Comparative Analysis of Chilling Injury in Banana Fruit During Storage: Physicochemical and Microstructural Changes, and Early Optical-Based Nondestructive Identification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physicochemical Property Analysis
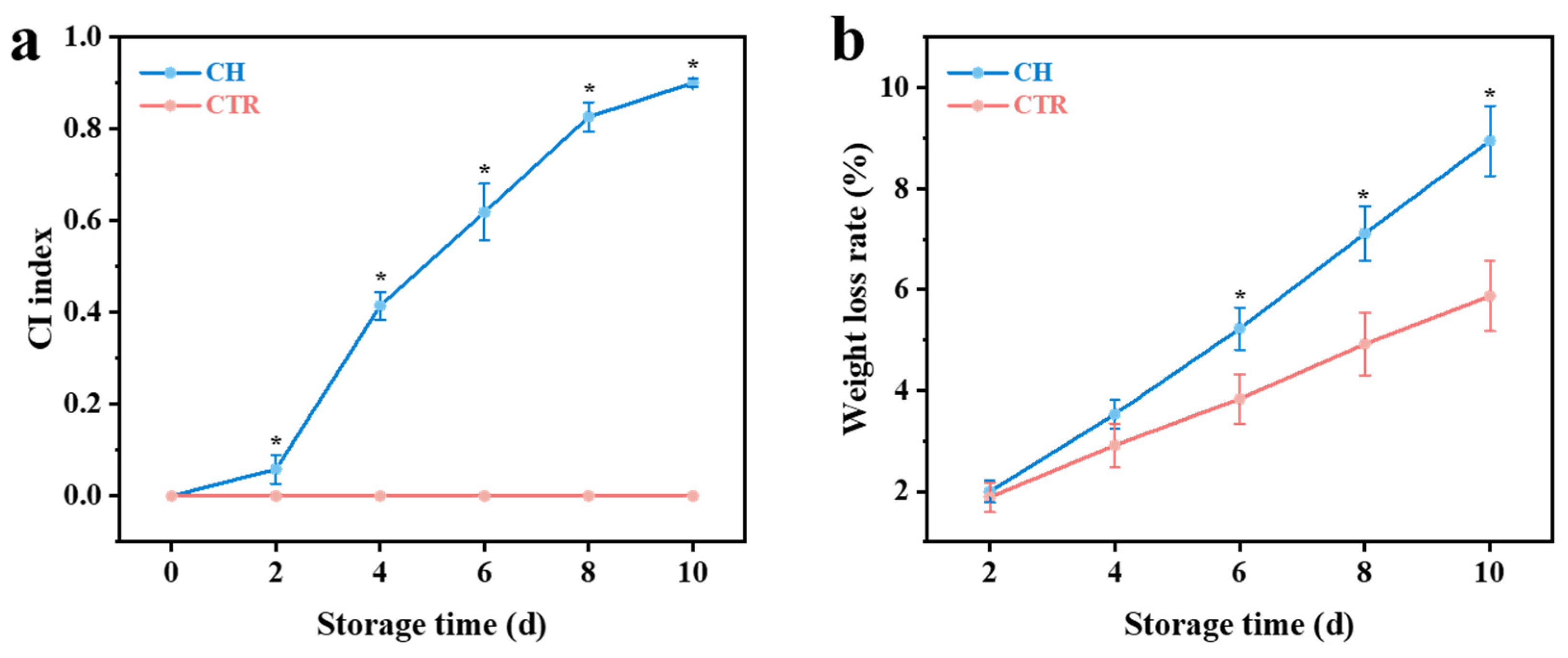
2.2.1. CI Index
2.2.2. Weight Loss Rate
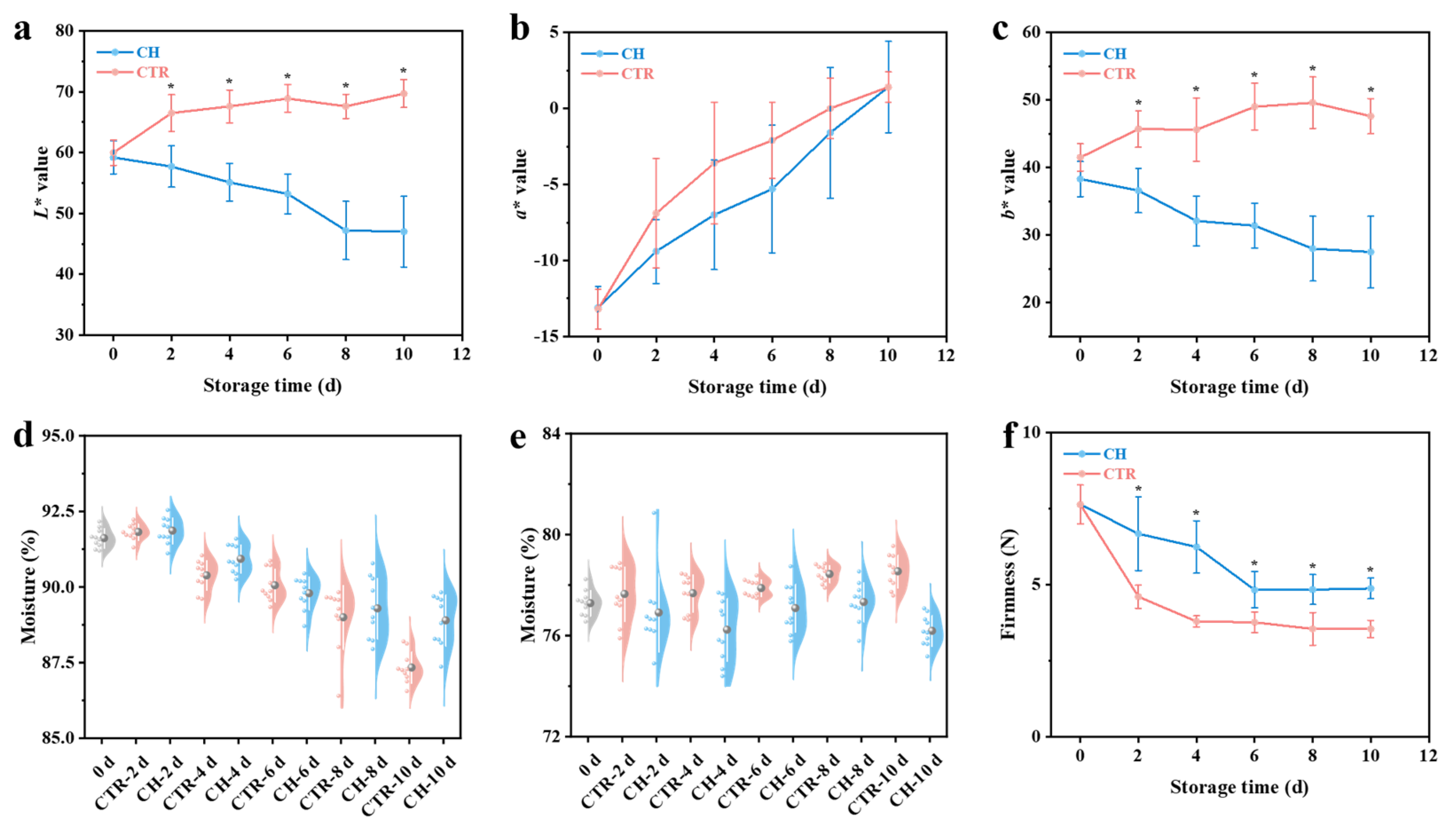
2.2.3. Peel Color
2.2.4. Moisture Content
2.2.5. Pulp Firmness
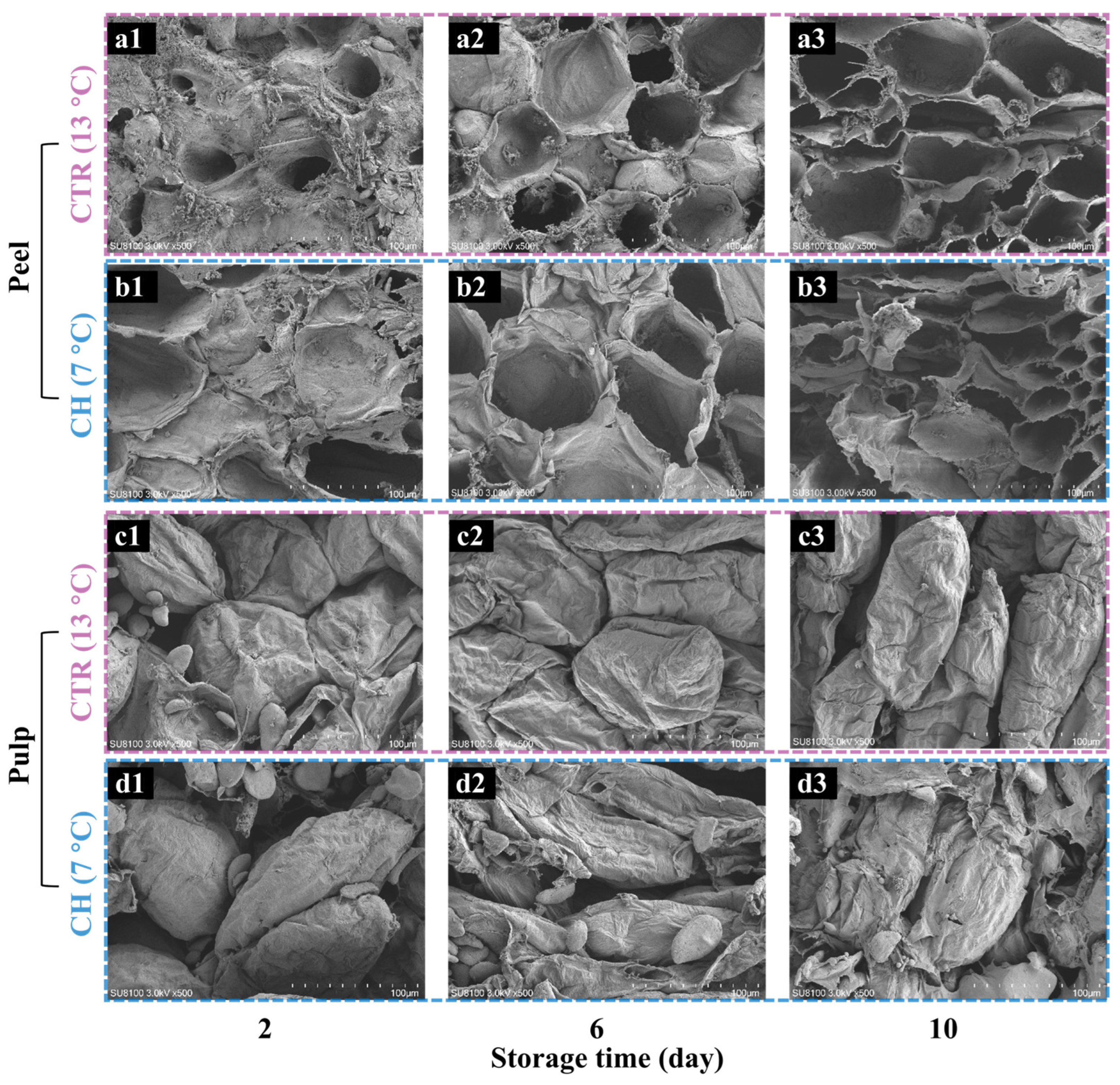
2.3. Microstructural Property Analysis
2.4. Optical Property Analysis
2.4.1. Hyperspectral Microscope Imaging
2.4.2. Hyperspectral Imaging
2.5. Statistical Analysis and Software
3. Results and Discussion
3.1. Changes in Physicochemical Properties
3.2. Changes in Microstructural Properties
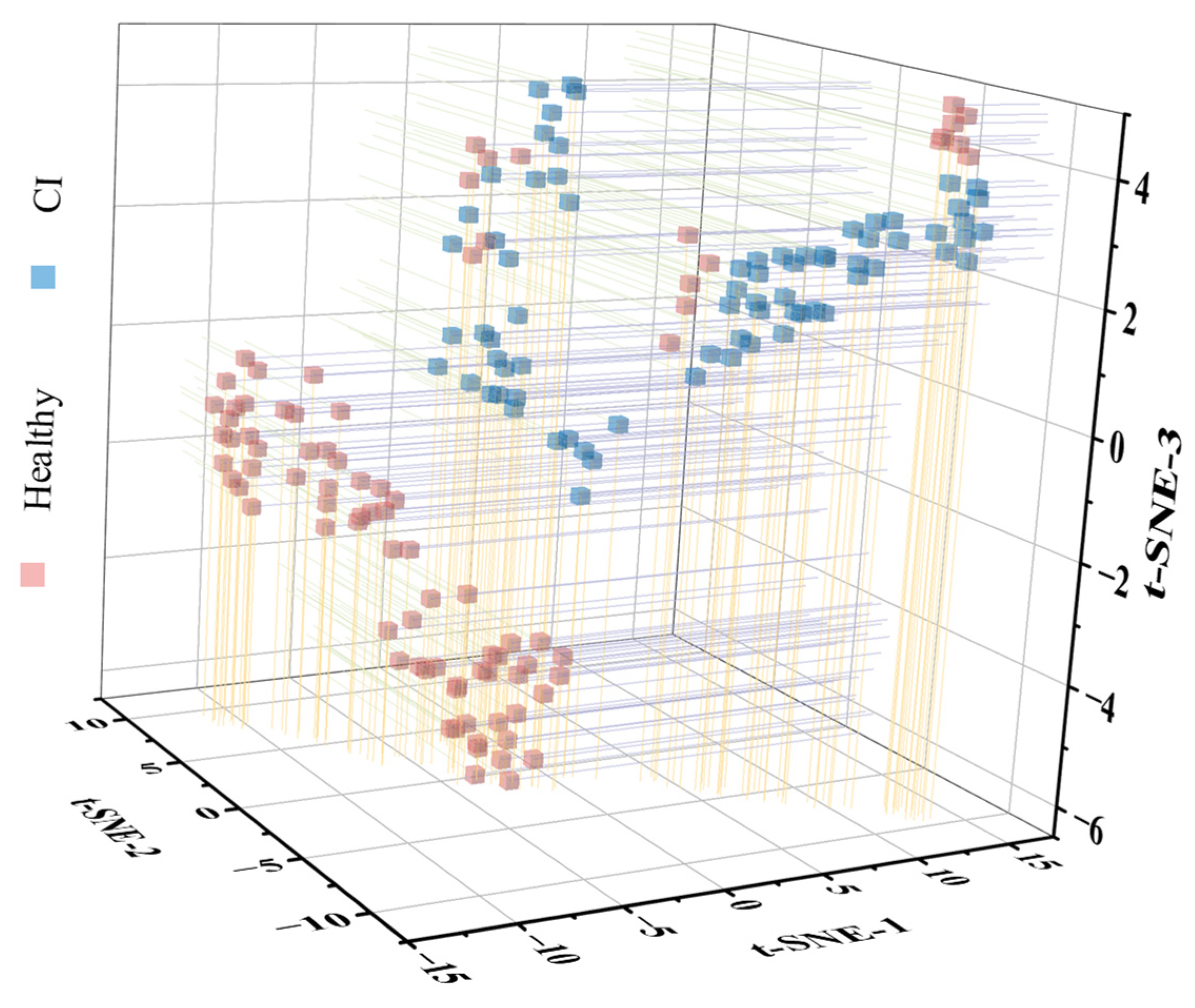
3.3. Identification of Chilling Injury Using Hyperspectral Microscope Imaging
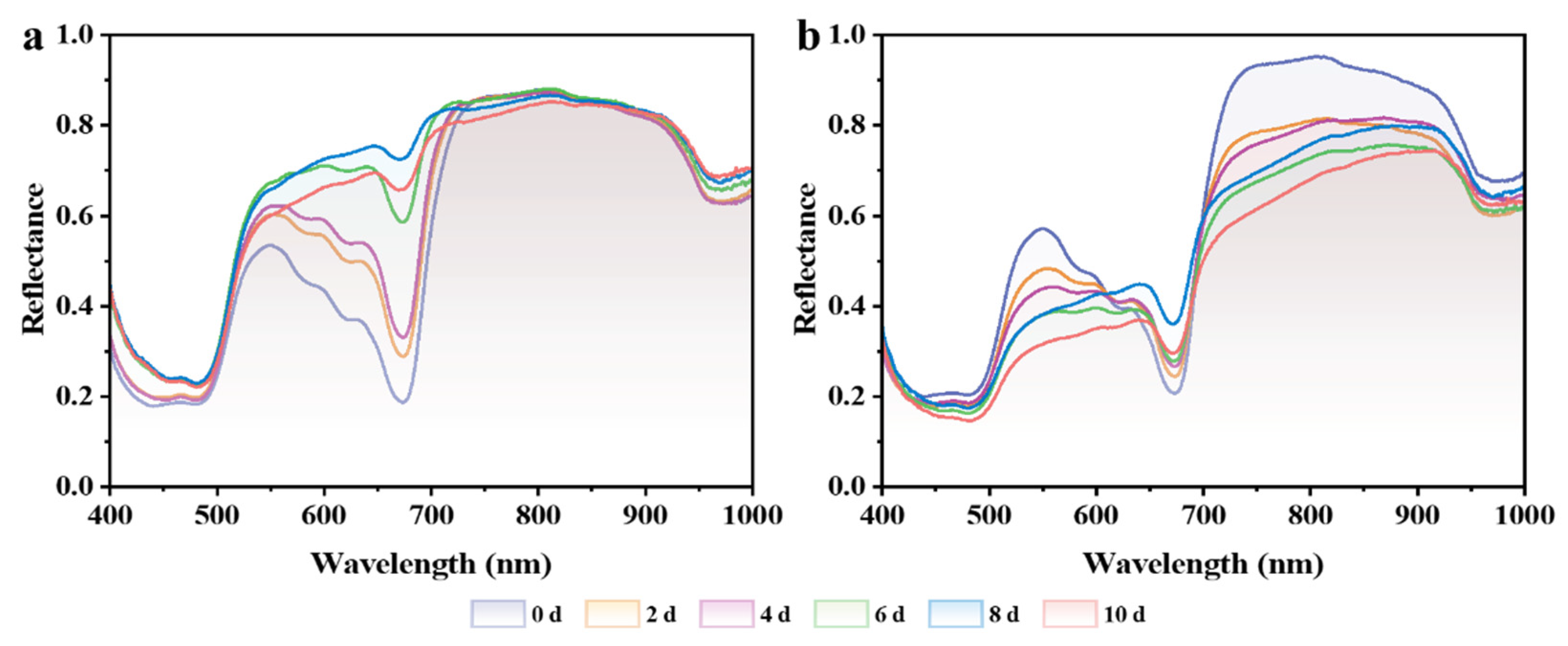
3.4. Early Nondestructive Identification of Chilling-Injured Banana Fruit Using Hyperspectral Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bomfim, N.S.; Ferreira, R.S.; Oliveira, J.S.; Alfenas, R.C.G. Green banana biomass anti-obesogenic, anti-hyperlipidemic, antidiabetic, and intestinal function potential effects: A systematic review. Nutr. Rev. 2024, 83, e293–e303. [Google Scholar] [CrossRef]
- Hilaluddin; Mondal, S.; Rakshit, S.; Pal, K.; Santra, S.; Goswami, D.; Mondal, S.P.; Halder, S.K.; Mondal, K.C. Production of glutathione from probiotic Bacillus amyloliquefaciens KMH10 using banana peel extract. Bioresour. Technol. 2023, 376, 128910. [Google Scholar] [CrossRef]
- Li, Q.; Lin, H.; Lin, H.; Lin, M.; Wang, H.; Wei, W.; Chen, J.; Lu, W.; Shao, X.; Fan, Z. The metabolism of membrane lipid participates in the occurrence of chilling injury in cold-stored banana fruit. Food Res. Int. 2023, 173, 113415. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, X.; Wu, J.; He, Y.; Yang, H. Metabolic analysis of salicylic acid-induced chilling tolerance of banana using NMR. Food Res. Int. 2020, 128, 108796. [Google Scholar] [CrossRef]
- Bugaud, C.; Joannès-Dumec, C.; Louisor, J.; Tixier, P.; Salmon, F. Preharvest temperature affects chilling injury in dessert bananas during storage. J. Sci. Food Agric. 2016, 96, 2384–2390. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, Y.; Lin, H.; Shi, J.; Chen, Y.; Wang, H. Inhibitory effects of propyl gallate on membrane lipids metabolism and its relation to increasing storability of harvested longan fruit. Food Chem. 2017, 217, 133–138. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Ndlovu, P.F.; Magwaza, L.S.; Tesfay, S.Z.; Mphahlele, R.R. Destructive and rapid non-invasive methods used to detect adulteration of dried powdered horticultural products: A review. Food Res. Int. 2022, 157, 111198. [Google Scholar] [CrossRef]
- Ge, W.; Zhao, Y.; Kong, X.; Sun, H.; Luo, M.; Yao, M.; Wei, B.; Ji, S. Combining salicylic acid and trisodium phosphate alleviates chilling injury in bell pepper (Capsicum annuum L.) through enhancing fatty-acid desaturation efficiency and water retention. Food Chem. 2020, 327, 127057. [Google Scholar] [CrossRef]
- Jiang, M.; You, S.; Sha, H.; Bai, B.; Zhang, L.; Tu, K.; Peng, J.; Song, L.; Lan, W.; Pan, L. Detection of Alternaria alternata infection in winter jujubes based on optical properties and their correlation with internal quality parameters during storage. Food Chem. 2023, 409, 135298. [Google Scholar] [CrossRef]
- You, S.; Jiang, M.; Lan, W.; Chen, M.; Bai, B.; Zhang, L.; Tu, K.; Song, L.; Pan, L. Assessment of the optical properties with physicochemical properties and cell wall polysaccharides of ‘Korla’ pear flesh during Alternaria alternata-induced disease development. Food Chem. 2023, 409, 135302. [Google Scholar] [CrossRef]
- Rizzolo, A.; Bianchi, G.; Vanoli, M.; Lurie, S.; Spinelli, L.; Torricelli, A. Electronic nose to detect volatile compound profile and quality changes in ‘spring Belle’ peach (Prunus persica L.) during cold storage in relation to fruit optical properties measured by time-resolved reflectance spectroscopy. J. Agric. Food. Chem. 2013, 61, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.; Vanoli, M.; Dagar, A.; Weksler, A.; Lovati, F.; Zerbini, P.E.; Spinelli, L.; Torricelli, A.; Feng, J.; Rizzolo, A. Chilling injury in stored nectarines and its detection by time-resolved reflectance spectroscopy. Postharvest Biol. Technol. 2011, 59, 211–218. [Google Scholar] [CrossRef]
- Chu, X.; Zhang, K.; Wei, H.; Ma, Z.; Fu, H.; Miao, P.; Jiang, H.; Liu, H. A Vis/NIR spectra-based approach for identifying bananas infected with Colletotrichum musae. Front. Plant Sci. 2023, 14, 1180203. [Google Scholar] [CrossRef]
- Zaukuu, J.Z.; Mensah, S.; Mensah, E.T.; Akomanin-Mensah, F.; Wiredu, S.; Kovacs, Z. Combining NIR spectroscopy with chemometrics for discriminating naturally ripened banana and calcium carbide ripened banana. NPJ Sci. Food 2024, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Kuroki, S.; Ikehata, A. Selective detection of starch in banana fruit with Raman spectroscopy. Food Chem. 2023, 401, 134166. [Google Scholar] [CrossRef]
- Wang, Z.; Song, S.; Zhao, M.; Zuo, C.; Wang, M.; Zhao, J.; Song, J.; Tu, K.; Lan, W.; Song, D. New insights into the relationship between optical response and physicochemical properties in apple flesh: Hyperspectral microscope imaging technology. Food Res. Int. 2024, 194, 114940. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, Q.; Zhang, W.; Sun, Y.; Hu, P.; Tu, K. Detection of cold injury in peaches by hyperspectral reflectance imaging and artificial neural network. Food Chem. 2016, 192, 134–141. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, S.; Zhou, D.; Ding, C.; Wang, J.; Zhou, H.; Tu, K.; Pan, L. Nondestructive detection of weight loss rate, surface color, vitamin C content, and firmness in mini-Chinese cabbage with nanopackaging by Fourier Transform-near infrared spectroscopy. Foods 2021, 10, 2309. [Google Scholar] [CrossRef]
- Wang, J.; Huo, Y.; Wang, Y.; Zhao, H.; Li, K.; Liu, L. Grading detection of “Red Fuji” apple in Luochuan based on machine vision and near-infrared spectroscopy. PLoS ONE 2022, 17, e0271352. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Jin, S.; Dong, W.; Zhang, Y.; Chen, W.; Shi, L.; Cao, S.; Yang, Z. γ-Aminobutyric acid treatment induced chilling tolerance in postharvest kiwifruit (Actinidia chinensis cv. Hongyang) via regulating ascorbic acid metabolism. Food Chem. 2023, 404, 134661. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, L.; Qiu, D.; Zhang, N.; Bi, F. Changes of morphology, chemical compositions, and the biosynthesis regulations of cuticle in response to chilling injury of banana fruit during storage. Front. Plant Sci. 2021, 12, 792384. [Google Scholar] [CrossRef]
- Xiao, L.; Jiang, X.; Deng, Y.; Xu, K.; Duan, X.; Wan, K. Study on characteristics and lignification mechanism of postharvest banana fruit during chilling injury. Foods 2023, 12, 1097. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Z.; Zhu, W.; Ni, X.; Wang, J.; Fu, L.; Chen, J.; Li, T.; Tang, L.; Yang, Y.; et al. The MaNAP1-MaMADS1 transcription factor module mediates ethylene-regulated peel softening and ripening in banana. Plant Cell 2024, 37, koae282. [Google Scholar] [CrossRef]
- Sun, P.; Zhu, Z.; Jin, Z.; Xie, J.; Miao, H.; Liu, J. Molecular characteristics and functional identification of a key alpha-amylase-encoding gene AMY11 in Musa acuminata. Int. J. Mol. Sci. 2024, 25, 7832. [Google Scholar] [CrossRef]
- Li, A.; Chen, J.; Lin, Q.; Zhao, Y.; Duan, Y.; Wai, S.; Song, C.; Bi, J. Transcription factor MdWRKY32 participates in starch-sugar metabolism by binding to the MdBam5 promoter in apples during postharvest storage. J. Agric. Food Chem. 2021, 69, 14906–14914. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, Y.; Guo, R.; Jiao, L.; Wang, K.; Zhu, Q.; Dong, D. In situ detection of fruit spoilage based on volatile compounds using the mid-infrared fiber-optic evanescent wave spectroscopy. Front. Plant Sci. 2022, 13, 991883. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Kim, S.W. Current only-based fault diagnosis method for industrial robot control cables. Sensors 2022, 22, 1917. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Y.; Sun, Y.; Zhou, H.; Chen, K. Identification of apple varieties using a multichannel hyperspectral imaging system. Sensors 2020, 20, 5120. [Google Scholar] [CrossRef]
- Kim, M.; Hong, J.; Lee, D.; Kim, S.; Chun, H.S.; Cho, Y.H.; Kim, B.H.; Ahn, S. Discriminant analysis of the geographical origin of Asian red pepper powders using second-derivative FT-IR spectroscopy. Foods 2021, 10, 1034. [Google Scholar] [CrossRef]
- Rahman, D.Y.; Sarian, F.D.; Wijk, A.; Martinez-Garcia, M.; Maarel, M.J.E.C. Thermostable phycocyanin from the red microalga Cyanidioschyzon merolae, a new natural blue food colorant. J. Appl. Phycol. 2017, 29, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wei, W.; Yang, Y.; Wu, C.; Chen, J.; Lu, W.; Kuang, J.; Shan, W. E3 ligase MaNIP1 degradation of NON-YELLOW COLORING1 at high temperature inhibits banana degreening. Plant Physiol. 2023, 192, 1969–1981. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Wang, C.; Chan, D.; Kim, M.S. Development of hyperspectral imaging technique for the detection of chilling injury in cucumbers; spectral and image analysis. Appl. Eng. Agric. 2006, 22, 101–111. [Google Scholar] [CrossRef]
- Guo, X.; Tseung, C.; Zare, A.; Liu, T. Hyperspectral image analysis for the evaluation of chilling injury in avocado fruit during cold storage. Postharvest Biol. Technol. 2023, 206, 112548. [Google Scholar] [CrossRef]
- Cen, H.; Lu, R.; Zhu, Q.; Mendoza, F. Nondestructive detection of chilling injury in cucumber fruit using hyperspectral imaging with feature selection and supervised classification. Postharvest Biol. Technol. 2016, 111, 352–361. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, S.H.; Lee, M.A.; Chung, Y.B.; Yang, J.H.; Cho, J.; Min, S.G. Chemometrics and neural networks for estimating the chilling injury severity of kimchi cabbage (Brassica rapa L. ssp. pekinensis) based on hyperspectral images. LWT 2024, 207, 116601. [Google Scholar] [CrossRef]
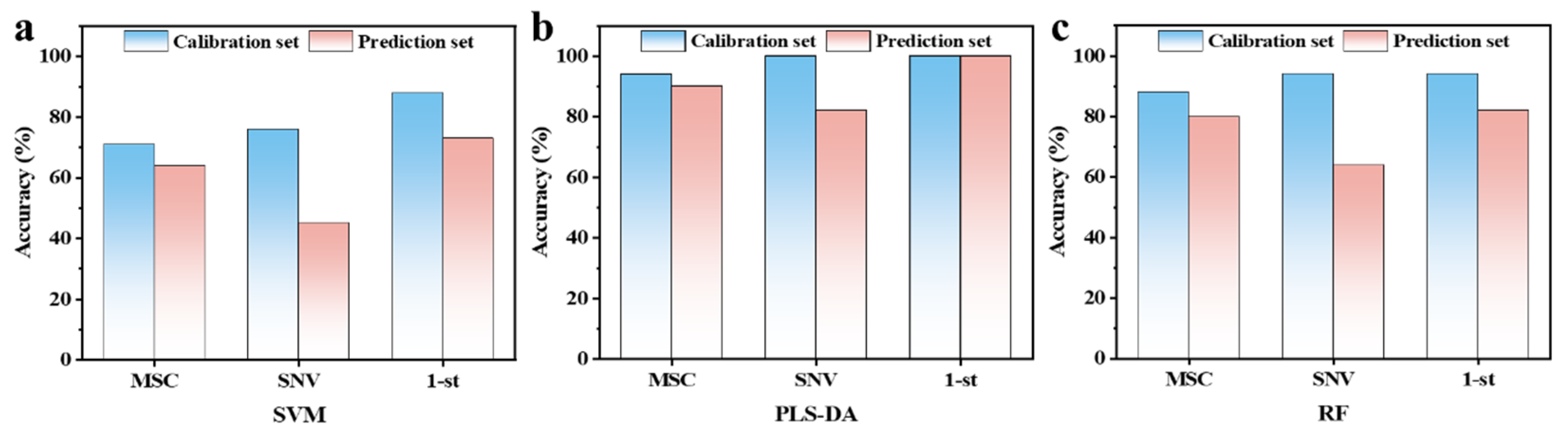
| Model | Preprocessing | Calibration Set Accuracy (%) | Prediction Set Accuracy (%) | ||||
|---|---|---|---|---|---|---|---|
| Healthy | CI | Overall | Healthy | CI | Overall | ||
| SVM | Raw | 91 | 87 | 89.2 | 79 | 86 | 82.3 |
| 1-st | 86 | 98 | 92 | 75 | 98 | 85.4 | |
| 2-nd | 86 | 97 | 91.5 | 77 | 98 | 87.5 | |
| PLS-DA | Raw | 94 | 93 | 93.8 | 87 | 80 | 83.3 |
| 1-st | 98 | 100 | 99 | 97 | 100 | 98.5 | |
| 2-nd | 91 | 100 | 95.4 | 90 | 98 | 93.7 | |
| RF | Raw | 98 | 89 | 93.8 | 91 | 83 | 87.7 |
| 1-st | 100 | 98 | 99 | 97 | 100 | 98.5 | |
| 2-nd | 100 | 95 | 97.9 | 97 | 93 | 95.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Hu, L.; Zhao, J.; He, J.; Wen, A.; Lv, D.; Xu, Z.; Lan, W.; Pan, L. Comparative Analysis of Chilling Injury in Banana Fruit During Storage: Physicochemical and Microstructural Changes, and Early Optical-Based Nondestructive Identification. Foods 2025, 14, 1319. https://doi.org/10.3390/foods14081319
Ma H, Hu L, Zhao J, He J, Wen A, Lv D, Xu Z, Lan W, Pan L. Comparative Analysis of Chilling Injury in Banana Fruit During Storage: Physicochemical and Microstructural Changes, and Early Optical-Based Nondestructive Identification. Foods. 2025; 14(8):1319. https://doi.org/10.3390/foods14081319
Chicago/Turabian StyleMa, Hui, Lingmeng Hu, Jingyuan Zhao, Jie He, Anqi Wen, Daizhu Lv, Zhi Xu, Weijie Lan, and Leiqing Pan. 2025. "Comparative Analysis of Chilling Injury in Banana Fruit During Storage: Physicochemical and Microstructural Changes, and Early Optical-Based Nondestructive Identification" Foods 14, no. 8: 1319. https://doi.org/10.3390/foods14081319
APA StyleMa, H., Hu, L., Zhao, J., He, J., Wen, A., Lv, D., Xu, Z., Lan, W., & Pan, L. (2025). Comparative Analysis of Chilling Injury in Banana Fruit During Storage: Physicochemical and Microstructural Changes, and Early Optical-Based Nondestructive Identification. Foods, 14(8), 1319. https://doi.org/10.3390/foods14081319









