1. Introduction
Green tea is a non-fermented tea made from new buds of the
Camellia plant through the processes of withering, cooling, kneading, and drying. It is one of the most important types of tea worldwide. Research shows that green tea contains a variety of beneficial components for the human body, including polyphenols, vitamins, proteins, amino acids, lipids, polysaccharides, and mineral elements. Green tea also has antioxidant, anti-inflammatory, calming, and blood pressure-lowering properties; promotes metabolism; and prevents cardiovascular diseases [
1,
2,
3,
4,
5].
Superfine grinding technology is a novel technique that employs mechanical or fluid power to break down objects into powders up to particle sizes at the micron, submicron, and nanometer levels [
6]. This process modifies the structure and specific surface area of the materials, leading to new advantageous properties that the raw materials did not possess. Currently, the main methods of superfine grinding include jet milling [
7], vibration milling [
8], and ball milling [
9].
Superfine green tea powder (SGTP) is green tea powder treated with specific technologies, as raw material is ground into powder with a particle size between 1 nm and 100 μm using modern superfine grinding technology [
10,
11]. Apart from being consumed directly, SGTP is a high-quality additive in various food products used to impart natural green color and tea flavor; it finds applications in bread, ice cream, milk, and other food items. Compared with green tea extract, SGTP retains the nutritional components of green tea more effectively. Moreover, it is better suited for producing various foods and beverages with green tea flavors. Therefore, it has gained widespread application in the field of food processing [
12].
The analytic hierarchy process (AHP) is a multi-level decision analysis method proposed by Saaty. This method calculates the weights of individual indicators by constructing matrixes for pairwise comparisons. It is widely used to assign weight values to various indicators in a multi-criteria evaluation system [
13,
14]. Being an extension of AHP, the AHP–fuzzy comprehensive evaluation approach determines weights through AHP and makes evaluations using fuzzy methods. It effectively converts qualitative evaluations into quantitative evaluations, enabling comprehensive assessments [
15]. The AHP–fuzzy comprehensive evaluation approach is widely used in fields such as risk assessment [
16], solution selection [
17], product design [
18], and process optimization [
19].
Response surface methodology (RSM) is a statistical method that utilizes an appropriate experimental design and fits a quadratic regression equation to establish the functional relationship between factors and response values. By analyzing the regression equation, optimal process parameters can be determined [
20]. This method is widely used in food formulation optimization [
21,
22].
Chlorophyll is the main pigment in green tea, and its content is one of the most important indexes for judging the quality of green tea powder [
23]. Caffeine is the main bitter substance in tea [
24]; generally, the caffeine content in tea ranges from 2% to 4% [
25]. Moderate amounts of caffeine can complex with tea polyphenols, amino acids, and other components to synthesize refreshing substances. High-quality green tea powder should have a refreshing taste with less bitterness, so slightly lower caffeine content is beneficial to the quality of SGTP. Tea polyphenols are the main antioxidant components of tea, with the effect of scavenging reactive oxygen radicals and inhibiting lipid peroxidation [
26,
27]. Higher tea polyphenols content is favorable to the quality of tea powder. Free amino acids, being important components, contribute to the fresh taste of tea and provide a refreshing mouthfeel. Free amino acids positively determine SGTP quality [
28]. Chlorophyll, caffeine, tea polyphenols, and free amino acids are important components of green tea, and their contents affect the quality of green tea powder. The contents of these components have been used as parts of the indexes for judging the quality of tea powder in previous studies [
29,
30,
31,
32,
33].
Currently, there is extensive research on the production processes of green tea, black tea, and oolong tea in the industry [
34,
35,
36,
37,
38,
39]. Despite the known benefits of SGTP and the potential of superfine grinding technology, few studies have been conducted on the SGTP production process compared to green tea, black tea and oolong tea, and even fewer on optimizing the ball milling process specifically for SGTP. This study seeks to fill this gap by identifying the optimal ball milling conditions—grinding time, rotation speed, and ball-to-material ratio—that maximize the health-promoting components of SGTP. By doing so, we aim to provide a scientific basis for enhancing the quality and health benefits of superfine green tea powder, aligning with the growing consumer demand for functional foods that support health and well-being.
This study utilized ‘Longjing 43’ green tea as the raw material and employed the ball milling method to produce SGTP. The chlorophyll, caffeine, tea polyphenols (TPs), and total free amino acids (TFAA) contents were employed as indicators to investigate the effects of variables (grinding time, rotation speed, and ball-to-material ratio) on the nutrient composition of SGTP. An AHP–fuzzy comprehensive evaluation system applicable to SGTP was established by combining the AHP with fuzzy comprehensive evaluation. The SGTP production process was optimized using the RSM. In addition, we carried out particle size analysis and morphological observation for the SGTP prepared under optimal process conditions and analyzed its main component contents, dissolution characteristics, and antioxidant capacity. The results provide technical support for improving the quality of green tea powder and screening the optimal parameters of the three processes (grinding time, rotation speed, and ball-to-material ratio) in the ball milling method, and they also provide a reference for the application of superfine grinding technology in food processing.
2. Materials and Methods
2.1. Materials and Chemicals
‘Longjing 43’ green tea was purchased in August 2022 from Yuyao Zhu Tea Factory (Ningbo, China). Fresh tea shoots, consisting of two leaves and a bud, were plucked by hand from the tea plantation of Yuyao Zhu Tea Factory (Ningbo, China) in May 2022. The feedstock was first steamed and dried and then stored in a refrigerated room.
Ethanol absolute, acetone, methanol, disodium hydrogen phosphate dodecahydrate, potassium dihydrogen phosphate, chloride dihydrate, L-theanine, folin phenol, sodium carbonate, gallic acid, magnesium oxide, ferrous sulfate, and potassium sodium tartrate tetrahydrate were analytical reagents (ARs) purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Ninhydrin (AR) was purchased from Shanghai Titan Scientific Co., Ltd. (Shanghai, China). The caffeine standard (LC) was purchased from Tanmo Quality Inspection Technology Co., Ltd. (Changzhou, China).
2.2. Sample Preparation
In the first phase, ‘Longjing 43’ green tea (LJ43) was subjected to primary grinding using a stone mill (Quanzhou Shishanhai Co., Ltd., Quanzhou, China), followed by sieving through a 450 μm sieve (Shaoxing Shangyu Shengchao Instrument Equipment Co., Ltd., Shaoxing, China). The next step was grinding in an XQM-2 vertical planetary ball mill (Changsha Tianchuang Powder Technology Co., Ltd., Changsha, China). Stainless steel grinding balls with a diameter of 5–15 mm were used. The grinding process operated for 10 min in the forward direction, followed by 10 min in the reverse direction, with a 1 min resting time in between. This sequence was repeated several times to finally obtain SGTP samples. This marked the end of the first phase.
The second phase included analyzing the principal components (chlorophyll, caffeine, polyphenols, and amino acids), as well as scanning electron microscopy (SEM) analysis (size and morphology). During this phase, the ground material passed through a stone mill and a high-speed pulverizer (Dongguan Fangtai Electric Appliance Co., Ltd., Dongguan, China). The LJ43 was placed in a high-speed pulverizer; crushed for 30 s to obtain ordinary green tea powder (OGTP); ground in a stone mill; and passed through a 450 μm sieve to obtain coarse green tea powder (CGTP) without superfine grinding. Then, the CGTP was superfine ground according to the optimal process obtained from the first phase to produce BSGTP; the LJ43 was not subjected to any treatment as a control.
2.3. Determination of Major SGTP Component Contents
The caffeine content was determined referring to the national standard, GB/T 8312-2013 [
40], and caffeine (99.8%, LC) was used as the standard. Standard curve: y = 1465.9x − 1233.1 (R
2 = 0.9998). The instruments used in the experiment were an Acquity UPLC H-Class HPLC (Waters Corporation, Milford, MA, USA) and an Athena C18-WP column (CNW Technologies GmbH, Dusseldorf, Germany). Chromatographic conditions: UV detection wavelength: 280 nm; mobile phase: mix 600 mL of methanol with 1400 mL of distilled water and filter through a 0.45 μm membrane; flow rate: 1.0 mL/min; column temperature: 40 °C; injection volume: 10 μL.
The chlorophyll content was determined referring to the agricultural industry standard, NY/T 3082-2017 [
41]. The TPs content was determined referring to the national standard, GB/T 8313-2018 [
42]. The TFAA content was determined referring to the national standard, GB/T 8314-2013 [
43]. The instrument used in the experiment was a T6 New Century UV-Vis Spectrophotometer (Beijing Puxi General Instrument Co., Ltd., Beijing, China). The chlorophyll content was determined using UV spectrophotometry. An ethanol–acetone (1:1,
v/
v) mixture was used as the extractant, and the detection wavelengths were set at 645 nm and 663 nm. The TPs content was determined according to the UV spectrophotometric method using gallic acid as calibrant. The detection wavelength was set at 765 nm. The chlorophyll content was determined using UV spectrophotometry with L-theanine as the standard. The detection wavelength was set at 570 nm.
2.4. AHP–Fuzzy Comprehensive Evaluation of SGTP
Traditional evaluation methods are inherently subjective due to their susceptibility to the personal preferences of sensory judges. To address this limitation, this study establishes an AHP–fuzzy comprehensive evaluation system that combines AHP with traditional evaluation methods for SGTP. Using the AHP, the weights of various indicators can be determined. Next, the multilevel fuzzy evaluation approach was used to score and evaluate the SGTP indicators. Lastly, the SGTP quality was assessed based on the sample’s comprehensive scores from both methods.
2.4.1. AHP to Determine the Weight of Each Indicator
A 4th-order judgment matrix was established using four evaluation indicators: chlorophyll content, caffeine content, TPs content, and TFAA content. Ten experts from the fields of botany, tea studies, food engineering, and biology were invited as reviewers. The matrix indicators were discussed and scored on a scale of 1–5 based on their relative importance. For instance, if A was considered significantly more important than B and assigned a score of 3, then B would receive a score of 1/3, equivalent to 0.33. The relative weights of the evaluation indicators were then calculated, and the consistency of the results was verified. The scoring method for the judgment matrix is detailed in
Table 1.
2.4.2. Fuzzy Comprehensive Evaluation of SGTP
After using the AHP to determine the weight value of each index, 10 experts from the botany, tea studies, food engineering, and biology fields were invited as reviewers to score the SGTP samples obtained after different grinding processes. The final results were taken as the average scores provided by the 10 reviewers. The specific scoring method is shown in
Table 2.
2.5. Single-Factor Experiment
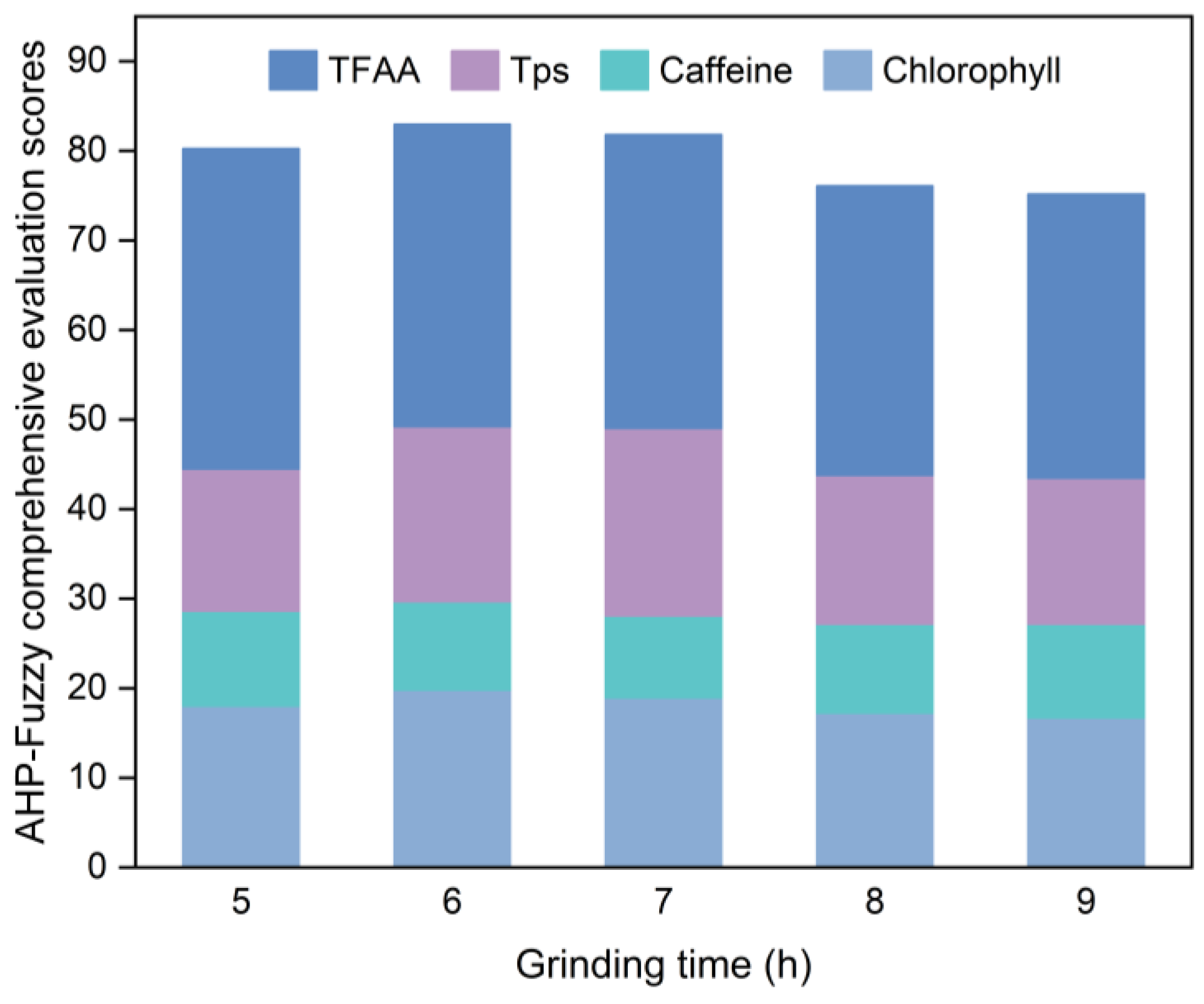
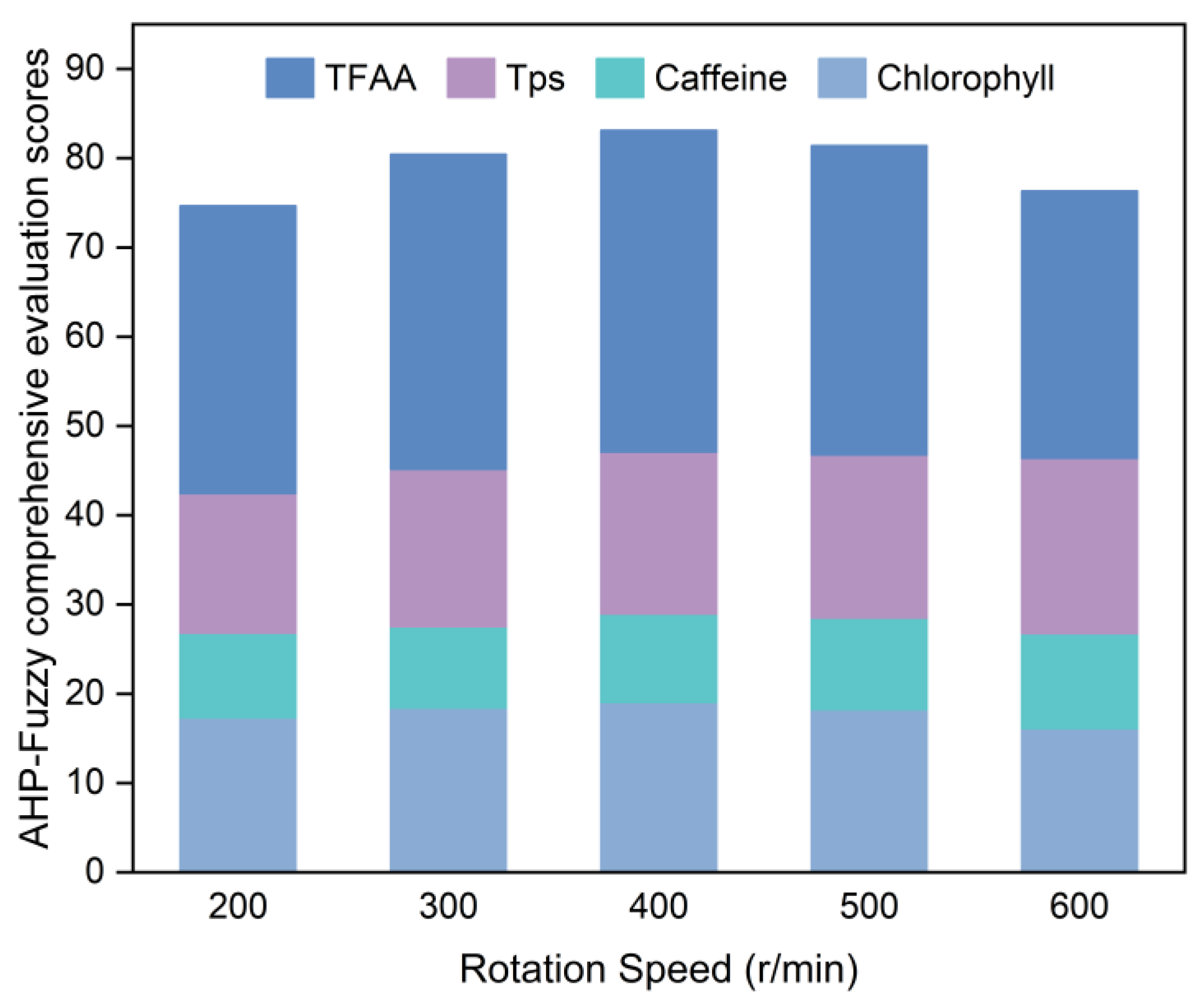
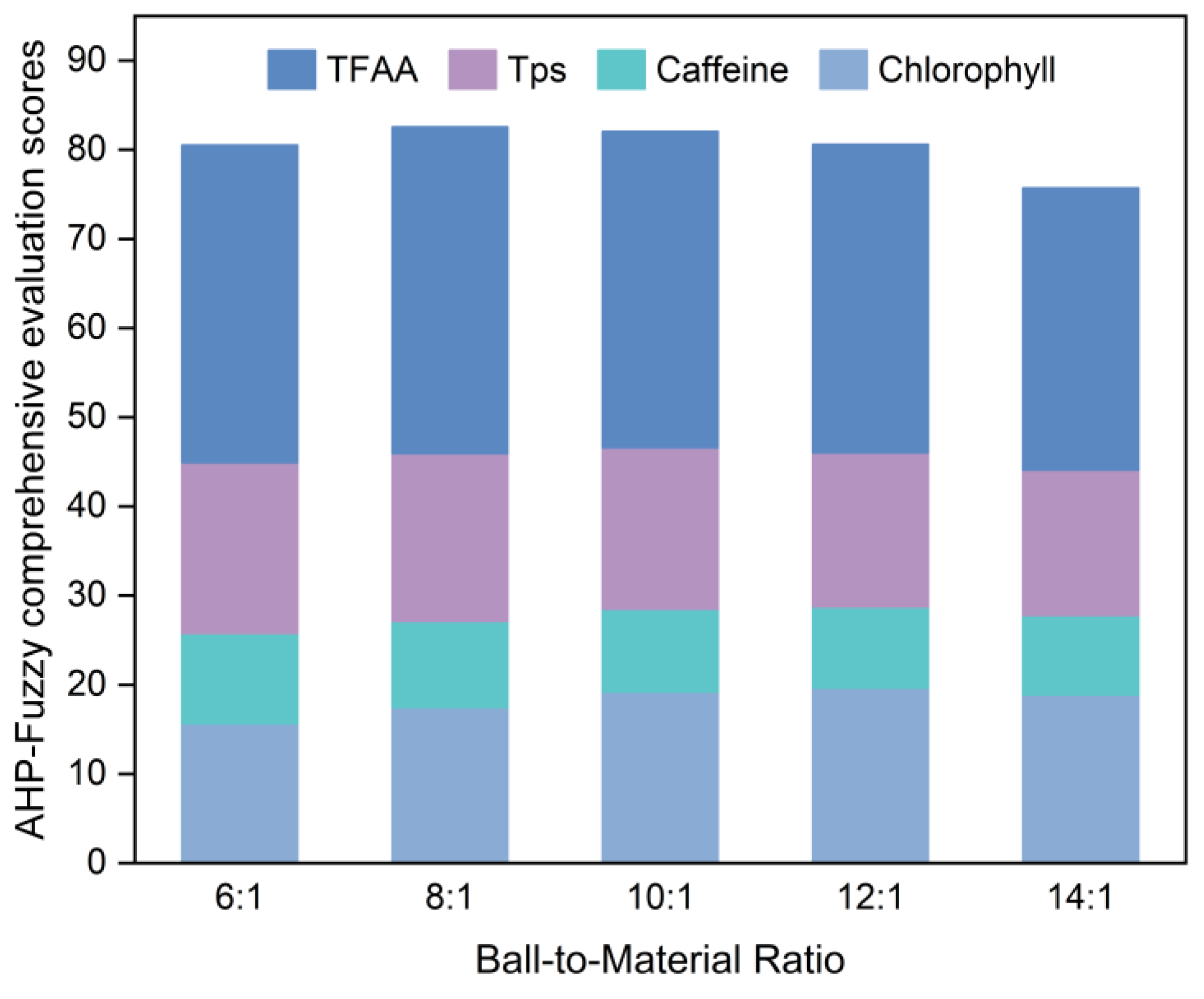
The effects of grinding time, rotation speed, and ball-to-material ratio (mass ratio) on the chlorophyll, caffeine, TPs, and TFAA contents in SGTP were investigated through single-factor experiments by controlling the variables.
The CGTP was placed in an XQM-2 vertical planetary ball mill for superfine grinding. The default grinding conditions were a grinding time of 7 h, rotation speed of 400 r/min, and ball-to-material ratio of 10:1. After obtaining the SGTP samples under different conditions, the samples were tested for the chlorophyll, caffeine, TPs, and TFAA contents. The samples in each group were scored according to the method described in
Section 2.4, and then, the optimal single-factor conditions for the preparation of SGTP were determined using the AHP–fuzzy comprehensive evaluation score. The method is detailed in
Table 3.
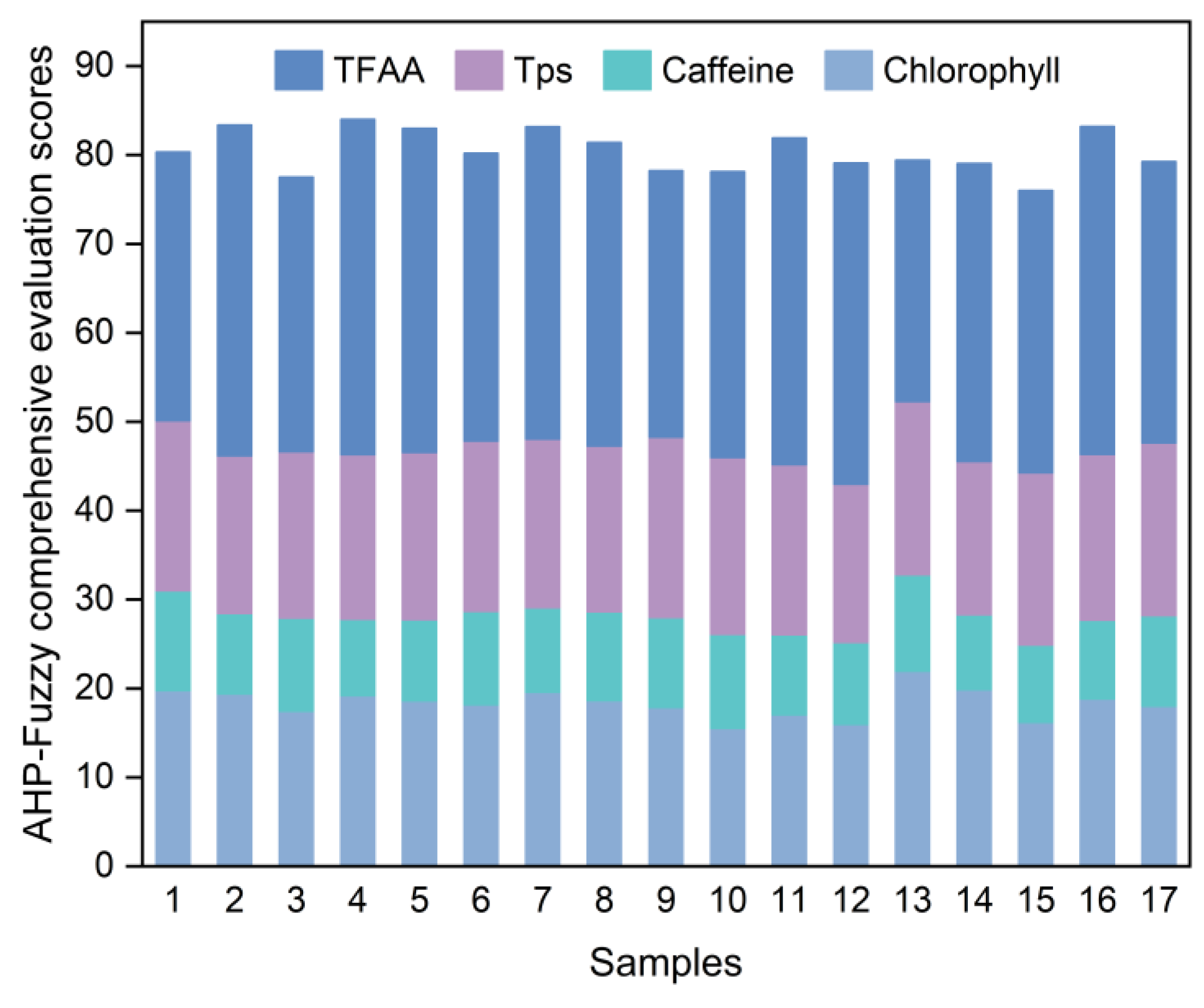
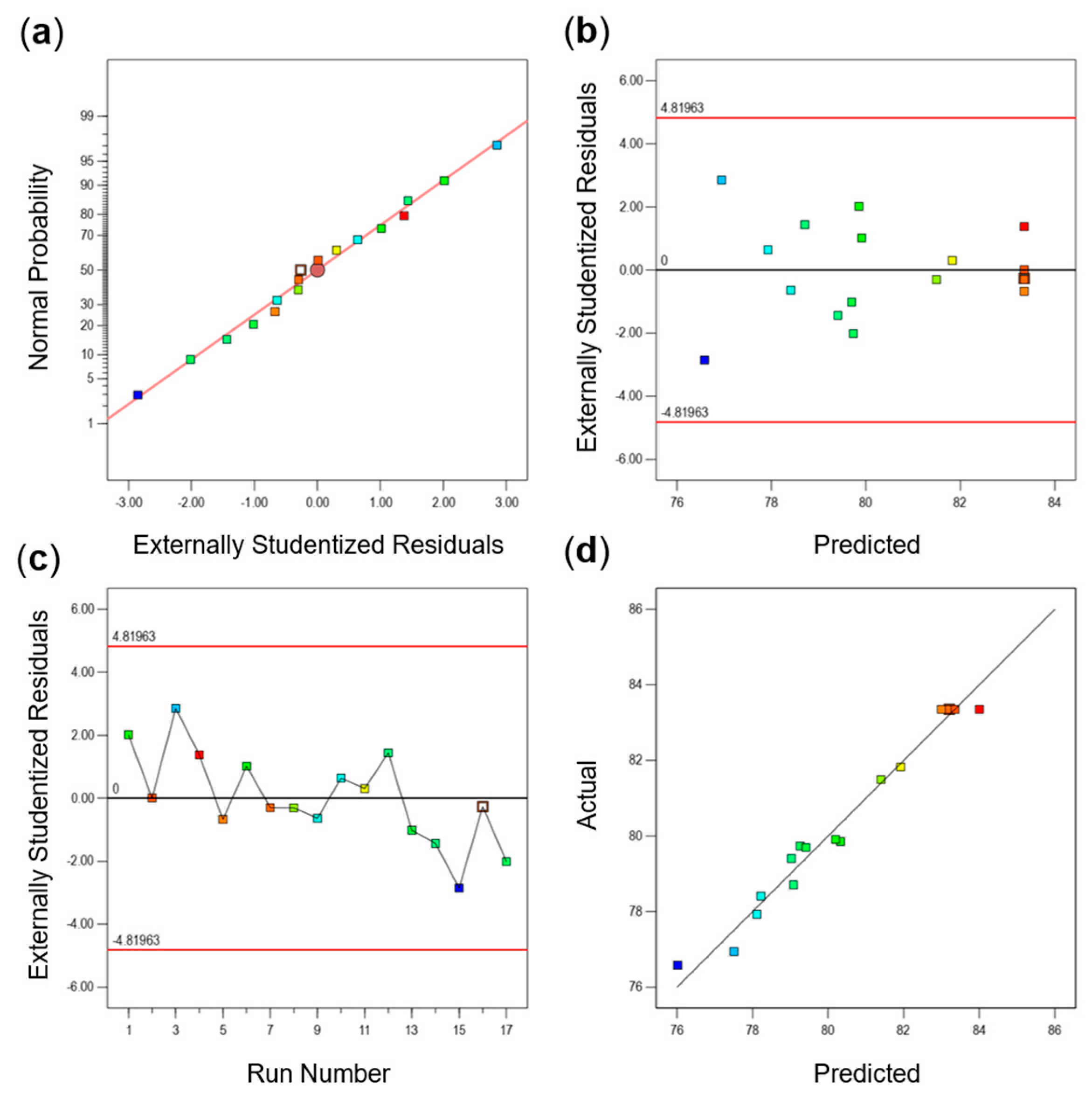
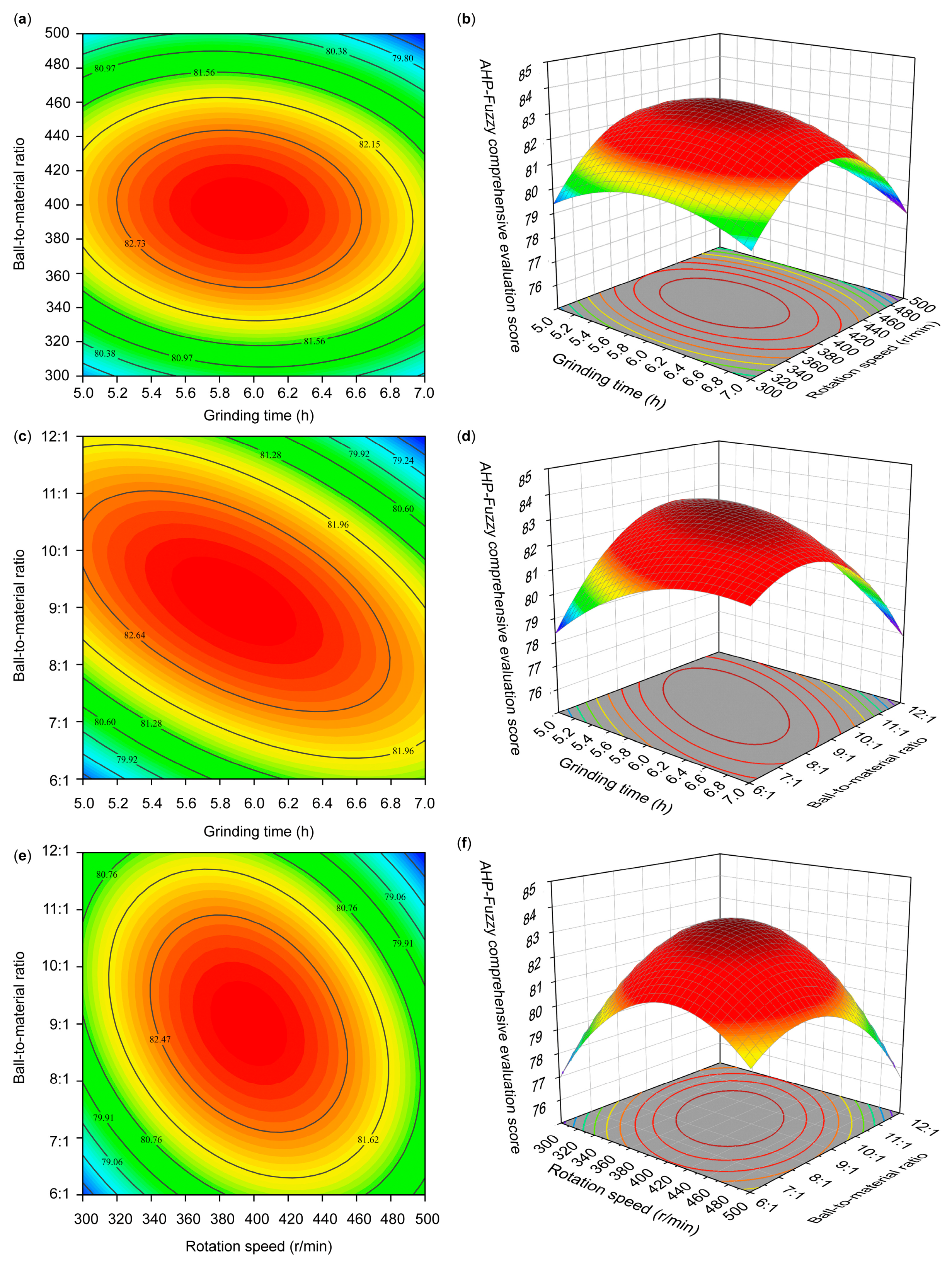
2.6. Response Surface Optimization Experiment
Based on the previous single-factor experiments, a Box–Behnken model was employed to design a three-factor, three-level response surface optimization experiment (
Table 4). This experiment aimed to determine the optimal grinding technique for producing SGTP.
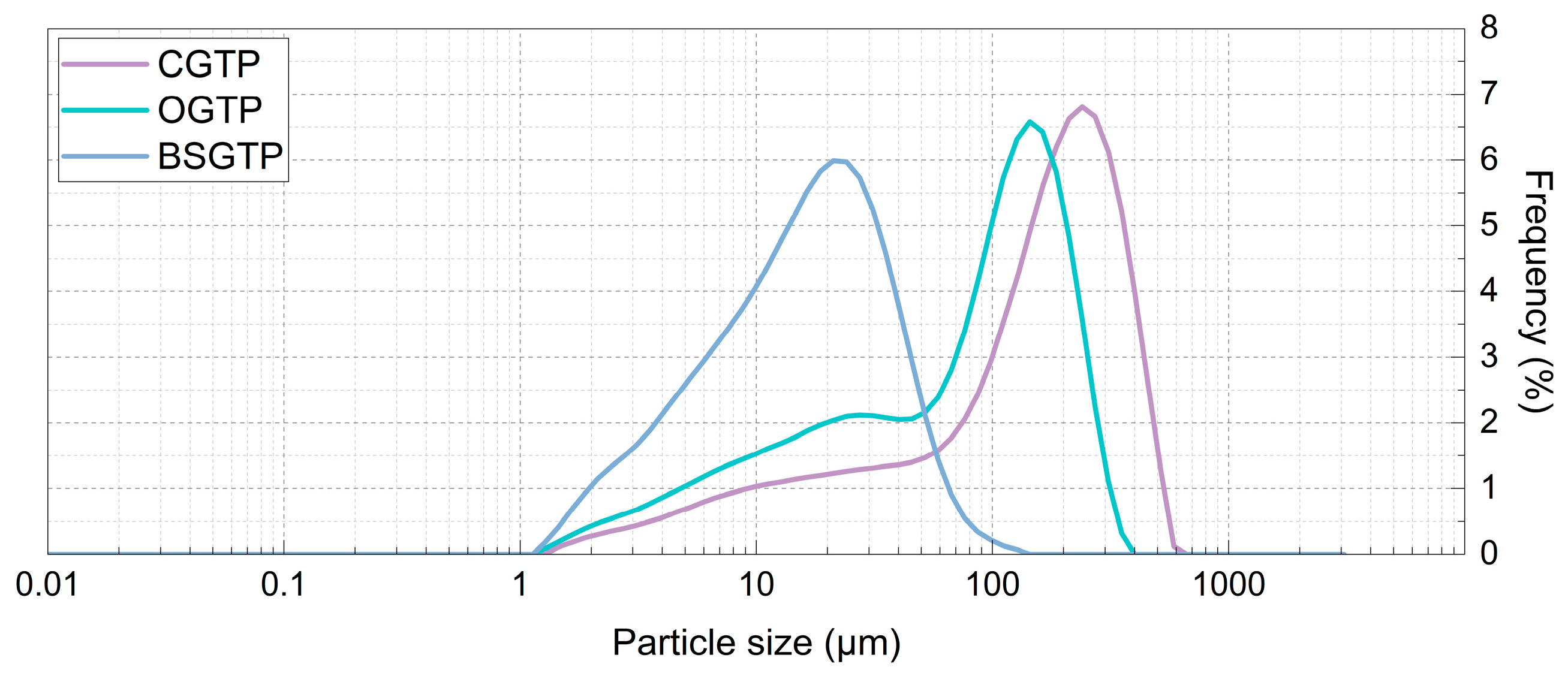
2.7. Particle Size Analysis of BSGTP
An appropriate amount of sample was weighed and dispersed using distilled water as a dispersant. After the sample and distilled water were mixed thoroughly, the sample was analyzed for particle size. The instrument used in the experiment was a Mastersizer 3000 laser particle size analyzer (Malvern Panalytical Ltd., Malvern, UK). The instrument measurement parameters were set as follows: ultrasonic intensity, 10 W/cm
2; refractive index, 1.52; upper and lower lines, 10–20%; rotation speed, 2500 r/min. The D
10 (diameter of 10% of particles below this value), D
50 (median particle size, diameter of 50% of particles below this value), D
90 (diameter of 90% of particles below this value), D
(3, 2) (surface weighed mean diameter), D
(4, 3) (volume-weighted mean diameter), SPAN (distribution width), and specific surface area of the powders were measured under these conditions, and the cell wall breakage ratio (Φ) was calculated using the following equation:
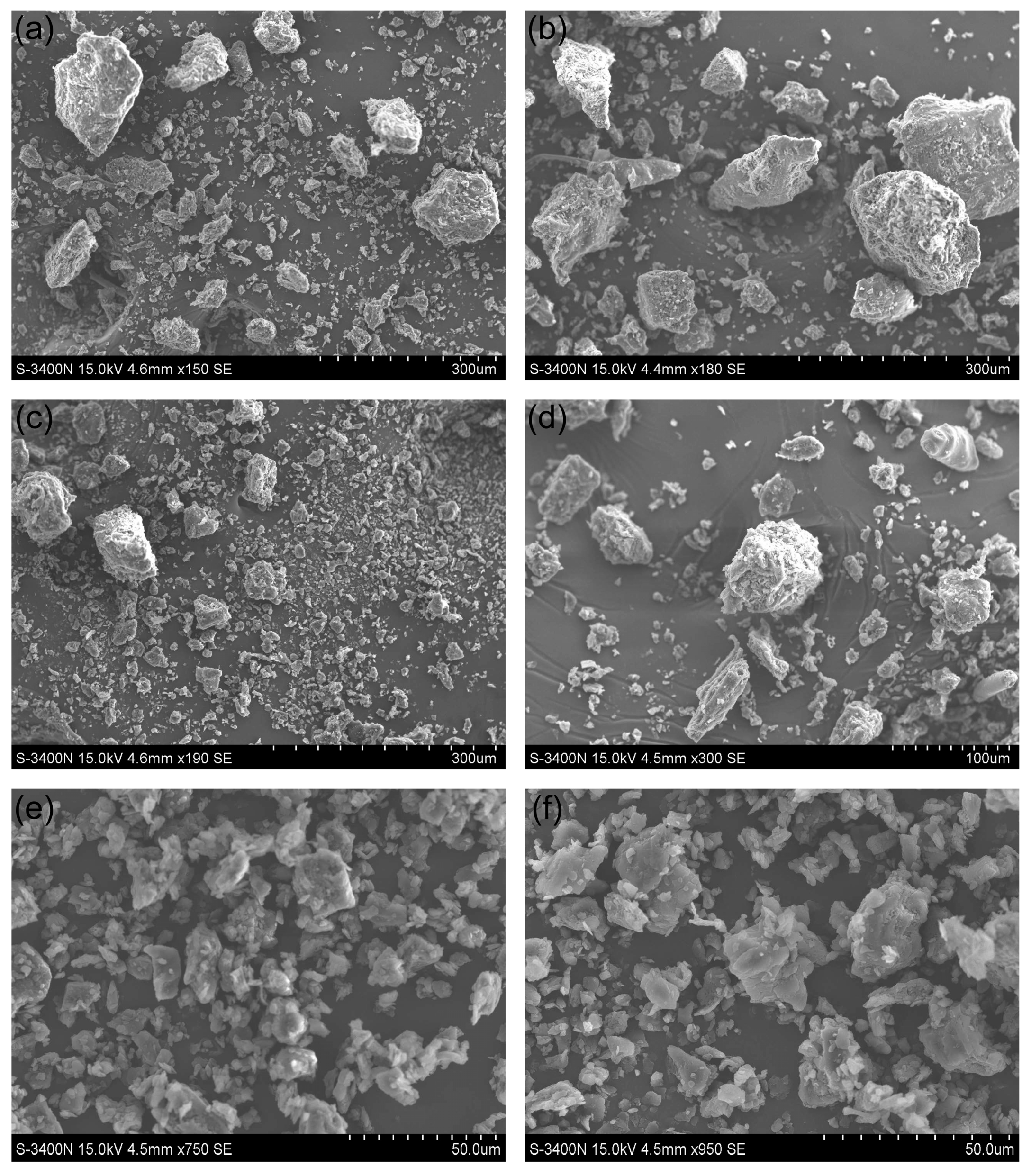
2.8. Morphological Observation of BSGTP
A suitable carrier table size was selected to paste the conductive adhesive, and then, an appropriate amount of sample was dialed onto the conductive adhesive using a dissecting needle. The samples were fixed via gold spraying with an E-1010 ion sputtering system (Hitachi, Ltd., Tokyo, Japan). The microstructural characteristics of the samples were then observed under an S-3400N scanning electron microscope (Hitachi, Ltd., Tokyo, Japan).
2.9. Determination of Major BSGTP Component Contents
The TPs content was determined using a spectrophotometric method [
44]. Ferrous tartrate solution was used as the color developer, in which Fe
2+ can form blue-violet compounds with polyphenols. The detection wavelength was set at 540 nm. The instrument used in the experiment was a T6 New Century UV–Vis Spectrophotometer (Beijing Puxi General Instrument Co., Ltd., Beijing, China). The chlorophyll, caffeine, and TFAA contents were determined using the same methods described in
Section 2.3.
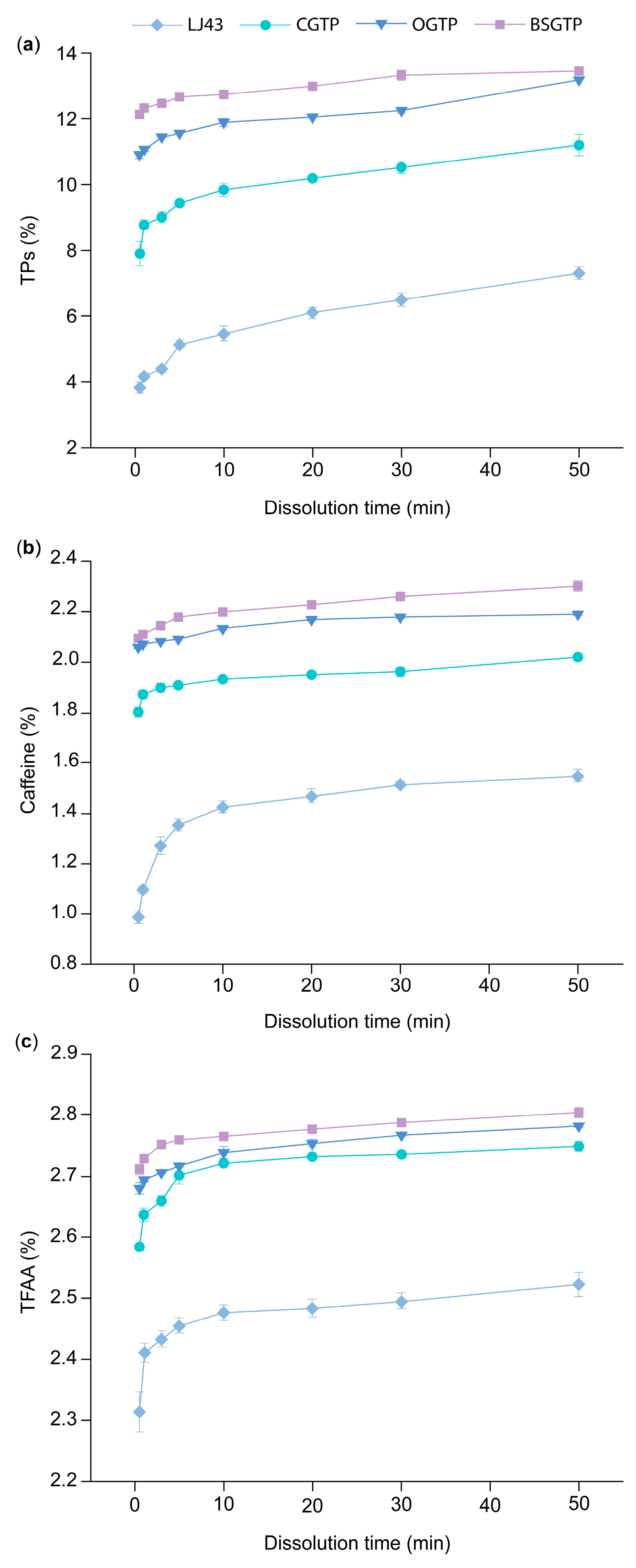
2.10. Dissolution Determination of BSGTP
After weighing 0.3 g of the sample in a 50 mL centrifuge tube, 45 mL of distilled water at 37 °C was added, followed immediately by a 37 °C constant-temperature water bath. The samples were extracted for 0.5 min, 1 min, 3 min, 5 min, 10 min, 20 min, 30 min, and 50 min. After extraction, the test solution was centrifuged at 10,000×
g for 10 min, and the supernatant was passed through a 0.45 μm filter membrane. The filtrate was transferred into a 50 mL volumetric flask and shaken well after adding distilled water to the scale; then, the sample dissolution solution was obtained. It was used to determine the dissolution of the major components of different samples at 37 °C. The TPs, caffeine, and TFAA contents in the sample dissolution solution were determined using the same methods described in
Section 2.9.
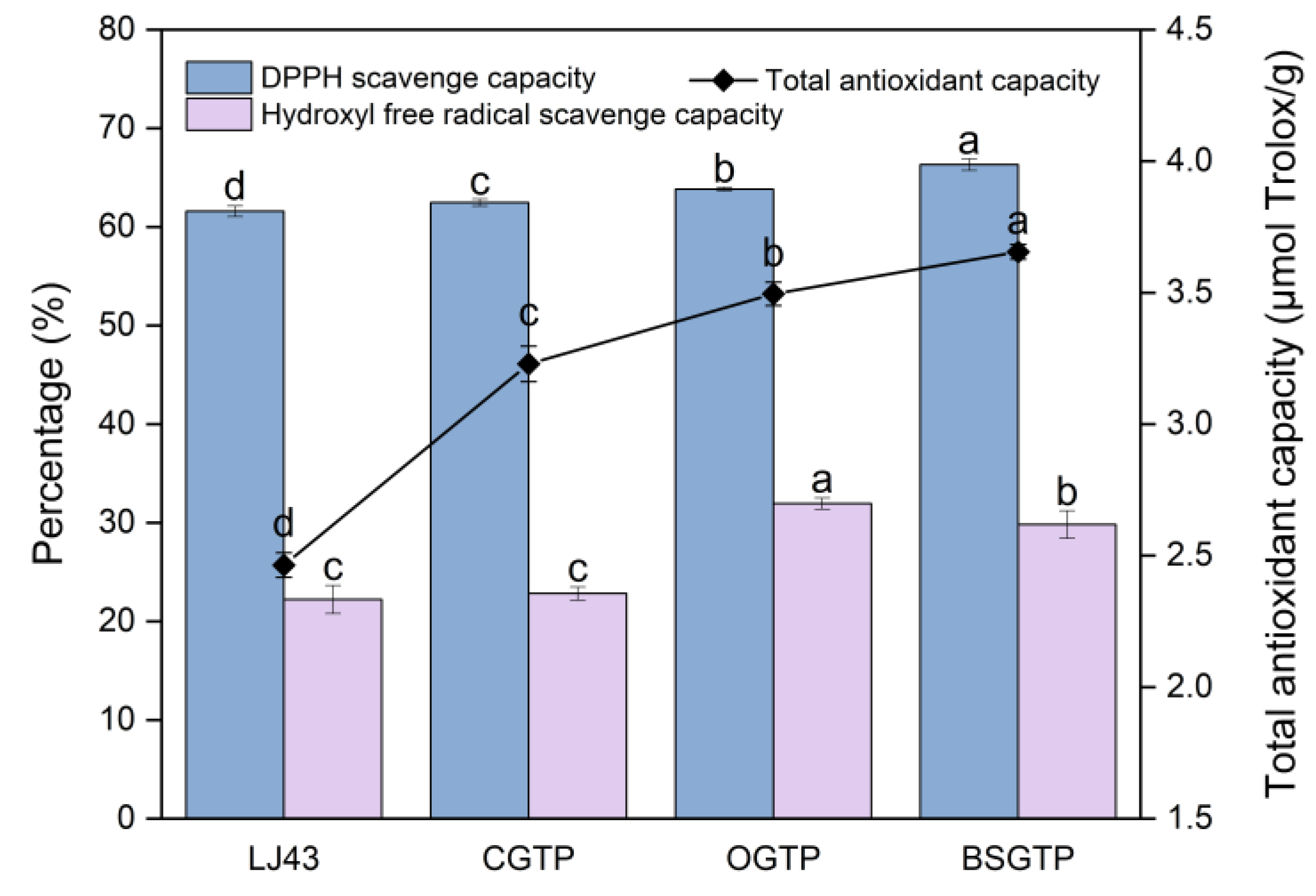
2.11. Study of Antioxidant Capacity of BSGTP
The total antioxidant capacity of the samples was determined using the FRAP-2-G kit (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China). The DPPH free-radical-scavenging capacity of the samples was determined using the BC4750 kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). The hydroxyl free-radical-scavenging capacity of the samples was determined using the QZQ-2-G kit (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China). The instrument used in the experiment was a T6 New Century UV–Vis Spectrophotometer (Beijing Puxi General Instrument Co., Ltd., Beijing, China).
2.12. Statistical Analysis
All experiments were replicated three times, and the final results were averaged. Microsoft Excel 2019 was used for data statistics, SPSS Statistics 25 for data processing, Design Expert 13 for response surface experimental design and the generation of response surface images, and OriginPro 2024 and Adobe Illustrator 2020 for graphing.
4. Conclusions
In this study, the effect of the superfine grinding technique on the quality of SGTP was investigated. An AHP–fuzzy comprehensive evaluation system for SGTP was established, and the effects of three factors (grinding time, rotation speed, and ball-to-material ratio) on the main components of SGTP were investigated. The results showed that grinding time, rotation speed, and ball-to-material ratio all significantly affect the main components of SGTP and thus can all affect the quality of SGTP. The ranking of the three factors’ influence on the quality of SGTP is as follows: ball-to-material ratio > grinding time > rotation speed. The SGTP production process was optimized using the RSM. The optimal conditions for preparing SGTP were a grinding time of 5.85 h, a rotation speed of 397 r/min, and a ball-to-material ratio of 9.2:1. Under these conditions, the sample’s AHP–fuzzy comprehensive evaluation score was 83.3519, closely aligning with the predicted value (83.3630) from the model. In addition, compared with green tea powder made using the traditional crushing method, SGTP possesses strong advantages in terms of particle size, the content and dissolution of major components, and antioxidant capacity. The parameters of SGTP are as follows: median particle size (D50) of 17 μm, chlorophyll content of 4.77 mg/g, caffeine content of 2.40%, TPs content of 15.97%, TFAA of 2.84%, total antioxidant capacity of 3.65 μmol Trolox/g, DPPH free-radical-scavenging capacity of 66.31%, and hydroxyl free-radical-scavenging capacity of 29.83%.
In the future, it will be necessary to conduct a new optimization study of the preparation process to verify the effect of sensory evaluation (color, taste, aroma, etc.) on the quality of SGTP. In addition, the effects of temperature changes during the superfine grinding process on the main components of tea powder are unknown, and a correlation analysis between different evaluation indexes of tea powder has not been conducted. Therefore, future research should focus on further analyzing the existing results and incorporating sensory evaluation into the evaluation system to find the optimal ball milling process parameters.