foods14081335
APA StyleWang, N., Wu, P., & Chen, X. D. (2025). New Insights into a Conceptual Bionic Colonic Bioreactor: A Model, ‘Probiotics in Human Colon’, Showing How Probiotics Alleviate Constipation from a Bioprocess Engineering Perspective. Foods, 14(8), 1335. https://doi.org/10.3390/foods14081335








