Sustainable Extraction of Fresh Banana Inflorescence by ASE: Optimization and Characterization of Anthocyanin Rich Extracts by LC-UV-MS/MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Banana Inflorescence Samples
2.2. Chemicals
2.3. Sample Preparation
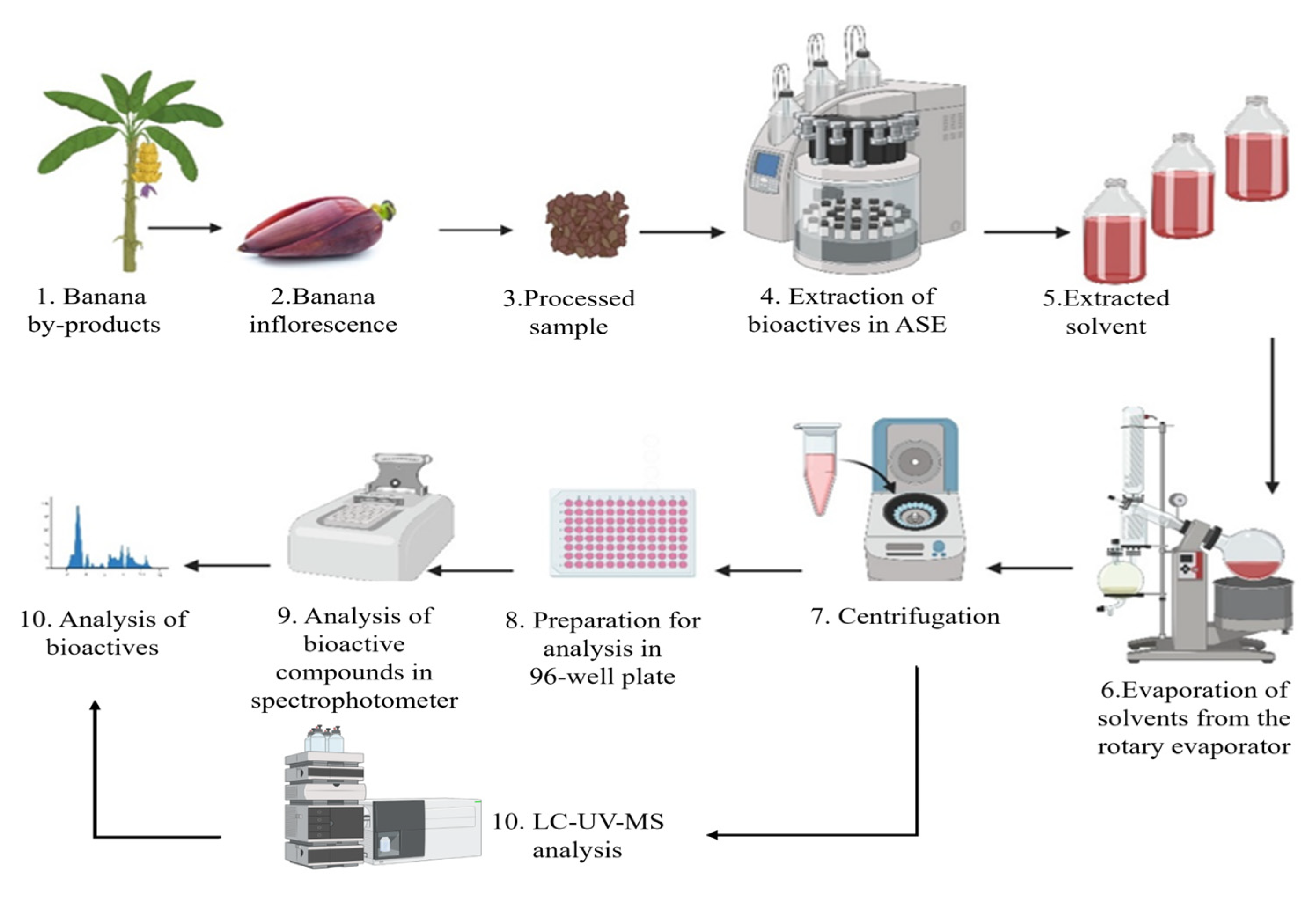
2.4. Experimental Process
2.5. Physical Properties of Banana Inflorescence
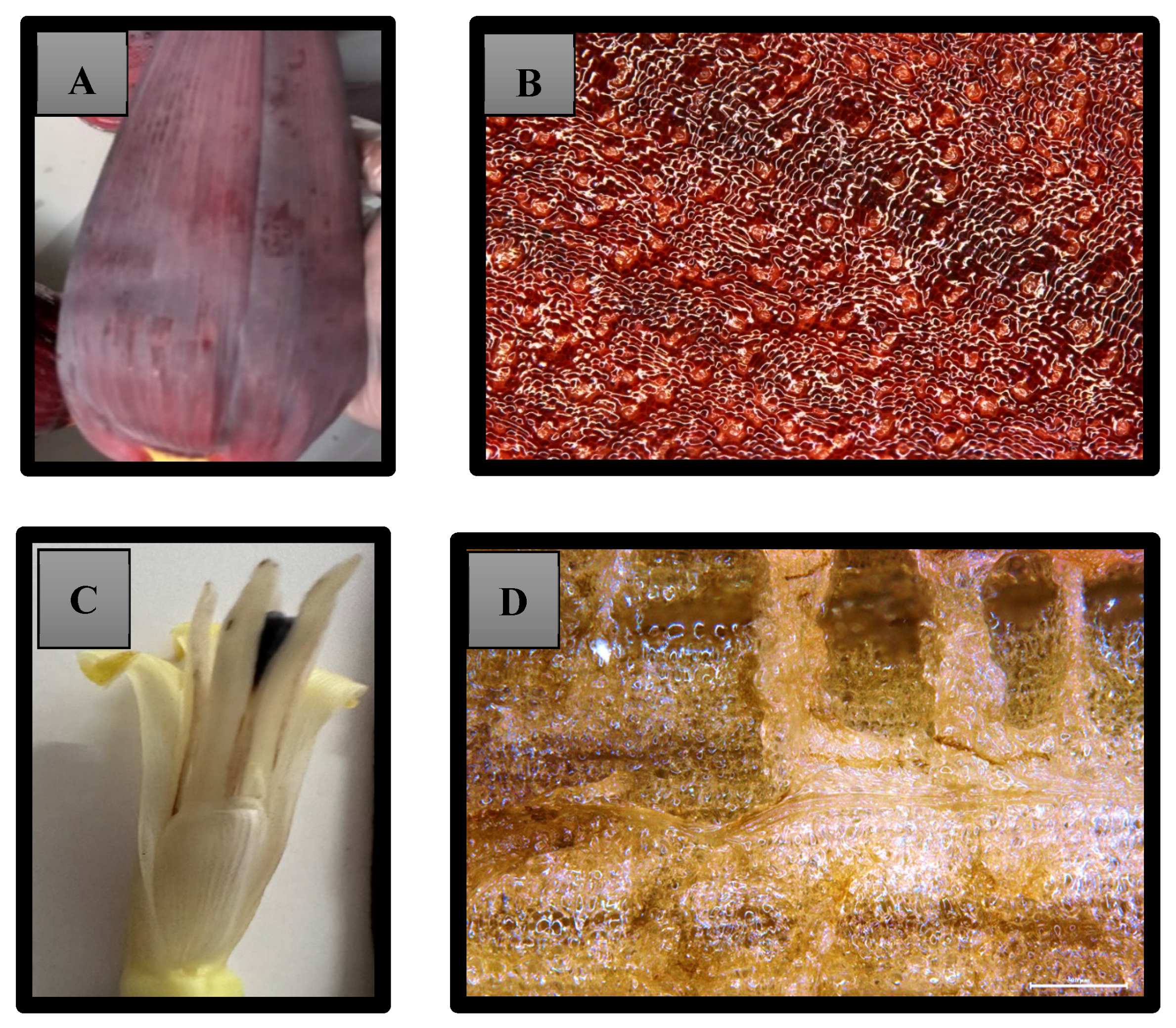
2.5.1. Morphology
2.5.2. Moisture Content
2.5.3. Ash Content
2.5.4. Microstructure
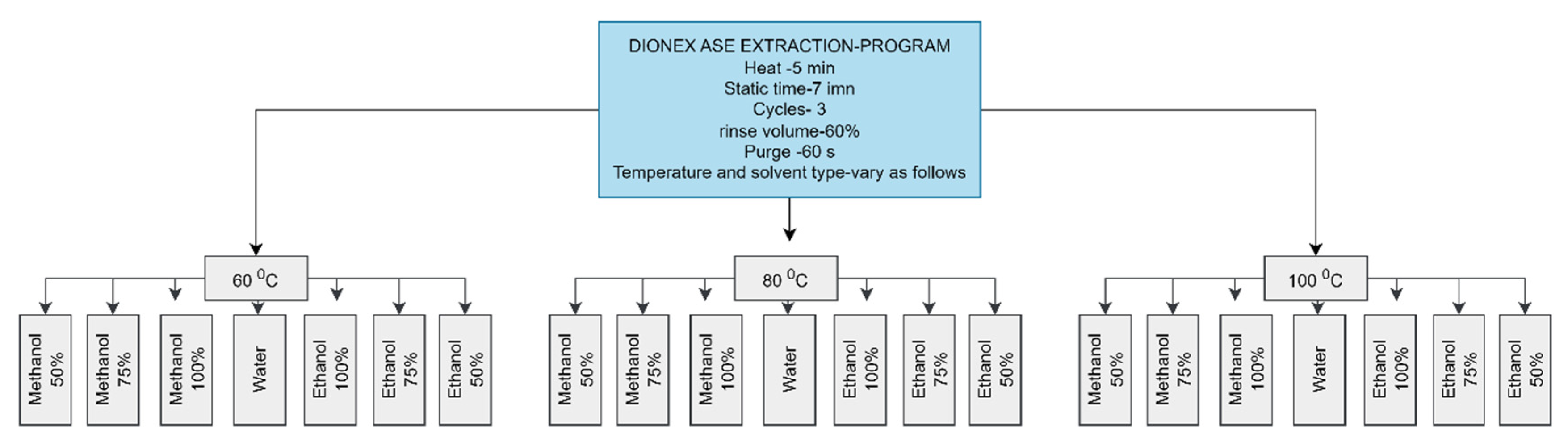
2.6. Extraction
2.7. Bioactive Properties of Extracts
2.7.1. Total Phenolic Content (Folin–Ciocalteau Method)
2.7.2. Total Antioxidant Activity (DPPH Analysis)
2.7.3. Total Monomeric Anthocyanin Content
2.7.4. Characterization of Anthocyanin by LC-MS Analysis
2.8. Statistical Analysis
3. Results and Discussions
3.1. Morphology
3.2. Microstructure
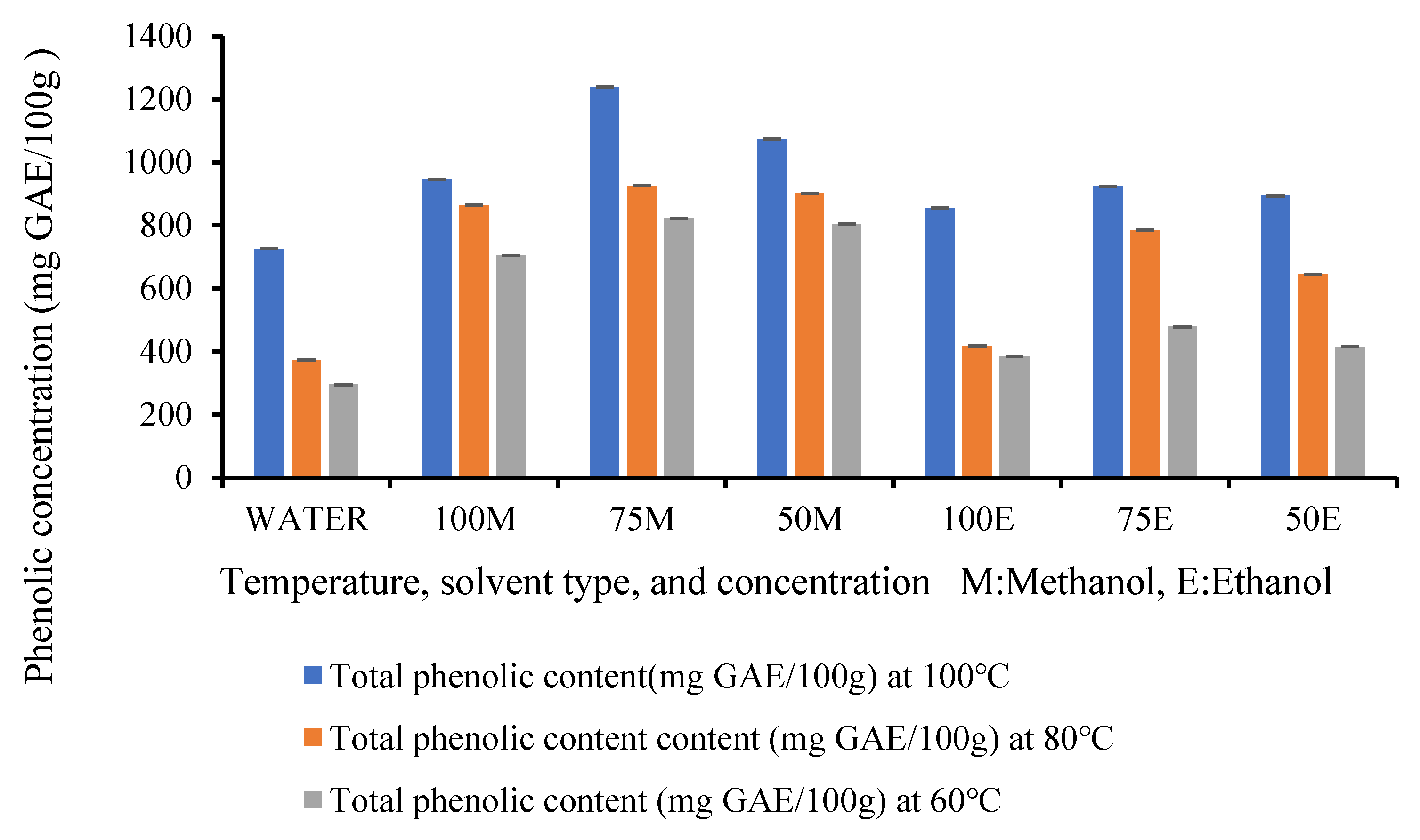
3.3. Total Phenolic Content (TPC)
3.4. Antioxidant Activity
3.5. Total Monomeric Anthocyanin Content
3.6. Anthocyanin Profile of Fresh Banana Inflorescence Extract
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef]
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara, M. An international regulatory review of food health-related claims in functional food products labeling. J. Funct. Foods 2020, 68, 103896. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Re-thinking functional food development through a holistic approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Qamar, S.; Shaikh, A. Therapeutic potentials and compositional changes of valuable compounds from banana—A review. Trends Food Sci. Technol. 2018, 79, 1–9. [Google Scholar] [CrossRef]
- Amutha, K.; Sudha, A.; Saravanan, D. Characterization of Natural Fibers Extracted from Banana Inflorescence Bracts. J. Nat. Fibres 2020, 19, 872–881. [Google Scholar] [CrossRef]
- Choudhury, N.; Nickhil, C.; Deka, S.C. Comprehensive review on the nutritional and therapeutic value of banana by-products and their applications in food and non-food sectors. Food Biosci. 2023, 56, 103416. [Google Scholar] [CrossRef]
- Bharathi, S.D.; Jacob, S. Comprehensive treatment strategy for banana inflorescence bract to synthesize biodiesel and bioethanol through fungal biorefinery. Waste Biomass Valoris. 2023, 15, 417–436. [Google Scholar] [CrossRef]
- Begum, Y.A.; Deka, S.C. Banana flower: A potential source of functional ingredients and its health benefitial effect. In Applied Food Science and Engineering with Industrial Applications; Apple Academics Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Pongsuwan, C.; Boonsuk, P.; Sermwittayawong, D.; Aiemcharoen, P.; Mayakun, J.; Kaewtatip, K. Banana inflorescence waste fiber: An effective filler for starch-based bioplastics. Ind. Crops Prod. 2022, 180, 114731. [Google Scholar] [CrossRef]
- Agcaoili, J. Level of Acceptability of Banana Inflorescence (Musa paradisiaca) Pickle. Herculean J. 2023, 1, 1. [Google Scholar] [CrossRef]
- Panyayong, C.; Srikaeo, K. Effects of hydrocolloids on the qualities of pureed banana inflorescences prepared for individuals with dysphagia. Food Hydrocoll. Health 2023, 3, 100129. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, R.; Gosewade, S. Bananas as underutilized fruit having huge potential as raw materials for food and non-food processing industries: A brief review. Pharma Innov. J. 2018, 7, 574–580. [Google Scholar]
- Nadumane, V.K.; Timsina, B. Anticancer potential of banana flower extract: An in vitro study. Bangladesh J. Pharmacol. 2014, 9, 628–635. [Google Scholar] [CrossRef]
- Ramu, R.; Shirahatti, P.S.; Zameer, F.; Ranganatha, L.V.; Nagendra Prasad, M.N. Inhibitory effect of banana (Musa sp. var. Nanjangud rasa bale) flower extract and its constituents Umbelliferone and Lupeol on α-glucosidase, aldose reductase and glycation at multiple stages. S. Afr. J. Bot. 2014, 95, 54–63. [Google Scholar] [CrossRef]
- Begum, Y.A.; Baishya, P.; Das, M.J.; Deka, S.C. Novel approach for the development of dietary fibre-anthocyanin enriched functional bread from culinary banana bract. Int. J. Food Sci. Technol. 2020, 55, 3455–3462. [Google Scholar] [CrossRef]
- Begum, Y.A.; Deka, S.C. Ultrasound-assisted extracted dietary fibre from culinary banana bract as matrices for anthocyanin: Its preparation, characterization and storage stability. J. Food Sci. Technol. 2020, 57, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Yimyam, S.; Suppansan, P.; Jankajonchai, K. Effectiveness of banana flower beverage on breast milk production among mothers of preterm neonates. J. Neonatal Nurs. 2023, 29, 755–759. [Google Scholar] [CrossRef]
- Kalwar, P.R.; Kolte, S.S.; Thaker, K. Identification of Progesterone in the Banana Inflorescence of Musa paradisiaca L. Species by Using HPTLC GC-MS Techniques. Anal. Chem. Lett. 2021, 11, 39–54. [Google Scholar] [CrossRef]
- Alam, M.; Biswas, M.; Hasan, M.M.; Hossain, M.F.; Zahid, M.A.; Al-Reza, M.S.; Islam, T. Quality attributes of the developed banana flour: Effects of drying methods. Heliyon 2023, 9, e18312. [Google Scholar] [CrossRef]
- Celli, G.B.; Brooks, M.S.-L. Impact of extraction and processing conditions on betalains and comparison of properties with anthocyanins—A current review. Food Res. Int. 2017, 100, 501–509. [Google Scholar] [CrossRef]
- Basumatary, S.; Nath, N. Assessment of chemical compositions and in vitro antioxidant properties of Musa balbisiana Colla inflorescence. Int. J. Pharm. Res. 2018, 10, 80–85. [Google Scholar]
- Gayathry, K.; John, J.A. Phenolic profile, antioxidant, and hypoglycaemic potential of pseudostem and inflorescence extracts of three banana cultivars. Biomass Convers. Biorefinery 2023, 15, 2387–2395. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Prestes, R.C.; Kubota, E.H.; Scapin, G.; Mazutti, M.A. Evaluation of antioxidant activity of extracts of banana inflorescences (Musa cavendishii). CYTA J. Food 2015, 13, 498–505. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Truong, V.; Hu, Z.; Thompson, R.; Yencho, G.; Pecota, K. Pressurized liquid extraction and quantification of anthocyanins in purple-fleshed sweet potato genotypes. J. Food Compos. Anal. 2012, 26, 96–103. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Salces, R.; Korta, E.; Barranco, A.; Berrueta, L.; Gallo, B.; Vicente, F. Determination of polyphenolic profiles of Basque cider apple varieties using accelerated solvent extraction. J. Agric. Food Chem. 2001, 49, 3761–3767. [Google Scholar] [CrossRef]
- Azian, M.; Anisa, A.I.; Iwai, Y. Mechanisms of ginger bioactive compounds extract using Soxhlet and accelerated water extraction. Int. J. Chem. Mater. Sci. Eng. 2014, 8, 438–442. [Google Scholar]
- Oliveira, A.M.B.; Viganó, J.; Sanches, V.L.; Rostagno, M.A.; Martínez, J. Extraction of potential bioactive compounds from industrial Tahiti lime (Citrus latifólia Tan.) by-product using pressurized liquids and ultrasound-assisted extraction. Food Res. Int. 2022, 157, 111381. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive phenolic compounds from agri-food wastes: An update on green and sustainable extraction methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Rajha, H.N.; Ziegler, W.; Louka, N.; Hobaika, Z.; Vorobiev, E.; Boechzelt, H.G.; Maroun, R.G. Effect of the drying process on the intensification of phenolic compounds recovery from grape pomace using accelerated solvent extraction. Int. J. Mol. Sci. 2014, 15, 18640–18658. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Yu, L. Free radical scavenging properties of conjugated linoleic acids. J. Agric. Food Chem. 2001, 49, 3452–3456. [Google Scholar]
- Bashmil, Y.M.; Ali, A.; Bk, A.; Dunshea, F.R.; Suleria, H.A. Screening and Characterization of Phenolic Compounds from Australian Grown Bananas and Their Antioxidant Capacity. Antioxidants 2021, 10, 1521. [Google Scholar] [CrossRef]
- Sheng, Z.-W.; Ma, W.-H.; Jin, Z.-Q.; Bi, Y.; Sun, Z.-G.; Dou, H.-T.; Li, J.; Han, L. Investigation of dietary fiber, protein, vitamin E and other nutritional compounds of banana flower of two cultivars grown in China. Afr. J. Biotechnol. 2010, 9, 3888–3895. [Google Scholar]
- Chiang, S.H.; Yang, K.M.; Lai, Y.C.; Chen, C.W. Evaluation of the in vitro biological activities of Banana flower and bract extracts and their bioactive compounds. Int. J. Food Prop. 2021, 24, 1–16. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Kubota, E.H.; Prestes, R.C.; Mello, R.O.; Rosa, C.S.; Scapin, G.; Ferreira, S. Development and evaluation of pork burger with added natural antioxidant based on extract of banana inflorescence (Musa cavendishii). CYTA J. Food 2016, 14, 280–288. [Google Scholar] [CrossRef]
- Barros, F.; Dykes, L.; Awika, J.; Rooney, L. Accelerated solvent extraction of phenolic compounds from sorghum brans. J. Cereal Sci. 2013, 58, 305–312. [Google Scholar] [CrossRef]
- Kumar, N.; Sami, R.; Khojah, E.; Aljahani, A.H.; Al-Mushhin, A.A. Effects of drying methods and solvent extraction on quantification of major bioactive compounds in pomegranate peel waste using HPLC. Sci. Rep. 2022, 12, 8000. [Google Scholar] [CrossRef]
- Boulekbache-Makhlouf, L.; Medouni, L.; Medouni-Adrar, S.; Arkoub, L.; Madani, K. Effect of solvents extraction on phenolic content and antioxidant activity of the byproduct of eggplant. Ind. Crops Prod. 2013, 49, 668–674. [Google Scholar] [CrossRef]
- Ramu, R.; Shirahatti, P.S.; Anilakumar, K.R.; Nayakavadi, S.; Zameer, F.; Dhananjaya, B.L.; Nagendra Prasad, M.N. Assessment of nutritional quality and global antioxidant response of banana (Musa sp. CV. Nanjangud Rasa Bale) pseudostem flower. Pharmacogn. Res. 2017, 9, S74–S83. [Google Scholar] [CrossRef]
- Amornlerdpison, D.; Choommongkol, V.; Narkprasom, K.; Yimyam, S. Bioactive compounds and antioxidant properties of banana inflorescence in a beverage for maternal breastfeeding. Appl. Sci. 2021, 11, 343. [Google Scholar] [CrossRef]
- Begum, Y.A.; Deka, S.C. Chemical profiling and functional properties of dietary fibre rich inner and outer bracts of culinary banana flower. J. Food Sci. Technol. 2019, 56, 5298–5308. [Google Scholar] [CrossRef] [PubMed]
- Boonsiriwit, A.; Lee, M.; Kim, M.; Inthamat, P.; Siripatrawan, U.; Lee, Y.S. Hydroxypropyl methylcellulose/microcrystalline cellulose biocomposite film incorporated with butterfly pea anthocyanin as a sustainable pH-responsive indicator for intelligent food-packaging applications. Food Biosci. 2021, 44, 101392. [Google Scholar] [CrossRef]
- Sharif, N.; Khoshnoudi-Nia, S.; Jafari, S.M. Nano/microencapsulation of anthocyanins; a systematic review and meta-analysis. Food Res. Int. 2020, 132, 109077. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tejera, E.; Battino, M. Malvidin: Advances in the Resources, Biosynthesis Pathway, Bioavailability, Bioactivity, and Pharmacology. In Handbook of Dietary Flavonoids; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–35. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar]
- Lee, H.-Y.; Kim, J.-S. Cherry fruit anthocyanins cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside protect against blue light-induced cytotoxicity in HaCaT cells. Appl. Biol. Chem. 2023, 66, 3. [Google Scholar] [CrossRef]
- Rajan, V.K.; Ragi, C.; Muraleedharan, K. A computational exploration into the structure, antioxidant capacity, toxicity and drug-like activity of the anthocyanidin “Petunidin”. Heliyon 2019, 5, e02115. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Akgun, B. Petunidin: Advances on Resources, Biosynthesis Pathway, Bioavailability, Bioactivity, and Pharmacology. In Handbook of Dietary Flavonoids; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–34. [Google Scholar]
- Yücetepe, M.; Özaslan, Z.T.; Karakuş, M.Ş.; Akalan, M.; Karaaslan, A.; Karaaslan, M.; Başyiğit, B. Unveiling the multifaceted world of anthocyanins: Biosynthesis pathway, natural sources, extraction methods, copigmentation, encapsulation techniques, and future food applications. Food Res. Int. 2024, 187, 114437. [Google Scholar] [CrossRef]


| Morphology | Fresh |
|---|---|
| Length (cm) | 32 ± 4.00 |
| Width (cm) | 12 ± 3.00 |
| Weight (Kg) | 1.23 ± 0.97 |
| Moisture % | 92.96 ± 0.15 |
| Ash content % | 0.59 ± 0.01 |
| Colour—HEX (#) | 533B3C |
| Colour (L*) | 27.46 ± 0.05 |
| a* | 11.13 ± 0.11 |
| b* | 3.53 ± 0.11 |
| c* | 11.8 ± 0.24 |
| h* | 17.6 ± 0.21 |
| Temperature | Solvent Type | Total Phenolic Content (mg GAE/100 g DW) | DPPH Scavenging Activity (IC 50) mg/mL |
|---|---|---|---|
| 100 °C | Water | 725.7 ± 12.0 h i | 3.70 ± 0.02 a b c d e |
| Methanol | 945.6 ± 21.2 c | 2.58 ± 0.03 i | |
| 75% Methanol | 1239.6 ± 20.8 a | 2.21 ± 0.03 j | |
| 50% Methanol | 1073.1 ± 18.3 b | 2.45 ± 0.05 i j | |
| Ethanol | 855.3 ± 39.4 o f | 3.47 ± 0.11 d e f g | |
| 75% Ethanol | 923.2 ± 6.8 c d e | 3.27 ± 0.05 a b c d e | |
| 50% Ethanol | 894.1 ± 10.9 c d e | 3.37 ± 0.03 f g | |
| 80 °C | Water | 372.8 ± 14.3 l | 3.91 ± 0.01a b |
| Methanol | 864.6 ± 20.8 d e f | 3.44 ± 0.03 e f g | |
| 75% Methanol | 926.3 ± 19.7 c d | 2.93± 0.04 h | |
| 50% Methanol | 902.4 ± 29.4 c d e | 3.37 ± 0.04 f g | |
| Ethanol | 417.5 ± 19.4 k l | 3.82 ± 0.05 a b c | |
| 75% Ethanol | 784.7 ± 26.2 g h | 3.60 ± 0.03 b c d e f | |
| 50% Ethanol | 644.6 ± 35.5 j | 3.74 ± 0.02 a b c d e | |
| 60 °C | Water | 295.1 ± 12.0 m | 3.95 ± 0.02 a |
| Methanol | 704.9 ± 31.8 i j | 3.72 ± 0.02 a b c d e | |
| 75% Methanol | 822.9 ± 20.8 f g | 3.51 ± 0.05 c d e f g | |
| 50% Methanol | 804.86 ± 25.12 f g | 3.53 ± 0.01 c d e f g | |
| Ethanol | 385.4 ± 20.8 l | 3.90 ± 0.05 a b | |
| 75% Ethanol | 478.8 ± 10.4 k | 3.76 ± 0.04 a b c d | |
| 50% Ethanol | 415.9 ± 10.5 k l | 3.47 ± 0.14 c d e f g |
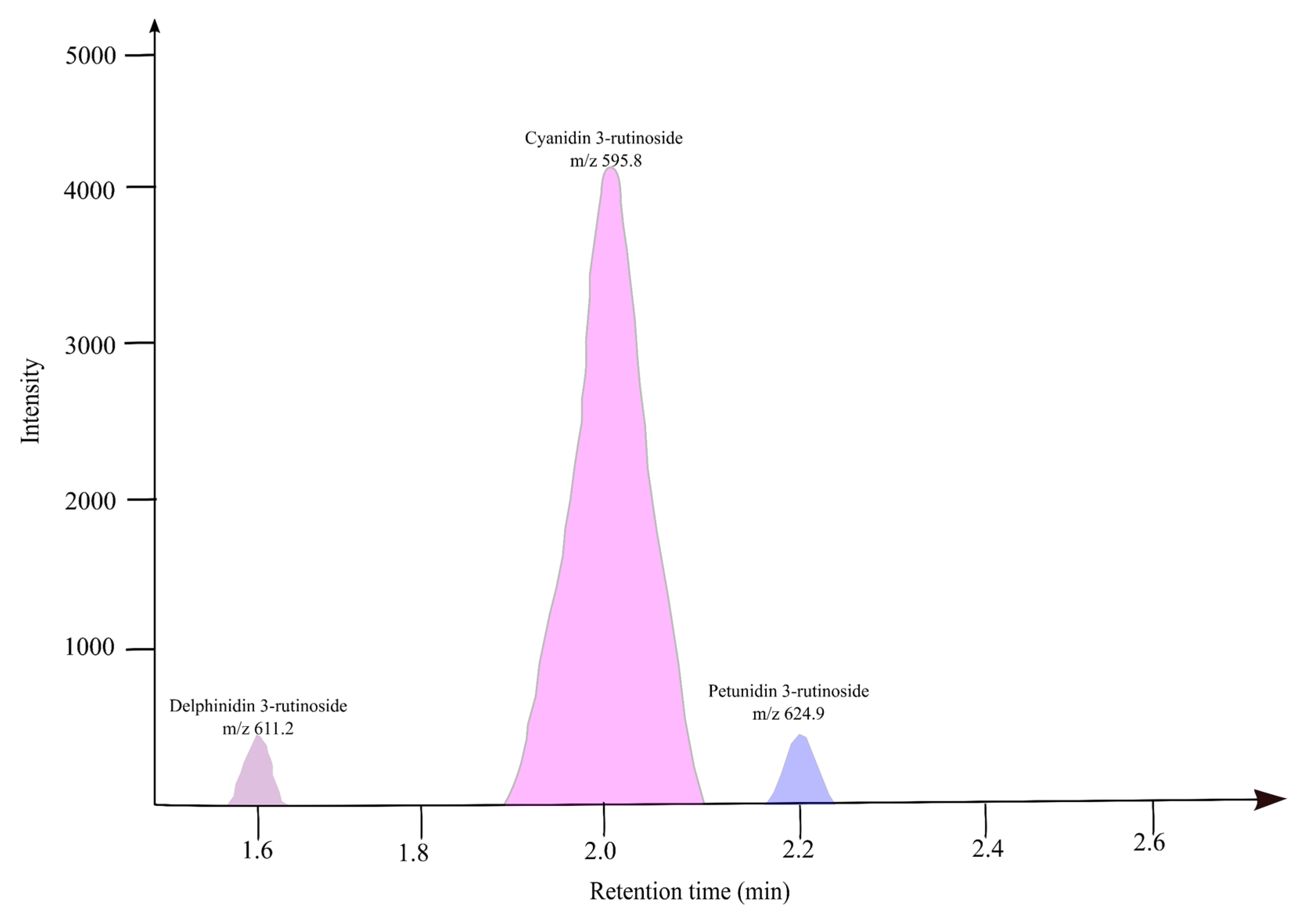
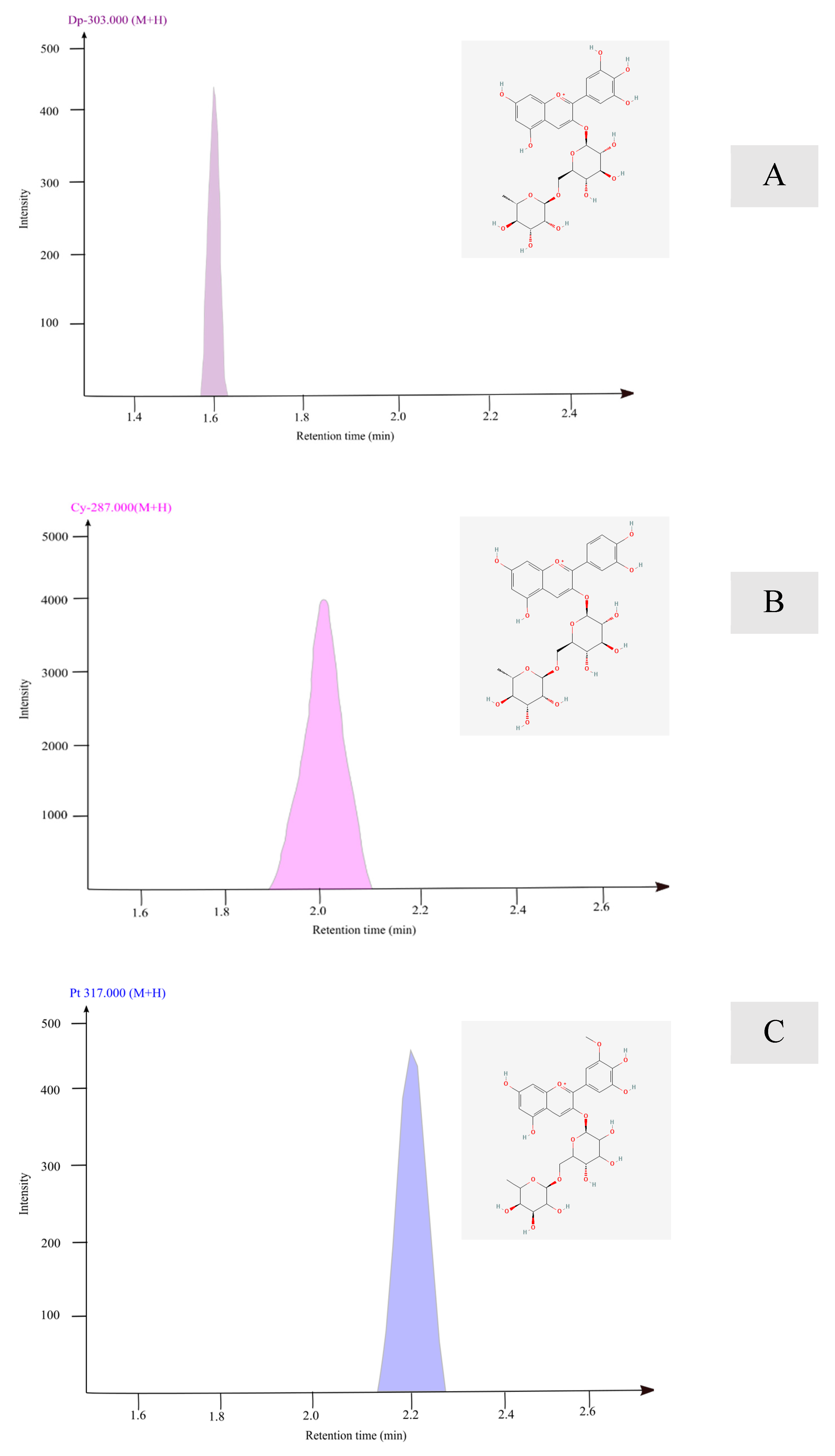
| Peak | Retention Time (min) | Molecular (m/z) | Fragments (m/z) | Compound Name | Anthocyanin% | Concentration as Cyanidin Equivalent µg/mL |
|---|---|---|---|---|---|---|
| 1 | 1.6 | 611.2 | 302.7 | Dp-3-rutinoside | 4.90 ± 0.01 | 0.082 |
| 2 | 2.0 | 595.8 | 287.7 | Cy-3-rutinoside | 90.03 ± 0.07 | 1.546 |
| 3 | 2.2 | 624.9 | 317.7 | Pt-3-rutinoside | 4.90 ± 0.05 | 0.082 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senevirathna, N.; Hassanpour, M.; O’Hara, I.; Karim, A. Sustainable Extraction of Fresh Banana Inflorescence by ASE: Optimization and Characterization of Anthocyanin Rich Extracts by LC-UV-MS/MS. Foods 2025, 14, 1299. https://doi.org/10.3390/foods14081299
Senevirathna N, Hassanpour M, O’Hara I, Karim A. Sustainable Extraction of Fresh Banana Inflorescence by ASE: Optimization and Characterization of Anthocyanin Rich Extracts by LC-UV-MS/MS. Foods. 2025; 14(8):1299. https://doi.org/10.3390/foods14081299
Chicago/Turabian StyleSenevirathna, Nuwanthi, Morteza Hassanpour, Ian O’Hara, and Azharul Karim. 2025. "Sustainable Extraction of Fresh Banana Inflorescence by ASE: Optimization and Characterization of Anthocyanin Rich Extracts by LC-UV-MS/MS" Foods 14, no. 8: 1299. https://doi.org/10.3390/foods14081299
APA StyleSenevirathna, N., Hassanpour, M., O’Hara, I., & Karim, A. (2025). Sustainable Extraction of Fresh Banana Inflorescence by ASE: Optimization and Characterization of Anthocyanin Rich Extracts by LC-UV-MS/MS. Foods, 14(8), 1299. https://doi.org/10.3390/foods14081299







