Detection of Mechanically Separated Meat from Pork in Meat-Containing Foods by Targeted LC-MS/MS Analysis
Abstract
1. Introduction
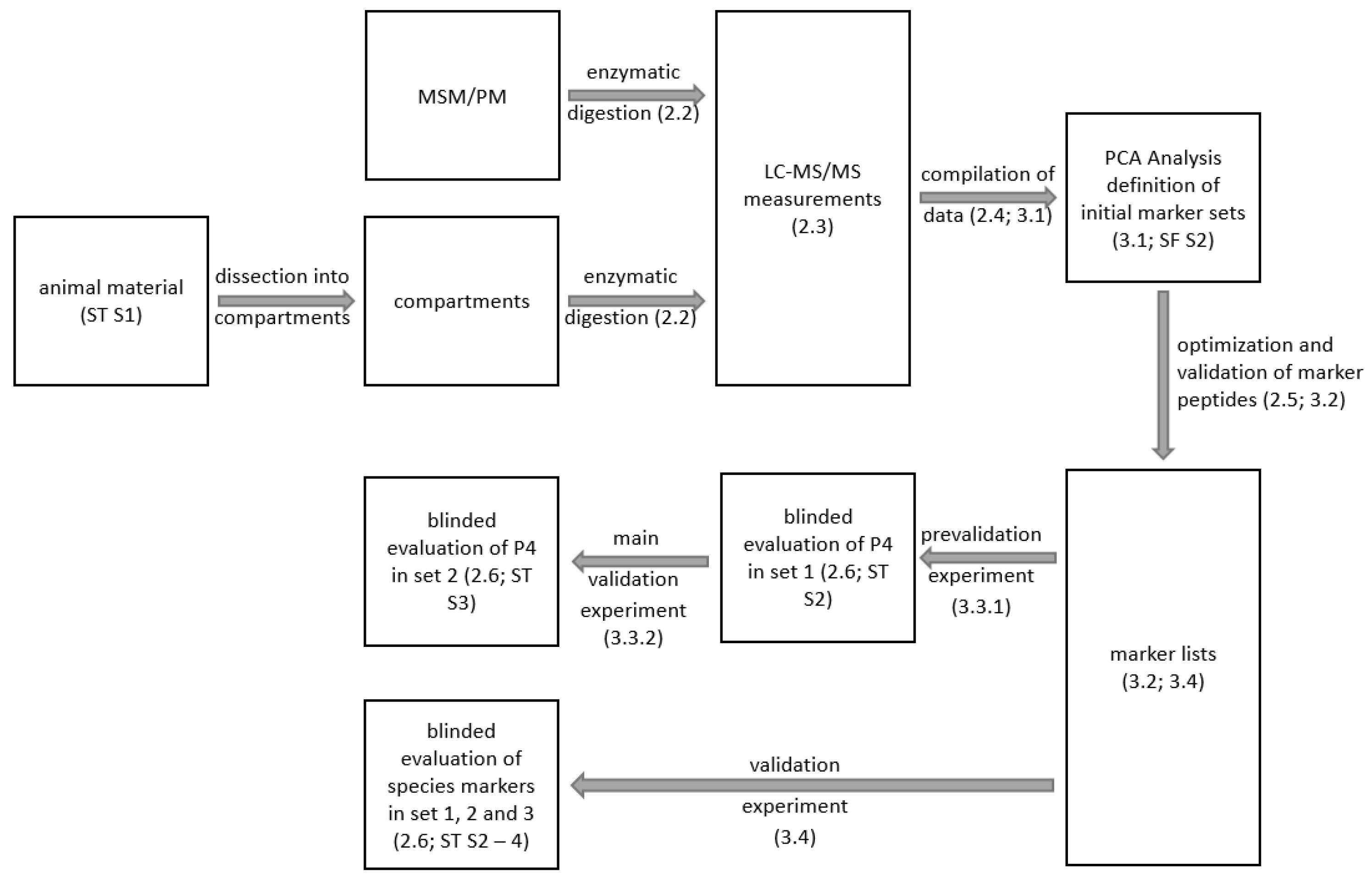
2. Materials and Methods
2.1. Animal and Raw Material
2.2. Sample Preparation
2.3. LC-MS/MS
2.4. Definition of Specific Marker Ions (MarkerView)
- One-fold-charged molecules are not accepted as candidates;
- The frequency of occurrence of every candidate marker ion must be 100% in one of the groups (meat, tendon, skin, MSM, etc.);
- Four pMRM transitions could be allocated;
- In pure tissue, each pMRM transition must be at least ten times higher than the minimum acceptable signal intensity of 50 counts or SNR of 3 (Supplementary Table S7);
- Marker ions identified as peptides must show a minimal size of six amino acids.
2.5. Identification and Verification of Specific Marker Ions
2.6. Samples for the Validation of the MSM Assay and the Species-Specific Markers
2.7. Assignment of Samples
2.8. Statistical Analysis
2.9. Calcium Content
3. Results
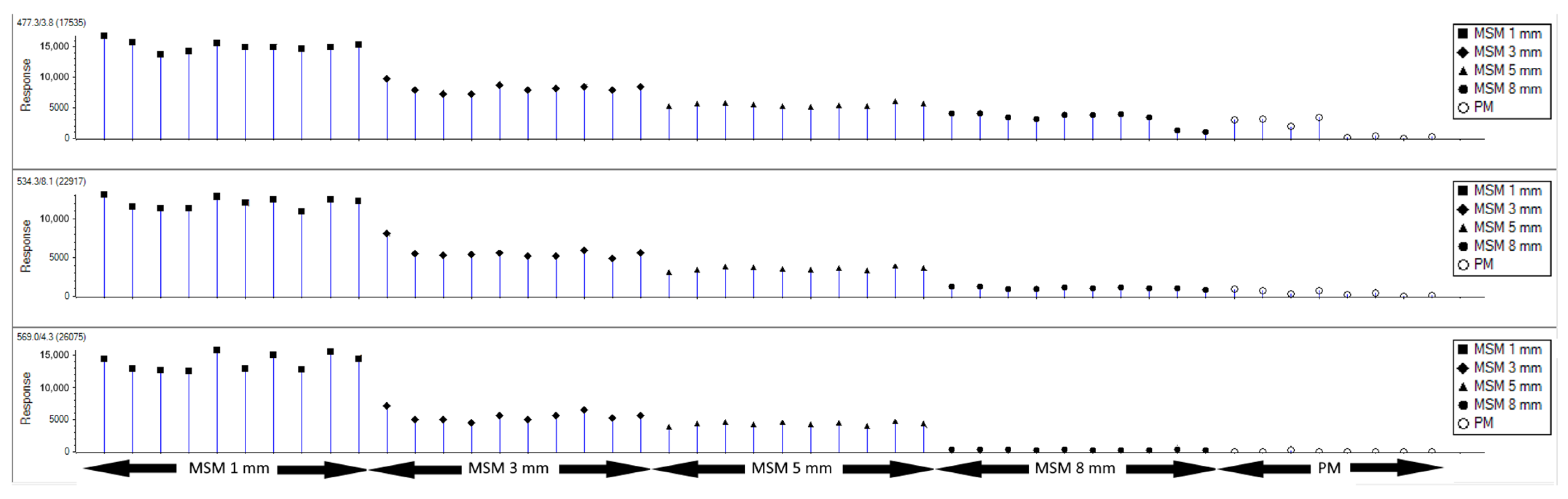
3.1. Definition of Specific Marker Ions for Porcine MSM
3.2. Identification of the Potential Marker Ions by LC-MS/MS
3.3. Validation of M3
3.3.1. Blinded Pre-Validation of M3 in Samples with Different Amounts of MSM (Set 1; Fitness for Purpose)
3.3.2. Blinded Validation of M3 in Industrially Produced Sausages (Set 2; Precision and Trueness)
3.3.3. Evaluation of Potential Protegrin Sources Other than MSM (Fitness for Purpose, Selectivity and Sensitivity)
3.3.4. Calcium Contents of 1, 3, 5, and 8 mm MSM (Fitness for Purpose, Measurement, and Uncertainty)
3.4. Species Authentication
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CE | Collision energy |
| cps | Counts per second |
| IDA | Information dependent acquisition |
| kDa | Kilodalton |
| LC | Liquid chromatography |
| m/z | Mass per charge ratio |
| MS | Mass spectrometry |
| MSM | Mechanically separated meat |
| MS/MS | Tandem mass spectrometry |
| (p)MRM | (Pseudo-)multiple-reaction monitoring |
| RT | Retention time |
| SNR | Signal to noise ratio |
| TOF | Time of flight |
References
- U.S. Food & Drug: Economically Motivated Adulteration (Food Fraud) Research Publications. Available online: https://www.fda.gov/food/economically-motivated-adulteration-food-fraud/economically-motivated-adulteration-food-fraud-research-publications (accessed on 7 February 2025).
- Commission Regulation (EC) No 853/2004. Available online: https://eur-lex.europa.eu/eli/reg/2004/853/oj/eng (accessed on 7 February 2025).
- Directorate-General for Health and Food Safety: Directorate for Health and Food Audits and Analysis, Mechanically Separated Meat—Overview Report. Publications Office. 2015. Available online: https://data.europa.eu/doi/10.2772/60722 (accessed on 1 April 2025).
- Commission Regulation (EC) No 2074/2005 (Current Version from 14 December 2019). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02005R2074-20191214 (accessed on 4 April 2025).
- Stanislavski, D. Fleischwirtschaft: Nicht Sachgerecht. Available online: https://www.fleischwirtschaft.de/forschung/nachrichten/standpunkt-nicht-sachgerecht-53672 (accessed on 6 February 2025).
- Tremlova, B.; Sarha, P.; Pospiech, M.; Buchtova, H.; Randulova, Z. Histological analysis of different kinds of mechanically recovered meat. Arch. Für Leb. 2006, 57, 85–91. [Google Scholar]
- Mohamed, M.A.; Zahran, D.A.; Kassem, G.M.; Emara, M.; Mansour, N. Detection of mechanically recovered poultry meat (MRPM) in traditional egyptian luncheon (emulsion type sausage). Pol. J. Food Nutr. Sci. 2016, 66, 17–23. [Google Scholar] [CrossRef]
- Langen, M.; Horn, D. Bildatlas Histologie Fleisch und Fleischerzeugnisse: Befunde beurteilen—Ergebnisse Sicher Bewerten, 1st ed.; Behrs Verlag: Hamburg, Germany, 2020. [Google Scholar]
- Bender, H. Lebensmittelzeitung: Hähnchen-Urteil Erzürnt Branche. Available online: https://www.lebensmittelzeitung.net/politik/nachrichten/kennzeichnungsrecht-haehnchen-urteil-erzuernt-gefluegelbranche-179553 (accessed on 6 February 2025).
- Regulation (EC) No 2017/625. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02017R0625-20220128 (accessed on 7 February 2025).
- Xiong, W.; McFarland, M.A.; Pirone, C.; Parker, C.H. Selection of Tree Nut Allergen Peptide Markers: A Need for Improved Protein Sequence Databases. J. AOAC Int. 2019, 102, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Perner, S.P. Investigation of Reduced ELISA Recovery of Almond and Hazelnut Traces from Roasted Nut Samples by SDS-PAGE and Mass Spectrometry. J. AOAC Int. 2019, 102, 1271–1279. [Google Scholar] [CrossRef]
- Ruhland, M.; Klinger, R. Food Fraud: A Simple and Efficient LC–MS/MS Approach for Peptide-Based Food Authentication. J. AOAC Int. 2019, 102, 1303–1308. [Google Scholar]
- Fiorino, G.M.; Fresch, M.; Brümmer, I.; Losito, I.; Arlorio, M.; Brockmeyer, J.; Monaci, L. Mass Spectrometry-Based Untargeted Proteomics for the Assessment of Food Authenticity: The Case of Farmed Versus Wild-Type Salmon. J. AOAC Int. 2019, 102, 1339–1345. [Google Scholar] [CrossRef]
- Lasch, P.; Uhlig, S.; Uhlig, C.; Wilhelm, C.; Bergmann, N.; Wittke, S. Development and In-House Validation of an LC–MS and LC–MS/MS Assay for the Determination of Food Fraud for Different Fish Species. J. AOAC Int. 2019, 102, 1330–1338. [Google Scholar] [CrossRef]
- Brümmer, I.; Klußmann, A.; Brockmeyer, J. Authentizität von Lebensmitteln—Real” tuna? Isobaric labelling as a method for identifying marker peptides for authenticity verification. Dtsch. Lebensm.-Rundsch. 2018, 114, 156–163. [Google Scholar]
- Stader, C.; Judas, M.; Jira, W. A rapid UHPLC-MS/MS screening method for the detection of the addition of porcine blood plasma to emulsion-type pork sausages. Anal. Bioanal. Chem. 2019, 411, 6697–6709. [Google Scholar] [CrossRef]
- Jira, W.; Behnke, T.; Brockmeyer, J.; Frost, K.; Hiller, E.; Möllers, M.; Niedzwiecka, A.; Pöpping, B.; Uhlig, S.; Weidner, M.; et al. Inter-laboratory Validation of an HPLC–MS/MS Method for the Detection of Microbial Transglutaminase in Meat and Meat Products. Food Anal. Methods 2022, 15, 2323–2334. [Google Scholar] [CrossRef]
- Tian, L.; Bilamjian, S.; Liu, L.; Akiki, C.; Cuthbertson, D.J.; Anumol, T.; Bayen, S. Development of a LC-QTOF-MS based dilute-and-shoot approach for the botanical discrimination of honeys. Anal. Chim. Acta 2024, 1304, 342536. [Google Scholar] [CrossRef]
- Beteinakis, S.; Papachristodoulou, A.; Stathopoulos, P.; Mikros, E.; Halabalaki, M. A multilevel LC-HRMS and NMR correlation workflow towards foodomics advancement: Application in table olives. Talanta 2024, 280, 126641. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhu, Y.; Zhang, H.; Zhang, X.; Li, Y.; Yao, Q.; Cai, Q.; Hu, Y. Differentiation of three commercial tuna species through GC-Q-TOF and UPLC-Q/Orbitrap mass spectrometry-based metabolomics and chemometrics. Food Chem. 2024, 452, 139603. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Wei, X.; Li, X.; Wei, X.; Wu, S.; Huang, W.; Koidis, A.; Xu, Z.; Lei, H. Untargeted metabolomics by liquid chromatography-mass spectrometry for food authentication: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2455–2488. [Google Scholar] [CrossRef]
- Xu, L.; Xu, Z.; Liao, X. A review of fruit juice authenticity assessments: Targeted and untargeted analyses. Crit. Rev. Food Sci. Nutr. 2022, 62, 6081–6102. [Google Scholar] [CrossRef]
- Wilhelm, C.; Hofsommer, M.; Wittke, S. Detection of Mechanically Separated Meat from Chicken in Sausages and Cold Meat by Targeted LC–MS/MS Analysis. Food Anal. Methods 2022, 15, 1899–1908. [Google Scholar] [CrossRef]
- The Grocer: Chicken Suppliers Face £80m Losses over FSA Wishbone Meat Rule Change. Available online: https://www.thegrocer.co.uk/news/chicken-suppliers-face-80m-losses-over-fsa-wishbone-meat-rule-change/691667.article (accessed on 7 March 2025).
- Field, R. Bone marrow measurements for mechanically recovered products from machines that press bones. Meat Sci. 1999, 51, 205–214. [Google Scholar] [CrossRef]
- Branscheid, W.; Judas, M.; Höreth, R. The morphological detection of bone and cartilage particles in mechanically separated meat. Meat Sci. 2009, 81, 46–50. [Google Scholar] [CrossRef]
- Troeger, K.; Wachsmann, G. Detection of carefully mechanically recovered meat (3-mm meat) in cooked sausage. Mitteilungsblatt Der Fleischforsch. Kulmb. 2007, 46, 169–174. [Google Scholar]
- Kokryakov, V.N.; Harwig, S.S.; Panyutich, E.A.; Shevchenko, A.A.; Aleshina, G.M.; Shamova, O.V.; Korneva, H.A.; Lehrer, R.I. Protegrins: Leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993, 327, 231–236. [Google Scholar] [CrossRef]
- Zhao, C.; Gains, T.; Lehrer, R.I. Identification of a new member of the protegrin family by cDNA cloning. FEBS Lett. 1994, 346, 285–288. [Google Scholar]
- Gour, S.; Kumar, V.; Singh, A.; Gadhave, K.; Goyal, P.; Pandey, J.; Giri, R.; Yadav, J.K. Mammalian antimicrobial peptide protegrin-4 self assembles and forms amyloid-like aggregates: Assessment of its functional relevance. J. Pept. Sci. 2019, 25, e3151. [Google Scholar] [CrossRef]
- Ng, P.C.; Ruslan, N.A.S.A.; Chin, L.X.; Ahmad, M.; Abu Hanifah, S.; Abdullah, Z.; Khor, S.M. Recent advances in halal food authentication: Challenges and strategies. J. Food Sci. 2021, 87, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Higgs, R.E.; Butler, J.P.; Han, B.; Knierman, M.D. Quantitative Proteomics via High Resolution MS Quantification: Capabilities and Limitations. Int. J. Proteom. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Gallien, S.; Domon, B. Detection and quantification of proteins in clinical samples using high resolution mass spectrometry. Methods 2015, 81, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Faktor, J.; Sucha, R.; Paralova, V.; Liu, Y.; Bouchal, P. Comparison of targeted proteomics approaches for detecting and quantifying proteins derived from human cancer tissues. Proteomics 2017, 17, 1600323. [Google Scholar] [CrossRef] [PubMed]
- Shilov, I.V.; Seymour, S.L.; Patel, A.A.; Loboda, A.; Tang, W.H.; Keating, S.P.; Hunter, C.L.; Nuwaysir, L.M.; Schaeffer, D.A. The paragon algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol. Cell. Proteom. 2007, 6, 1638–1655. [Google Scholar] [CrossRef]
- Uhlig, S.; Colson, B.; Hettwer, K.; Simon, K.; Uhlig, C.; Wittke, S.; Stoyke, M.; Gowik, P. Valid machine learning algorithms for multiparameter methods. Accreditation Qual. Assur. 2019, 24, 271–279. [Google Scholar] [CrossRef]
- EN 16943:2017; DIN Media: Foodstuffs—Determination of Calcium, Copper, Iron, Magnesium, Manganese, Phosphorus, Sodium, Sulfur and Zinc by ICP-OES.; German Version. Available online: https://www.dinmedia.de/de/norm/din-en-16943/263496956 (accessed on 4 April 2025).
- ZDFmediathek: Wurst Unter Verdacht—Tönnies, Dubiose Lieferanten und Billigpampe. Available online: https://www.zdf.de/dokumentation/die-spur/toennies-separatorenfleisch-wurst-ernaehrung-100.html (accessed on 11 March 2025).
- Blanco, A.; Blanco, G. Medical Biochemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2022; ISBN 9780323915991. [Google Scholar]
- Perez-Palacios, T.; Caballero, D.; González-Mohíno, A.; Mir-Bel, J.; Antequera, T. Near Infrared Reflectance spectroscopy to analyse texture related characteristics of sous vide pork loin. J. Food Eng. 2019, 263, 417–423. [Google Scholar] [CrossRef]
- Wieja, K.; Kiełczyński, P.; Szymański, P.; Szalewski, M.; Balcerzak, A.; Ptasznik, S. Identification and investigation of mechanically separated meat (MSM) with an innovative ultrasonic method. Food Chem. 2021, 348, 128907. [Google Scholar] [CrossRef]
- Foodwatch: Wie Gutfried & Co. Schweinefleisch als Geflügelwurst Verkauft. Available online: https://www.foodwatch.org/de/wie-gutfried-co-schweinefleisch-als-gefluegelwurst-verkaufen (accessed on 10 February 2025).
- Bundesinstitut für Risikobewertung: Observe Hygiene Rules When Preparing Poultry Meat! Available online: https://www.bfr.bund.de/en/press_information/2006/26/observe_hygiene_rules_when_preparing_poultry_meat_-8347.html (accessed on 1 April 2025).

| Sequence Marker Peptide (Target) | Retention Time [min] | Precursor Ion [m/z] | CE | pMRM Transition No. (Product Ion, Charge State) | ||||
|---|---|---|---|---|---|---|---|---|
| (Charge State) | [V] | T1 | T2 | T3 | T4 | |||
| M1 | No sequence database match | 3.5 | 477.280 (+1) | 24 | 136.071 (+1) | 199.142 (+1) | 279.136 (+1) | 362.213 (+1) |
| M2 | No sequence database match | 7.5 | 543.290 (+1) | 26 | 217.120 (+1) | 318.184 (+1) | 364.192 (+1) | 415.243 (+1) |
| M3 | LDQPPKADEDPGTPKP | 3.8 | 568.953 (+3) | 24 | 298.674 (y6 +2) | 357.177 (b3 +1) | 596.340 (y6 +1) | 674.841 (y13 +2) |
| Sequence Marker Peptide (Target) | Retention Time [min] | Precursor Ion [m/z] (Charge State) | CE [V] | pMRM Transition No. (Product Ion, Charge State) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| C-SK04 | VGPAGPIGSRGPSG PP[Oxi]GPDGNKGE P[Oxi]GN | 6.7 | 819.730 (+3) | 40 | 303.130 (y3 +1) | 354.210 (a6 +1) | 382.209 (b5 +1) | 767.700 (y25 +3) |
| C-SK11 | VAVPGPMGPAGPR GLP[Oxi]GPP[Oxi]G AP[Oxi]GPQG | 11.0 | 779.399 (+3) | 32 | 471.220 (y5 +1) | 798.906 (+2) | 883.944 (+2) | 933.495 (b21 +2) |
| T-SK13 | No sequence database match | 3.8 | 545.617 (+3) | 20 | 147.076 (+1) | 493.583 (+3) | 646.822 (+2) | 744.883 (+2) |
| T-SK14 | No sequence database match | 1.6 | 453.730 (+2) | 20 | 228.135 (+1) | 299.171 (+1) | 608.278 (+1) | 736.337 (+1) |
| P-SK09 | VGPAGKEGPAGLP [Oxi]G | 8.8 | 611.825 (+2) | 30 | 533.780 (y12 +2) | 878.480 (+1) | 921.479 (b11 +1) | 1034.563(b12 +1) |
| P-SK10 | VAGAP[Oxi]GLP[Oxi] GPRG IP[Oxi]GPAG | 10.1 | 794.926 (+2) | 34 | 645.844 (14 +2) | 1007.527 (y11 +1) | 1175.653 (b13 +1) | 1290.680 (y14 +1) |
| C-ME06 | LLPAPGSPYGRA | 8.8 | 599.833 (+2) | 28 | 402.704 (y8 +2) | 486.749 (y10 +2) | 904.400 (y6 +1) | 972.490 (y10 +1) |
| C-ME09 | No sequence database match | 2.9 | 655.285 (+2) | 27 | 588.771 (+2) | 646.281 (+2) | 900.398 (+1) | 1047.453 (+1) |
| T-ME05 | LGQNPTNAEMNK | 4.9 | 658.817 (+2) | 30 | 299.171 (b3 +1) | 413.214 (b4 +1) | 904.419 (y8 +1) | 1018.462 (y9 +1) |
| T-ME12 | No sequence database match | 8.4 | 583.628 (+3) | 22 | 215.135 (+1) | 443.261 (+1) | 753.873 (+2) | 818.394 (+2) |
| P-ME16 | IKWGDAGATY | 6.9 | 541.270 (+2) | 23 | 283.129 (y2 +1) | 799.410 (b8 +1) | 840.352 (y8 +1) | 900.457 (b9 +1) |
| P-ME17 | FDQDDWKT | 5.2 | 527.727 (+2) | 22 | 263.103 (b2 +1) | 434.240 (y3 +1) | 792.352 (y6 +1) | 907.379 (y7 +1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilhelm, C.; Hofsommer, M.; Fischbach, N.; Wittke, S. Detection of Mechanically Separated Meat from Pork in Meat-Containing Foods by Targeted LC-MS/MS Analysis. Foods 2025, 14, 1317. https://doi.org/10.3390/foods14081317
Wilhelm C, Hofsommer M, Fischbach N, Wittke S. Detection of Mechanically Separated Meat from Pork in Meat-Containing Foods by Targeted LC-MS/MS Analysis. Foods. 2025; 14(8):1317. https://doi.org/10.3390/foods14081317
Chicago/Turabian StyleWilhelm, Christian, Mikko Hofsommer, Nadine Fischbach, and Stefan Wittke. 2025. "Detection of Mechanically Separated Meat from Pork in Meat-Containing Foods by Targeted LC-MS/MS Analysis" Foods 14, no. 8: 1317. https://doi.org/10.3390/foods14081317
APA StyleWilhelm, C., Hofsommer, M., Fischbach, N., & Wittke, S. (2025). Detection of Mechanically Separated Meat from Pork in Meat-Containing Foods by Targeted LC-MS/MS Analysis. Foods, 14(8), 1317. https://doi.org/10.3390/foods14081317





