Application of Chitosan and Its Derivatives in Postharvest Coating Preservation of Fruits
Abstract
1. Introduction
1.1. Chitosan
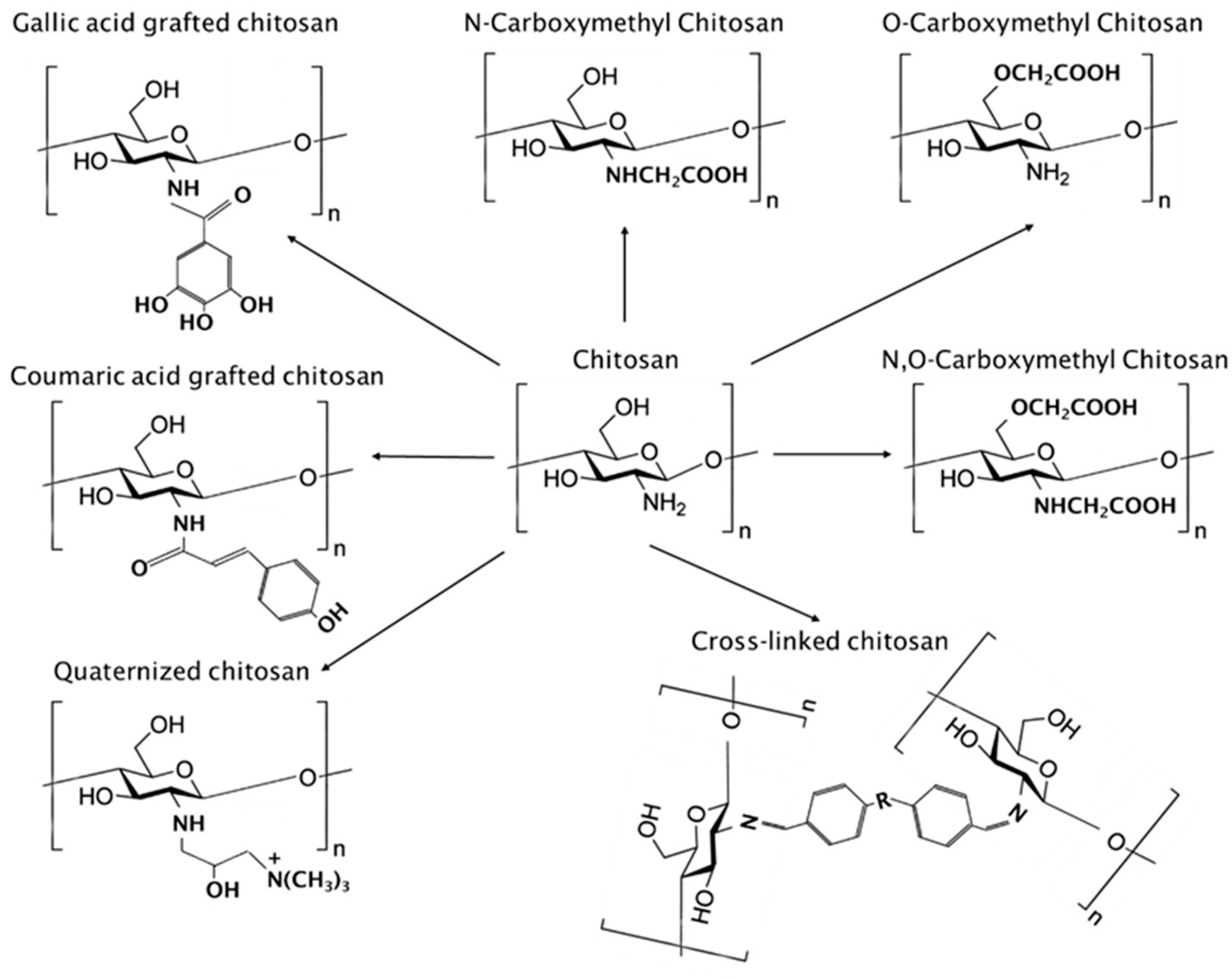
1.2. Modification of Chitosan
1.3. Preservation Mechanism of Chitosan-Based Coating
1.3.1. Physical Barrier
1.3.2. Antibacterial and Antifungal Activity
1.3.3. Physiological Metabolic Regulation
2. Application of Chitosan Coating in Fruit Preservation
2.1. Raw Chitosan
2.2. Chemically Modified Chitosan
2.2.1. Carboxymethyl Chitosan
2.2.2. Quaternized Chitosan
2.2.3. Grafted Chitosan
2.2.4. Cross-Linked Chitosan
2.2.5. Multiply Modified Chitosan
3. Application of Chitosan-Based Composite Coating in Fruit Preservation
4. Application of Chitosan-Based Nanocomposite Coating in Fruit Preservation
5. Challenges and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TSS | Total soluble solids |
| TA | Titratable acid |
| SSC | Soluble solids content |
| UV | Ultraviolet |
References
- Zhao, L.; Hu, Y.; Liang, L.; Dhanasekaran, S.; Zhang, X.; Yang, X.; Wu, M.; Song, Y.; Zhang, H. WSC1 regulates the growth, development, patulin production, and pathogenicity of Penicillium expansum infecting pear fruits. J. Agric. Food Chem. 2024, 72, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Luo, D.; Ba, L. Advances in the understanding of postharvest physiological changes and the storage and preservation of pitaya. Foods 2024, 13, 1307. [Google Scholar] [CrossRef] [PubMed]
- Malahlela, H.K.; Belay, Z.A.; Mphahlele, R.R.; Sigge, G.O.; Caleb, O.J. Recent advances in activated water systems for the postharvest management of quality and safety of fresh fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13317. [Google Scholar] [CrossRef] [PubMed]
- Ngolong Ngea, G.L.; Qian, X.; Yang, Q.; Dhanasekaran, S.; Ianiri, G.; Ballester, A.R.; Zhang, X.; Castoria, R.; Zhang, H. Securing fruit production: Opportunities from the elucidation of the molecular mechanisms of postharvest fungal infections. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2508–2533. [Google Scholar] [CrossRef]
- Kang, L.; Liang, Q.; Abdul, Q.; Rashid, A.; Ren, X.; Ma, H. Preparation technology and preservation mechanism of γ-CD-MOFs biaological packaging film loaded with curcumin. Food Chem. 2023, 420, 136142. [Google Scholar] [CrossRef]
- Anand, S.; Barua, M.K. Modeling the key factors leading to post-harvest loss and waste of fruits and vegetables in the agri-fresh produce supply chain. Comput. Electron. Agric. 2022, 198, 106936. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Liang, Q.; Kang, L.; Raza, H.; Chi, Z.; Chi, R.; Ren, X.; Ma, H. Preparation and characterization of ultrasound-assisted essential oil-loaded nanoemulsions stimulated pullulan-based bioactive film for strawberry fruit preservation. Food Chem. 2023, 422, 136254. [Google Scholar] [CrossRef]
- Xu, B.; Sylvain Tiliwa, E.; Yan, W.; Roknul Azam, S.M.; Wei, B.; Zhou, C.; Ma, H.; Bhandari, B. Recent development in high quality drying of fruits and vegetables assisted by ultrasound: A review. Food Res. Int. 2022, 152, 110744. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Xu, B.; Mujumdar, A.S.; Guo, Z. Light-emitting diodes (below 700 nm): Improving the preservation of fresh foods during postharvest handling, storage, and transportation. Compr. Rev. Food Sci. Food Saf. 2021, 21, 106–126. [Google Scholar] [CrossRef]
- Alenyorege, E.A.; Ma, H.; Ayim, I.; Zhou, C. Ultrasound decontamination of pesticides and microorganisms in fruits and vegetables: A review. J. Food Saf. Food Qual.-Arch. Leb. 2018, 69, 80–91. [Google Scholar] [CrossRef]
- Herman, R.A.; Ayepa, E.; Fometu, S.S.; Shittu, S.; Davids, J.S.; Wang, J. Mulberry fruit post-harvest management: Techniques, composition and influence on quality traits—A review. Food Control 2022, 140, 109126. [Google Scholar] [CrossRef]
- Tsegaye, M.; Alemu, T.; Dilnessa, A.; Tolessa, A.; Tantu, T.; Bekalu, Y.; Haile, F. Effect of storage condition on the nutritional and anti-nutritional composition of kurkura (Ziziphus mauritiana Lam.) fruit from North-Eastern Ethiopia. Heliyon 2023, 9, e17380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Luo, Z.; Wang, A.; Gu, X.; Lv, Z. Kinetic models applied to quality change and shelf life prediction of kiwifruits. LWT Food Sci. Technol. 2021, 138, 110610. [Google Scholar] [CrossRef]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of modified atmosphere packaging as a safety approach to fresh-cut fruits and vegetables—A review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Pu, Y.; Zhou, Q.; Yu, L.; Li, C.; Dong, Y.; Yu, N.; Chen, X. Longitudinal analyses of lignin deposition in green asparagus by microscopy during high oxygen modified atmosphere packaging. Food Packag. Shelf Life 2020, 25, 100536. [Google Scholar] [CrossRef]
- Mustapha, A.T.; Zhou, C.; Wahia, H.; Sarpong, F.; Nasiru, M.M.; Adegbemiga, Y.B.; Ma, H. Combination of thermal and dual-frequency sonication processes for optimum microbiological and antioxidant properties in cherry tomato. J. Food Process. Preserv. 2019, 44, e14325. [Google Scholar] [CrossRef]
- Zhou, W.; Sarpong, F.; Zhou, C. Use of ultrasonic cleaning technology in the whole process of fruit and vegetable processing. Foods 2022, 11, 2874. [Google Scholar] [CrossRef]
- Shahbaz, H.M.; Akram, K.; Ahn, J.-J.; Kwon, J.-H. Worldwide status of fresh fruits irradiation and concerns about quality, safety, and consumer acceptance. Crit. Rev. Food Sci. Nutr. 2015, 56, 1790–1807. [Google Scholar] [CrossRef]
- Antonio, A.L.; Cabo Verde, S.; Cerqueira, M.F. Raman measurements on gamma irradiated chestnut fruits. Radiat. Phys. Chem. 2025, 230, 112547. [Google Scholar] [CrossRef]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Apaliya, M.T.; Yang, Q. Augmentation of biocontrol agents with physical methods against postharvest diseases of fruits and vegetables. Trends Food Sci. Technol. 2017, 69, 36–45. [Google Scholar] [CrossRef]
- Cao, H.; Wang, X.; Liu, J.; Sun, Z.; Yu, Z.; Battino, M.; El-Seedi, H.; Guan, X. Mechanistic insights into the changes of enzyme activity in food processing under microwave irradiation. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2465–2487. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xu, B.; Yagoub, A.E.A.; Ma, H.; Sun, Y.; Xu, X.; Yu, X.; Zhou, C. Role of drying techniques on physical, rehydration, flavor, bioactive compounds and antioxidant characteristics of garlic. Food Chem. 2021, 343, 128404. [Google Scholar] [CrossRef] [PubMed]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1498–1513. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, Y.; Xu, C.; Jiang, Z.; Liu, J.; Sui, Y.; Zhang, H. Control of postharvest blue and gray mold in kiwifruit by Wickerhamomyces anomalus and its mechanism of antifungal activity. Postharvest Biol. Technol. 2023, 201, 112345. [Google Scholar] [CrossRef]
- Bartolini, S.; Pozzo, L.; Venturi, F.; Sanmartin, C.; Taglieri, I.; Macaluso, M.; Trivellini, A.; Orlando, M.; Zinnai, A.; Mensuali Sodi, A. Apple peel extracts as preservation solution to maintain the quality of fresh-cut apples. Eur. J. Hortic. Sci. 2022, 87, 1–9. [Google Scholar] [CrossRef]
- Cui, H.; Li, W.; Lin, L. Antibacterial activity of liposome containing curry plant essential oil against Bacillus cereusin rice. J. Food Saf. 2016, 37, e12302. [Google Scholar] [CrossRef]
- Abdulsalam, R.A.; Ijabadeniyi, O.A.; Sabiu, S. Fatty acid-modified chitosan and nanoencapsulation of essential oils: A snapshot of applications. Carbohydr. Res. 2024, 542, 109196. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Bacha, S.A.S.; Liang, Q.; Liu, Y.; Kang, L.; Chi, Z.; Chi, R.; Han, X.; Ekumah, J.-N.; et al. Preparation and functional characterization of pullulan-sodium alginate composite film enhanced with ultrasound-assisted clove essential oil Nanoemulsions for effective preservation of cherries and mushrooms. Food Chem. 2024, 457, 140048. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, X.; Zhang, C.; Shi, J.; Huang, X.; Li, Z.; Zou, X.; Gong, Y.; Holmes, M.; Povey, M.; et al. Agar/TiO2/radish anthocyanin/neem essential oil bionanocomposite bilayer films with improved bioactive capability and electrochemical writing property for banana preservation. Food Hydrocoll. 2022, 123, 107187. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, L.; Chen, Y.; Dhanasekaran, S.; Chen, X.; Zhang, X.; Yang, X.; Wu, M.; Song, Y.; Zhang, H. Study on the control effect and physiological mechanism of Wickerhamomyces anomalus on primary postharvest diseases of peach fruit. Int. J. Food Microbiol. 2024, 413, 110575. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Huang, X.; Shi, J.; Muhammad, A.; Zhai, X.; Xiao, J.; Li, Z.; Povey, M.; Zou, X. Study on cinnamon essential oil release performance based on pH-triggered dynamic mechanism of active packaging for meat preservation. Food Chem. 2023, 400, 134030. [Google Scholar] [CrossRef] [PubMed]
- Langer, S.E.; Marina, M.; Burgos, J.L.; Martínez, G.A.; Civello, P.M.; Villarreal, N.M. Calcium chloride treatment modifies cell wall metabolism and activates defense responses in strawberry fruit (Fragaria × ananassa, Duch). J. Sci. Food Agric. 2019, 99, 4003–4010. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Fang, J.; Fu, M.; Jiao, W.; Ren, P.; Yang, X. The role of 1-methylcyclopropylene (1-MCP) and salicylic acid (SA) in Induced resistance of postharvest fruits. Horticulturae 2023, 9, 108. [Google Scholar] [CrossRef]
- Pillai, A.R.S.; Eapen, A.S.; Zhang, W.; Roy, S. Polysaccharide-based edible biopolymer-based coatings for fruit preservation: A Review. Foods 2024, 13, 1529. [Google Scholar] [CrossRef]
- Tahir, H.E.; Zhihua, L.; Mahunu, G.K.; Xiaobo, Z.; Arslan, M.; Xiaowei, H.; Yang, Z.; Mariod, A.A. Effect of gum arabic edible coating incorporated with African baobab pulp extract on postharvest quality of cold stored blueberries. Food Sci. Biotechnol. 2019, 29, 217–226. [Google Scholar] [CrossRef]
- Ding, F.; Long, S.; Huang, X.; Shi, J.; Povey, M.; Zou, X. Emerging pickering emulsion films for bio-based food packaging applications. Food Packag. Shelf Life 2024, 42, 101242. [Google Scholar] [CrossRef]
- Ul Haq, Z.; Khaliq, G.; Irshad, A.; El-Beltagi, H.S.; Mahmoud Ismail, A.; El-Mogy, M.M. Effect of beeswax and lemon grass oil on Alternaria alternata and post-harvest quality of bitter gourd during storage. N. Z. J. Crop Hortic. Sci. 2024, 53, 384–404. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, Y.; Wang, T.; Ji, S.; Zhang, X.; Shen, T.; Huang, X.; Xiao, J.; Farag, M.A.; Shi, J.; et al. Quantum dots as advanced nanomaterials for food quality and safety applications: A comprehensive review and future perspectives. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13339. [Google Scholar] [CrossRef]
- Iftikhar, A.; Rehman, A.; Usman, M.; Ali, A.; Ahmad, M.M.; Shehzad, Q.; Fatim, H.; Mehmood, A.; Moiz, A.; Shabbir, M.A.; et al. Influence of guar gum and chitosan enriched with lemon peel essential oil coatings on the quality of pears. Food Sci. Nutr. 2022, 10, 2443–2454. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Xu, B.; Guo, Z. Fresh-cut orange preservation based on nano-zinc oxide combined with pressurized argon treatment. LWT Food Sci. Technol. 2021, 135, 112236. [Google Scholar] [CrossRef]
- Panahirad, S.; Dadpour, M.; Peighambardoust, S.H.; Soltanzadeh, M.; Gullón, B.; Alirezalu, K.; Lorenzo, J.M. Applications of carboxymethyl cellulose- and pectin-based active edible coatings in preservation of fruits and vegetables: A review. Trends Food Sci. Technol. 2021, 110, 663–673. [Google Scholar] [CrossRef]
- Xin, Y.; Yang, C.; Zhang, J.; Xiong, L. Application of whey protein-based emulsion coating treatment in fresh-cut apple preservation. Foods 2023, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.S.; Jaiswal, A.K.; Jaiswal, S. Lipid incorporated biopolymer based edible films and coatings in food packaging: A review. Curr. Res. Food Sci. 2024, 8, 100720. [Google Scholar] [CrossRef] [PubMed]
- Dayeh, N.; Vakilian, K.A.; Azadbakht, M. A fruit edible coating machine to protect the morphological, physiological, and biochemical properties of citrus fruits. Food Bioprod. Process. 2024, 148, 428–435. [Google Scholar] [CrossRef]
- Njombolwana, N.S.; Erasmus, A.; van Zyl, J.G.; du Plooy, W.; Cronje, P.J.R.; Fourie, P.H. Effects of citrus wax coating and brush type on imazalil residue loading, green mould control and fruit quality retention of sweet oranges. Postharvest Biol. Technol. 2013, 86, 362–371. [Google Scholar] [CrossRef]
- Tsague Donjio, R.; Aghofack Nguemezi, J.; Anoumaa, M.; Tafre Phounzong, E.; Kenfack, J.O.; Fonkou, T.; Pathare, P. Using response surface methodology to optimize edible coating formulations to delay ripening and preserve postharvest quality of tomatoes. J. Food Qual. 2023, 2023, 1019310. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, X.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; Shi, J. Effect of different coating methods on coating quality and mango preservation. Food Packag. Shelf Life 2023, 39, 101133. [Google Scholar] [CrossRef]
- Aziz, T.; Li, Z.; Naseeb, J.; Sarwar, A.; Zhao, L.; Lin, L.; Al Asmari, F. Role of bacterial exopolysaccharides in edible films for food safety and sustainability. Current trends and future perspectives. Ital. J. Food Sci. 2024, 36, 169–179. [Google Scholar] [CrossRef]
- Cui, H.; Yang, X.; Li, C.; Ye, Y.; Chen, X.; Lin, L. Enhancing anti-E. coli O157:H7 activity of composite phage nanofiber film by D-phenylalanine for food packaging. Int. J. Food Microbiol. 2022, 376, 109762. [Google Scholar] [CrossRef]
- Lin, L.; Mao, X.; Sun, Y.; Rajivgandhi, G.; Cui, H. Antibacterial properties of nanofibers containing chrysanthemum essential oil and their application as beef packaging. Int. J. Food Microbiol. 2019, 292, 21–30. [Google Scholar] [CrossRef]
- Cui, H.Y.; Wu, J.; Li, C.Z.; Lin, L. Anti-listeria effects of chitosan-coated nisin-silica liposome on Cheddar cheese. J. Dairy Sci. 2016, 99, 8598–8606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Long, Y.H.; Wang, Q.P.; Li, J.H.; An, H.M.; Wu, X.M.; Li, M. The effect of preharvest 28.6% chitosan composite film sprays for controlling the soft rot on kiwifruit. Hortic. Sci. 2019, 46, 180–194. [Google Scholar] [CrossRef]
- Kang, L.; Liang, Q.; Liu, Y.; Rashid, A.; Qayum, A.; Zhou, C.; Han, X.; Ren, X.; Chi, Z.; Chi, R.; et al. Preparation technology and preservation mechanism of novel Ag NPs-loaded ZIF-67 packaging film. Food Packag. Shelf Life 2024, 45, 101338. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, C.; Li, C.; Lin, L. Essential oils-based antibacterial agent against Escherichia coli O157:H7 biofilm on cucumber. J. Food Process. Preserv. 2017, 41, e13140. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Rashed, M.M.A.; Lin, L. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157:H7 biofilms on cucumber. Int. J. Food Microbiol. 2018, 266, 69–78. [Google Scholar] [CrossRef]
- Cui, H.; Surendhiran, D.; Li, C.; Lin, L. Biodegradable zein active film containing chitosan nanoparticle encapsulated with pomegranate peel extract for food packaging. Food Packag. Shelf Life 2020, 24, 100511. [Google Scholar] [CrossRef]
- Sahariah, P.; Papi, F.; Merz, K.L.; Sigurjonsson, O.E.; Meyer, R.L.; Nativi, C. Chitosan–saccharide conjugates for eradication of Pseudomonas aeruginosa biofilms. RSC Appl. Polym. 2024, 2, 461–472. [Google Scholar] [CrossRef]
- Godana, E.A.; Yang, Q.; Wang, K.; Zhang, H.; Zhang, X.; Zhao, L.; Abdelhai, M.H.; Guillaume Legrand, N.N. Bio-control activity of Pichia anomala supplemented with chitosan against Penicillium expansum in postharvest grapes and its possible inhibition mechanism. LWT Food Sci. Technol. 2020, 124, 109188. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, processing and modification techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef]
- Lin, L.; Xue, L.; Duraiarasan, S.; Haiying, C. Preparation of ε-polylysine/chitosan nanofibers for food packaging against Salmonella on chicken. Food Packag. Shelf Life 2018, 17, 134–141. [Google Scholar] [CrossRef]
- Pandey, R.; Mathur, G. Current trends in chitosan functionalization methods and their applications. Starch Stärke 2024, 77, 2300248. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Huang, K.X.; Zhou, L.Y.; Chen, J.Q.; Peng, N.; Chen, H.X.; Gu, H.Z.; Zou, T. Applications and perspectives of quaternized cellulose, chitin and chitosan: A review. Int. J. Biol. Macromol. 2023, 242, 124990. [Google Scholar] [CrossRef]
- Ren, X.; Hou, T.; Liang, Q.; Zhang, X.; Hu, D.; Xu, B.; Chen, X.; Chalamaiah, M.; Ma, H. Effects of frequency ultrasound on the properties of zein-chitosan complex coacervation for resveratrol encapsulation. Food Chem. 2019, 279, 223–230. [Google Scholar] [CrossRef]
- Mishra, A.; Omoyeni, T.; Singh, P.K.; Anandakumar, S.; Tiwari, A. Trends in sustainable chitosan-based hydrogel technology for circular biomedical engineering: A review. Int. J. Biol. Macromol. 2024, 276, 133823. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Recent advances in gelatine and chitosan complex material for practical food preservation application. Int. J. Food Sci. Technol. 2021, 56, 6279–6300. [Google Scholar] [CrossRef]
- Ding, F.; Hu, B.; Lan, S.; Wang, H. Flexographic and screen printing of carboxymethyl chitosan based edible inks for food packaging applications. Food Packag. Shelf Life 2020, 26, 100559. [Google Scholar] [CrossRef]
- Salah, M.; Huang, J.; Zhu, C.; Sobhy, M.; Farag, M.A.; Fang, Y.; Sobhy, R.; Walayat, N.; Khalifa, I.; Maqsood, S.; et al. Chitosan dual gel-like functionalized with flavonoid extract and cinnamaldehyde oil using dual cross-linking agents: Characterization, antioxidant, and antimicrobial effects. Curr. Res. Food Sci. 2024, 9, 100826. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Riaz, T.; Yasmin, I.; Leghari, A.A.; Amin, S.; Bilal, M.; Qi, X. Chitosan-based materials as edible coating of cheese: A review. Starch Stärke 2021, 73, 2100088. [Google Scholar] [CrossRef]
- Torkaman, S.; Rahmani, H.; Ashori, A.; Najafi, S.H.M. Modification of chitosan using amino acids for wound healing purposes: A review. Carbohydr. Polym. 2021, 258, 117675. [Google Scholar] [CrossRef] [PubMed]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2020, 23, 100439. [Google Scholar] [CrossRef]
- Tayebi, L.; Bayat, F.; Mahboubi, A.; Kamalinejad, M.; Haeri, A. Citric acid cross-linked biopolymeric nanofibers containing Zataria multiflora extract, an environmentally friendly active food packaging system. J. Food Meas. Charact. 2024, 18, 3458–3473. [Google Scholar] [CrossRef]
- Arslan, D.; Tuccitto, N.; Auditore, A.; Licciardello, A.; Marletta, G.; Riolo, M.; La Spada, F.; Taguali, S.C.; Calpe, J.; Meca, G.; et al. Chitosan-based films grafted with citrus waste-derived antifungal agents: An innovative and sustainable approach to enhance post-harvest preservation of citrus fruit. Int. J. Biol. Macromol. 2024, 264, 130514. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Tazdait, D.; Abdi, N.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Assessment of coating tomato fruit with shrimp shell chitosan and N,O-carboxymethyl chitosan on postharvest preservation. J. Food Meas. Charact. 2013, 7, 66–74. [Google Scholar] [CrossRef]
- Britto, D.d.; Assis, O.B.G. Chemical, biochemical, and microbiological aspects of chitosan quaternary salt as active coating on sliced apples. Food Sci. Technol. 2012, 32, 599–605. [Google Scholar] [CrossRef]
- Duarte, L.G.R.; Ferreira, N.C.A.; Fiocco, A.C.T.R.; Picone, C.S.F. Lactoferrin-chitosan-TPP nanoparticles: Antibacterial action and extension of strawberry shelf-life. Food Bioprocess Technol. 2022, 16, 135–148. [Google Scholar] [CrossRef]
- Fatima, M.; Mir, S.; Ali, M.; Hassan, S.; Ul Haq Khan, Z.; Waqar, K. Synthesis and applications of chitosan derivatives in food preservation—A review. Eur. Polym. J. 2024, 216, 113242. [Google Scholar] [CrossRef]
- Jimtaisong, A.; Saewan, N. Utilization of carboxymethyl chitosan in cosmetics. Int. J. Cosmet. Sci. 2013, 36, 12–21. [Google Scholar] [CrossRef]
- Almeida e Silva, T.; Gorup, L.F.; de Araújo, R.P.; Fonseca, G.G.; Martelli, S.M.; de Oliveira, K.M.P.; Faraoni, L.H.; de Arruda, E.G.R.; Gomes, R.A.B.; da Silva, C.H.M.; et al. Synergy of biodegradable polymer coatings with quaternary ammonium salts mediating barrier function against bacterial contamination and dehydration of eggs. Food Bioprocess Technol. 2020, 13, 2065–2081. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, Z.; Liao, Y.; Li, S.; Xue, Y.; Firempong, M.A.; Xu, Y.; Yu, J.; Smyth, H.D.C.; Xu, X. Improved intestinal absorption and oral bioavailability of astaxanthin using poly (ethylene glycol)-graft-chitosan nanoparticles: Preparation, in vitro evaluation, and pharmacokinetics in rats. J. Sci. Food Agric. 2021, 102, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Baswal, A.K.; Ramezanian, A.; Gill, K.S.; Mirza, A.A. Impact of carboxymethyl cellulose based edible coating on storage life and quality of guava fruit cv. ‘Allahabad Safeda’ under ambient storage conditions. J. Food Meas. Charact. 2021, 15, 4805–4812. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Chitrakar, B.; Wang, Y.; Xu, T.; Zhou, C. Preservation of duck eggs through glycerol monolaurate nanoemulsion coating. Curr. Res. Food Sci. 2021, 4, 752–757. [Google Scholar] [CrossRef]
- Li, M.; Yang, Z.; Zhai, X.; Li, Z.; Huang, X.; Shi, J.; Zou, X.; Lv, G. Incorporation of Lactococcus lactis and chia mucilage for improving the physical and biological properties of gelatin-based coating: Application for strawberry preservation. Foods 2024, 13, 1102. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Al-Gheethi, A.A.S.; Ghaleb, A.D.S.; Mahdi, A.A.; Al-Ansi, W.; Noman, A.E.; Al-Adeeb, A.; Odjo, A.K.O.; Du, Y.; Wei, M.; et al. Fabrication and characterization of chitosan/gelatin films loaded with microcapsules of Pulicaria jaubertii extract. Food Hydrocoll. 2022, 129, 107624. [Google Scholar] [CrossRef]
- Yang, Z.; Zou, X.; Li, Z.; Huang, X.; Zhai, X.; Zhang, W.; Shi, J.; Tahir, H.E. Improved postharvest quality of cold stored blueberry by edible coating based on composite gum arabic/roselle extract. Food Bioprocess Technol. 2019, 12, 1537–1547. [Google Scholar] [CrossRef]
- Cui, H.; Abdel-Samie, M.A.S.; Lin, L. Novel packaging systems in grape storage—A review. J. Food Process Eng. 2019, 42, e13162. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Zhang, J.; Shi, J.; Zou, X.; Huang, X.; Zhang, D.; Sun, Y.; Yang, Z.; Holmes, M.; et al. Natural biomaterial-based edible and pH-sensitive films combined with electrochemical writing for intelligent food packaging. J. Agric. Food Chem. 2018, 66, 12836–12846. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Dai, J.; Cui, H.; Lin, L. Edible films of pectin extracted from dragon fruit peel: Effects of boiling water treatment on pectin and film properties. Food Hydrocoll. 2024, 147, 109324. [Google Scholar] [CrossRef]
- Si, Z.; Hou, Z.; Vikhe, Y.S.; Thappeta, K.R.V.; Marimuthu, K.; De, P.P.; Ng, O.T.; Li, P.; Zhu, Y.; Pethe, K.; et al. Antimicrobial effect of a novel chitosan derivative and its synergistic effect with antibiotics. ACS Appl. Mater. Interfaces 2021, 13, 3237–3245. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Li, C.; Liu, R.; Lin, L. Fabrication of chitosan nanofibers containing tea tree oil liposomes against Salmonella spp. in chicken. LWT Food Sci. Technol. 2018, 96, 671–678. [Google Scholar] [CrossRef]
- Li, M.; Chen, C.; Xia, X.; Garba, B.; Shang, L.; Wang, Y. Proteomic analysis of the inhibitory effect of chitosan on Penicillium expansum. Food Sci. Technol. 2020, 40, 250–257. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Nasaj, M.; Chehelgerdi, M.; Asghari, B.; Ahmadieh-Yazdi, A.; Asgari, M.; Kabiri-Samani, S.; Sharifi, E.; Arabestani, M. Factors influencing the antimicrobial mechanism of chitosan action and its derivatives: A review. Int. J. Biol. Macromol. 2024, 277, 134321. [Google Scholar] [CrossRef]
- Cui, H.; Yuan, L.; Li, W.; Lin, L. Edible film incorporated with chitosan and Artemisia annua oil nanoliposomes for inactivation of Escherichia coli O157:H7 on cherry tomato. Int. J. Food Sci. Technol. 2016, 52, 687–698. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial actions and applications of chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Dasgupta, A.; Sarkar, J.; Panda, K.; Acharya, K. Chitosan nanoparticles: A positive modulator of innate immune responses in plants. Sci. Rep. 2015, 5, 15195. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as a potential natural compound to manage plant diseases. Int. J. Biol. Macromol. 2022, 220, 998–1009. [Google Scholar] [CrossRef]
- Dulta, K.; Agceli, G.K.; Singh, S.; Pandey, V.K.; Thakur, A.; Chauhan, P.K.; Aman, J.; Rustagi, S. Unveiling the effects of ZnO nanoparticle incorporated chitosan coating on postharvest quality of eggplant (Solanum melongena L.). Food Control 2025, 168, 110912. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Khaliq, G.; Ramzan, M.; Baloch, A.H. Effect of Aloe vera gel coating enriched with Fagonia indica plant extract on physicochemical and antioxidant activity of sapodilla fruit during postharvest storage. Food Chem. 2019, 286, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Flores-López, M.L.; Vieira, J.M.; C. Rocha, M.R.; Lagarón, J.M.; Cerqueira, M.A.; Jasso de Rodríguez, D.; Vicente, A.A. Postharvest quality improvement of tomato (Solanum lycopersicum L.) fruit using a nanomultilayer coating containing aloe vera. Foods 2023, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Jaimun, R.; Sangsuwan, J. Efficacy of chitosan-coated paper incorporated with vanillin and ethylene adsorbents on the control of anthracnose and the quality of Nam Dok Mai mango fruit. Packag. Technol. Sci. 2019, 32, 383–394. [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.; Li, J.; Li, M.; Xing, D.; An, H.; Wu, X.; Wu, Y. A chitosan composite film sprayed before pathogen infection effectively controls postharvest soft rot in kiwifruit. Agronomy 2020, 10, 265. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, N.; Zhou, Y.; Godana, E.A.; Dhanasekaran, S.; Gu, X.; Zhao, L.; Zhang, H. Transcriptome analysis reveals the mechanisms involved in the enhanced antagonistic efficacy of Rhodotorula mucilaginosa induced by chitosan. LWT Food Sci. Technol. 2021, 142, 110992. [Google Scholar] [CrossRef]
- Wang, Z.; Svyantek, A.; Miller, Z.; Jarrett, B.; Green, S.; Kapus, A. Postharvest treatment effects on ‘Somerset Seedless’ cold-hardy table grapes. Int. J. Fruit Sci. 2024, 24, 142–155. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Feng, C.; Li, X.; Liu, G.; Huang, Y.; Chen, R.; Tan, Y. Chitosan alleviates postharvest quality deterioration in fertile orange fruit by regulating membrane permeability and energy metabolism. J. Chin. Inst. Food Sci. Technol. 2023, 23, 250–258. [Google Scholar]
- Zheng, H.; Deng, W.; Yu, L.; Shi, Y.; Deng, Y.; Wang, D.; Zhong, Y. Chitosan coatings with different degrees of deacetylation regulate the postharvest quality of sweet cherry through internal metabolism. Int. J. Biol. Macromol. 2024, 254, 127419. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, S.; Gao, W.; Chen, X. Effects of chitosan with different molecular weights on storage quality and fungi inhibition of mini-cucumber. Food Control 2023, 153, 109905. [Google Scholar] [CrossRef]
- Alam, M.; Ali, S.; Shah, S.T.; Hussain, Z.; Ali, L.; Rab, A.; Muhammad, A.; Jadoon, S.A.; Ahmad, M.; Ahmad, I. Effect of chitosan on post-harvest shelf life of persimmon (Diospyros kaki L.). Acta Sci. Pol. Hortorum Cultus 2023, 22, 27–43. [Google Scholar] [CrossRef]
- Kaur, N.; Nikhanj, P. Investigating potential of edible coatings for microbial safety, quality and shelf life extension of fresh cut cucumbers: A statistical optimization approach. J. Stored Prod. Res. 2024, 108, 102393. [Google Scholar] [CrossRef]
- Su, J.; Zhang, W.; Moradi, Z.; Rouhi, M.; Parandi, E.; Garavand, F. Recent functionality developments of carboxymethyl chitosan as an active food packaging film material. Food Chem. 2025, 463, 141356. [Google Scholar] [CrossRef] [PubMed]
- Thanakkasaranee, S.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Jantrawut, P.; Ruksiriwanich, W.; Rose Sommano, S.; Punyodom, W.; et al. High substitution synthesis of carboxymethyl chitosan for properties improvement of carboxymethyl chitosan films depending on particle sizes. Molecules 2021, 26, 6013. [Google Scholar] [CrossRef]
- López-Maldonado, E.A.; Mavaei, M.; Dan, S.; Banitaba, S.N.; Gholamhosseinpour, M.; Hamedi, S.; Villarreal-Gómez, L.J.; Pérez-González, G.L.; Mashkouri, S.; Khademolqorani, S.; et al. Diverse applications of versatile quaternized chitosan salts: A review. Int. J. Biol. Macromol. 2024, 281, 136276. [Google Scholar] [CrossRef]
- Peng, N.; Ai, Z.; Fang, Z.; Wang, Y.; Xia, Z.; Zhong, Z.; Fan, X.; Ye, Q. Homogeneous synthesis of quaternized chitin in NaOH/urea aqueous solution as a potential gene vector. Carbohydr. Polym. 2016, 150, 180–186. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Du, H.; Li, Y.; Wen, Y.; Zhu, Z. A transparent p-coumaric acid-grafted-chitosan coating with antimicrobial, antioxidant and antifogging properties for fruit packaging applications. Carbohydr. Polym. 2024, 339, 122238. [Google Scholar] [CrossRef]
- Lee, C.R.; Lee, S.J.; Kim, T.I.; Chathuranga, K.; Lee, J.S.; Kim, S.; Kim, M.H.; Park, W.H. Chitosan-gallic acid conjugate edible coating film for perishable fruits. Food Chem. 2025, 463, 141322. [Google Scholar] [CrossRef]
- Jiang, S.; Qiao, C.; Liu, R.; Liu, Q.; Xu, J.; Yao, J. Structure and properties of citric acid cross-linked chitosan/poly(vinyl alcohol) composite films for food packaging applications. Carbohydr. Polym. 2023, 312, 120842. [Google Scholar] [CrossRef]
- Du, Y.; Shi, B.; Luan, X.; Wang, Y.; Song, H. Chitosan/cellulose nanocrystal biocomposite coating for fruit postharvest preservation. Ind. Crops Prod. 2023, 205, 117543. [Google Scholar] [CrossRef]
- He, C.; Yuan, L.; Bi, S.; Zhou, C.; Yang, Q.; Gu, J.; Yan, B.; He, J. Modified chitosan-based coating/packaging composites with enhanced antibacterial, antioxidant, and UV-resistant properties for fresh food preservation. ACS Appl. Mater. Interfaces 2024, 16, 48352–48362. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Sun, H.; Zhou, Y.; Tao, Y.; Zhang, C. Enhancement of antioxidant property of N-Carboxymethyl chitosan and its application in strawberry preservation. Molecules 2022, 27, 8496. [Google Scholar] [CrossRef]
- Pires, J.; Paula, C.D.d.; Souza, V.G.L.; Fernando, A.L.; Coelhoso, I. Understanding the barrier and mechanical behavior of different nanofillers in chitosan films for food packaging. Polymers 2021, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- Bansal, H.; Singh, S.; Sharma, A.; Kaur, K.; Yadav, K.; Bishnoi, M.; Singh, J.; Mehta, S.K. Enhancing grape preservation: The synergistic effect of curry leaf essential oil in buckwheat starch-chitosan composite coatings. Food Control 2025, 169, 111001. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Lekjing, S.; Noonim, P.; Charoenphun, N. Extension of quality and shelf life of tomatoes using chitosan coating incorporated with cinnamon oil. Foods 2024, 13, 1000. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, K.; Zhang, S.; Zhang, L.; Chang, J.; Jing, Z. Characterizations of water-soluble chitosan/curdlan edible coatings and the inhibitory effect on postharvest pathogenic fungi. Foods 2024, 13, 441. [Google Scholar] [CrossRef]
- Suresh, S.N.; Senthilkumar, P.; Pushparaj, C.; Sarangi, P.K.; Regina, V.R.; Subramani, R. Almond gum-chitosan nanocomposite as edible formulation for advancing postharvest longevity of fruits and vegetables. Polym. Adv. Technol. 2024, 35, e6453. [Google Scholar] [CrossRef]
- Yan, X.; Meng, F.; Wigati, L.P.; Van, T.T.; Phuong, N.T.H.; Koga, A.; Tanaka, F.; Tanaka, F. Improvement of cross-linked films based on chitosan/diepoxy-poly (ethylene glycol) incorporating trans-cinnamaldehyde essential oil: Preparation, properties, and application in banana storage. Int. J. Biol. Macromol. 2024, 263, 130299. [Google Scholar] [CrossRef]
- Shu, C.; Kim-Lee, B.; Sun, X. Chitosan coating incorporated with carvacrol improves postharvest guava (Psidium guajava) quality. Horticulturae 2024, 10, 80. [Google Scholar] [CrossRef]
- Shen, C.; Yang, X.; Wang, D.; Li, J.; Zhu, C.; Wu, D.; Chen, K. Carboxymethyl chitosan and polycaprolactone-based rapid in-situ packaging for fruit preservation by solution blow spinning. Carbohydr. Polym. 2024, 326, 121636. [Google Scholar] [CrossRef]
- Phuong, N.T.H.; Tanaka, F.; Wardana, A.A.; Van, T.T.; Yan, X.; Nkede, F.N.; Tanaka, F. Persimmon preservation using edible coating of chitosan enriched with ginger oil and visualization of internal structure changes using X-ray computed tomography. Int. J. Biol. Macromol. 2024, 262, 130014. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, Y.; Li, Z.; Sun, J.; Song, F.; Chen, J. Effects of chitosan grafted with gallic acid treatment on the postharvest physiology and biochemistry of Saimaiti apricots. J. Stored Prod. Res. 2024, 108, 102390. [Google Scholar] [CrossRef]
- Li, R.; Chen, C.; Chen, M.; Wu, R.; Sun, Y.; Zhu, B.; Yao, Z. Fabrication of carboxymethyl chitosan/oxidized carboxymethyl cellulose composite film and its assessment for coating preservation of strawberry. J. Food Sci. 2023, 88, 1865–1878. [Google Scholar] [CrossRef]
- Xiao, Z.; Ma, X.; Sun, P.; Kang, Y.; Niu, Y.; She, Y.; Zhao, D. Tomato preservation with essential oil microcapsules-chitosan coating. J. Food Meas. Charact. 2024, 18, 5928–5944. [Google Scholar] [CrossRef]
- Shen, C.; Chen, W.; Li, C.; Aziz, T.; Cui, H.; Lin, L. Topical advances of edible coating based on the nanoemulsions encapsulated with plant essential oils for foodborne pathogen control. Food Control 2023, 145, 109419. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Javed, M.; Huang, H.; Ma, Y.; Ettoumi, F.-e.; Wang, L.; Xu, Y.; El-Seedi, H.R.; Ru, Q.; Luo, Z. Construction of self-assembled nano cellulose crystals/chitosan nanobubbles composite hydrogel with improved gallic acid release property. Food Chem. 2024, 438, 137948. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Ai, S.; Guo, Z. Biobased composite films for strawberry preservation with bacterial antifouling and liquid resistance. Ind. Eng. Chem. Res. 2024, 63, 16672–16682. [Google Scholar] [CrossRef]
- Yin, C.; Sun, Z.; Yang, Y.; Cui, M.; Zheng, J.; Zhang, Y. Rapid in situ formation of κ-carrageenan-carboxymethyl chitosan-kaolin clay hydrogel films enriched with arbutin for enhanced preservation of cherry tomatoes. Int. J. Biol. Macromol. 2024, 273, 132957. [Google Scholar] [CrossRef]
- Tayel, A.A.; Ebaid, A.M.; Otian, A.M.; Mahrous, H.; El Rabey, H.A.; Salem, M.F. Application of edible nanocomposites from chitosan/fenugreek seed mucilage/selenium nanoparticles for protecting lemon from green mold. Int. J. Biol. Macromol. 2024, 273, 133109. [Google Scholar] [CrossRef]
- Taghipour, S.; Nia, A.E.; Hokmabadi, H.; Yahia, E.M. Quality evaluation of fresh pistachios (Pistacia vera L.) cultivars coated with chitosan/TiO2 nanocomposite. Int. J. Biol. Macromol. 2024, 258, 129055. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, S.; Nia, A.E.; Hokmabadi, H.; Martinez-Gomez, P. Physicochemical and quality characters of fresh pistachio (Pistacia vera L.) cultivars in response to chitosan/ZnO nanocomposite coating. Food Chem. 2024, 435, 137136. [Google Scholar] [CrossRef] [PubMed]
- Rashedy, A.A.; Abd El-Aziz, M.E.; Abd-Allah, A.S.E.; Hamed, H.H.; Emam, H.E.; Abd El-Moniem, E.A.A. Arabic gum/chitosan/Zn-NPs composite film maintains the quality of Hass avocado fruit by delaying ripening and activating enzymatic defense mechanisms. Sci. Rep. 2024, 14, 401. [Google Scholar] [CrossRef] [PubMed]
- Menezes, F.L.G.d.; Leite, R.H.d.L.; dos Santos, F.K.G.; Aria, A.I.; Aroucha, M.M.E. TiO2 incorporated into a blend of biopolymeric matrices improves film properties and affects the postharvest conservation of papaya fruits under UV light. Food Chem. 2024, 433, 137387. [Google Scholar] [CrossRef]
- da Silva, W.A.O.; Aroucha, E.M.M.; de Araujo, N.O.; dos Santos, F.K.G.; de Medeiros, J.F.; de Sousa, A.L.V.; Queiroz, L.P.d.O.; Leite, R.H.d.L. The shelf life of yellow passion fruit with an edible biocomposite coating based on chitosan, graphene oxide nanoparticles, and beeswax. Horticulturae 2024, 10, 756. [Google Scholar] [CrossRef]
- Wardana, A.A.; Wigati, L.P.; Marcellino, V.; Kusuma, G.; Yan, X.R.; Nkede, F.N.; Jothi, J.S.; Hang, N.P.T.; Tanaka, F.; Tanaka, F.; et al. The incorporation of chitosan nanoparticles enhances the barrier properties and antifungal activity of chitosan-based nanocomposite coating films. Int. J. Biol. Macromol. 2024, 280, 135840. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, R.; Zhou, Y.; Zhang, R.; Chen, S.; Yang, Q.; Gu, Y.; Han, L.; Yan, B. Janus biopolymer nanocomposite coating with excellent antibacterial and water/oxygen barrier performance for fruit preservation. Food Hydrocoll. 2024, 149, 109528. [Google Scholar] [CrossRef]
- Sharma, T.; Kaur, G.; Singh, A.; Kumar, V.; Dar, B.N. Enhancing litchi shelf life with gluten-based nanocomposite films: A comparative study of montmorillonite and starch nanocrystals reinforced with chitosan. Sci. Hortic. 2024, 332, 113239. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Wu, L.; Li, Y.; Li, H. Preparation and characterization of chitosan/dialdehyde carboxymethyl cellulose composite film loaded with cinnamaldehyde@zein nanoparticles for active food packaging. Int. J. Biol. Macromol. 2024, 261, 129586. [Google Scholar] [CrossRef]
- Rabasco-Vilchez, L.; Jimenez-Jimenez, F.; Possas, A.; Morcillo-Martin, R.; Perez-Rodriguez, F. Evaluation of the effect of nanocellulose edible coating on strawberries inoculated with Aspergillus flavus through image analysis. LWT Food Sci. Technol. 2024, 191, 115697. [Google Scholar] [CrossRef]
- Han, C.; Lederer, C.; McDaniel, M.; Zhao, Y. Sensory evaluation of fresh strawberries (Fragaria ananassa) coated with chitosan-based edible coatings. J. Food Sci. 2006, 70, S172–S178. [Google Scholar] [CrossRef]
- Hesami, A.; Kavoosi, S.; Khademi, R.; Sarikhani, S. Effect of chitosan coating and storage temperature on shelf-life and fruit quality of Ziziphus mauritiana. Int. J. Fruit Sci. 2021, 21, 509–518. [Google Scholar] [CrossRef]
- Riseh, R.S.; Vatankhah, M.; Hassanisaadi, M.; Shafiei-Hematabad, Z.; Kennedy, J.F. Advancements in coating technologies: Unveiling the potential of chitosan for the preservation of fruits and vegetables. Int. J. Biol. Macromol. 2024, 254, 127677. [Google Scholar]


| Fruit | Composition | Effect | Reference |
|---|---|---|---|
| Green grapes | Buckwheat starch + chitosan + curry leaf essential oil | Extend the shelf life to 12 days at room temperature and to 20 days under refrigeration storage. | [124] |
| Tomato | Chitosan + cinnamon oil | Modulate physicochemical qualities such as pH, TSS, TA, and TSS/TA ratios during 14-day storage. | [125] |
| Cherry tomato | Chitosan + curdlan | Maintain the quality of the postharvest cherry tomatoes at 10 days of storage. | [126] |
| Tomatoes and blueberry | Almond gum + chitosan | Extend the shelf life of tomatoes and blueberries to 25 days and 20 days. | [127] |
| Banana | Chitosan + diepoxy-polyethylene glycol + trans-cinnamaldehyde | Maintain the levels of TSS, TA, and appearance within 12–24 days. | [128] |
| Guava | Chitosan + carvacrol | Maintain the quality of guava with higher hardness, SSC, TA, and total phenol content, and lower weight loss. | [129] |
| Cherry tomato | Carboxymethyl chitosan + polycaprolactone | Maintain the weight loss, firmness loss, and color deepening during storage. | [130] |
| Persimmon | Chitosan + ginger oil | Inhibit changes in weight loss, respiration rate, ethylene formation, pH, and TSS during storage. | [131] |
| Saimaiti apricot | Chitosan + chitosan grafted with gallic acid | Extend the shelf life by 12 days compared to traditional refrigeration. | [132] |
| Strawberry | Carboxymethyl chitosan + oxidized carboxymethyl cellulose | Reduce the decay rate by about 42% compared to the control. | [133] |
| Fruit | Composition | Nanomaterial | Effect | Reference |
|---|---|---|---|---|
| Strawberry | Chitosan + carnauba wax | Resveratrol-encapsulated shellac nanoparticles | Enhance the mechanical and UV resistance properties and extend shelf life to 15 days. | [138] |
| Cherry tomato | Carboxymethyl chitosan + κ-carrageenan + arbutin | Kaolin clay | Enhance tensile strength from 20.60 MPa to 34.71 MPa and prolong storage for 9 days at 28 °C. | [139] |
| Lemon | Chitosan + fenugreek seed mucilage | Selenium nanoparticles | Eliminate green mold development after 10 days of coating. | [140] |
| Fresh pistachio | Chitosan | TiO2 | Extend the shelf life to 30 days. | [141] |
| Fresh pistachio | Chitosan | ZnO | Extend the shelf life to 35–40 days. | [142] |
| Avocado | Chitosan + arabic gum | Zinc nanoparticles | Inhibit weight loss, decay, and improve peel and pulp color. | [143] |
| Papaya | Chitosan + cassava starch | TiO2 | Reduce weight loss by 7.12 ± 1.57% and 5.27 ± 0.31% in light and darkness, respectively. | [144] |
| Passion fruit | Chitosan + beeswax | Graphene oxide | Enhance SSC and TA by 16.7% and 31.9% on day 8. | [145] |
| Tangerine | Chitosan | Chitosan nanoparticles | Inhibit the tendency of color and TSS. | [146] |
| Mango, banana and loquat | Quaternized chitosan | Aldehyde carboxycellulose nanofibres | Extend the shelf life of fruits by 5–10 days. | [147] |
| Lychee | Gluten + chitosan | Starch nanocrystals/montmorillonite | Maintain acceptable quality for three weeks. | [148] |
| Strawberry | Chitosan + dialdehyde carboxymethyl cellulose | Zein-loaded cinnamaldehyde nanoparticles | Extend the shelf life to 7 days. | [149] |
| Strawberry | Chitosan + raspberry leaf extract | Lignocellulosic nanofibers | Reduce weight loss by 26.4% compared to the uncoated group. | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Wang, X.; Zhang, J.; Li, C. Application of Chitosan and Its Derivatives in Postharvest Coating Preservation of Fruits. Foods 2025, 14, 1318. https://doi.org/10.3390/foods14081318
Dai L, Wang X, Zhang J, Li C. Application of Chitosan and Its Derivatives in Postharvest Coating Preservation of Fruits. Foods. 2025; 14(8):1318. https://doi.org/10.3390/foods14081318
Chicago/Turabian StyleDai, Limin, Xiaoshuai Wang, Jun Zhang, and Changwei Li. 2025. "Application of Chitosan and Its Derivatives in Postharvest Coating Preservation of Fruits" Foods 14, no. 8: 1318. https://doi.org/10.3390/foods14081318
APA StyleDai, L., Wang, X., Zhang, J., & Li, C. (2025). Application of Chitosan and Its Derivatives in Postharvest Coating Preservation of Fruits. Foods, 14(8), 1318. https://doi.org/10.3390/foods14081318








