Screening of Antifungal Lactic Acid Bacteria and Their Impact on the Quality and Shelf Life of Rye Bran Sourdough Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Strains
2.2. Strains and Growth Conditions
2.3. Screening of LAB for Their Antifungal Activity
2.3.1. Preliminary Screening
2.3.2. Further Screening
2.4. Preparation of Rye Bran Sourdough and Bread
2.5. Determination of TTA and Lactic and Acetic Acid Contents and Enumeration of LAB in Rye Bran Sourdough
2.6. Characterization of Rye Bran Bread Dough
2.7. Characterization of Rye Bran Bread
2.7.1. Determination of Texture
2.7.2. Determination of Specific Volume and Baking Loss Rate
2.7.3. Sensory Evaluation
2.7.4. Determination of Volatile Flavor Compounds
2.8. Antifungal Effect of LAB in Rye Bran Bread
2.9. Statistical Analysis
3. Results and Discussion
3.1. Screening of Antifungal LAB
3.2. Characterization of Rye Bran Sourdough
3.3. Characteristics of Rye Bran Bread Dough
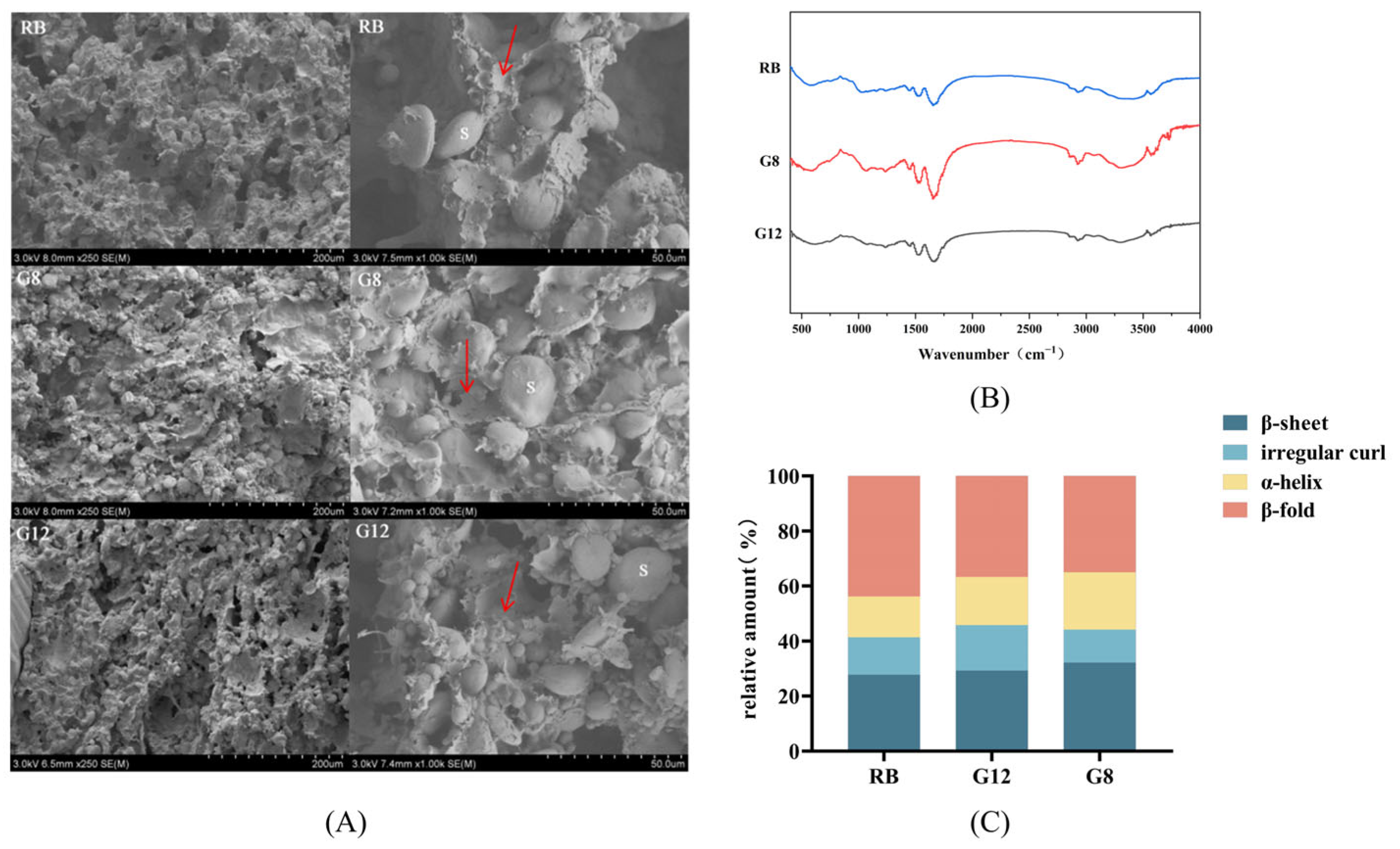
3.3.1. Microstructure of Rye Bran Bread Dough
3.3.2. Secondary Structure of Rye Bran Bread Dough
3.4. Characteristics of Rye Bran Bread
3.4.1. Texture of Rye Bran Bread
3.4.2. Baking Loss Rate and Specific Volume of Rye Bran Bread
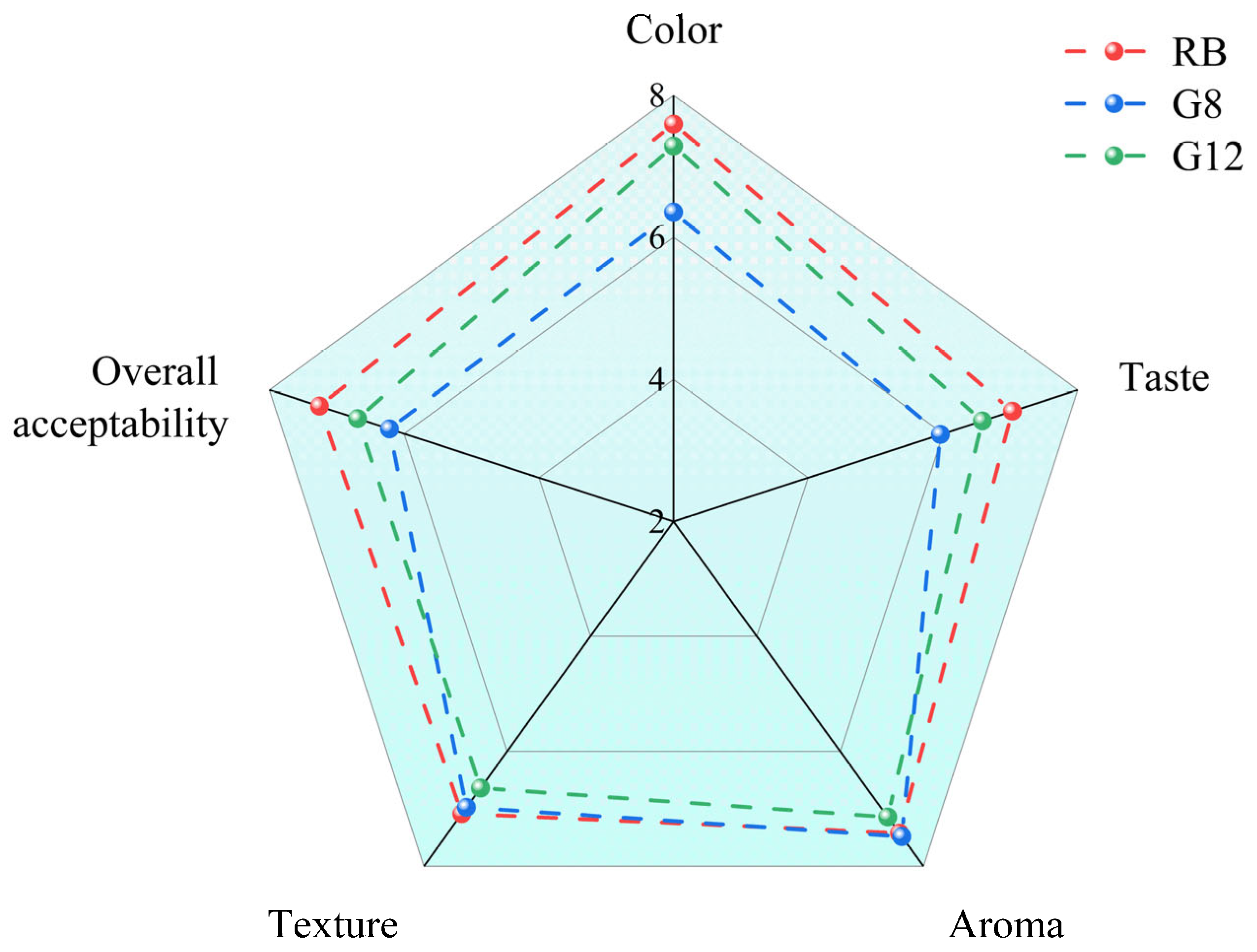
3.4.3. Sensory Evaluation of Rye Bran Bread
3.4.4. Volatile Flavor Compounds of Rye Bran Bread
3.5. Antifungal Effect of LAB in Rye Bran Sourdough Bread
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jonsson, K.; Andersson, R.; Bach Knudsen, K.E.; Hallmans, G.; Hanhineva, K.; Katina, K.; Kolehmainen, M.; Kyrø, C.; Langton, M.; Nordlund, E.; et al. Rye and health—Where do we stand and where do we go? Trends Food Sci. Technol. 2018, 79, 78–87. [Google Scholar] [CrossRef]
- Dziki, D. Rye Flour and Rye Bran: New Perspectives for Use. Processes 2022, 10, 293. [Google Scholar] [CrossRef]
- Saka, M.; Özkaya, B.; Saka, İ. The effect of bread-making methods on functional and quality characteristics of oat bran blended bread. Int. J. Gastron. Food Sci. 2021, 26, 100439. [Google Scholar] [CrossRef]
- Mohamad Asri, N.; Muhialdin, B.J.; Zarei, M.; Saari, N. Low molecular weight peptides generated from palm kernel cake via solid state lacto-fermentation extend the shelf life of bread. LWT 2020, 134, 110206. [Google Scholar] [CrossRef]
- Bianchi, A.; Venturi, F.; Palermo, C.; Taglieri, I.; Angelini, L.G.; Tavarini, S.; Sanmartin, C. Primary and secondary shelf-life of bread as a function of formulation and MAP conditions: Focus on physical-chemical and sensory markers. Food Packag. Shelf Life 2024, 41, 101241. [Google Scholar] [CrossRef]
- Noshirvani, N.; And Abolghasemi Fakhri, L. Advances in extending the microbial shelf-life of bread and bakery products using different technologies: A Review. Food Rev. Int. 2025, 41, 87–112. [Google Scholar] [CrossRef]
- Katina, K.; Laitila, A.; Juvonen, R.; Liukkonen, K.H.; Kariluoto, S.; Piironen, V.; Landberg, R.; Åman, P.; Poutanen, K. Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol. 2007, 24, 175–186. [Google Scholar] [CrossRef]
- Arslan-Tontul, S.; Çetin-Babaoğlu, H.; Aslan, M.; Tontul, I. Evaluation of refractance window-dried type 3 sourdough as an alternative to liquid sourdough in bread production. J. Cereal Sci. 2024, 116, 103882. [Google Scholar] [CrossRef]
- De Vuyst, L.; Vrancken, G.; Ravyts, F.; Rimaux, T.; Weckx, S. Biodiversity, ecological determinants, and metabolic exploitation of sourdough microbiota. Food Microbiol. 2009, 26, 666–675. [Google Scholar] [CrossRef]
- Illueca, F.; Moreno, A.; Calpe, J.; Nazareth, T.D.M.; Dopazo, V.; Meca, G.; Quiles, J.M.; Luz, C. Bread biopreservation through the addition of lactic acid bacteria in sourdough. Foods 2023, 12, 864. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Guo, Z.; Ji, N.; Sun, Q.; Liu, T.; Li, Y. Antifungal activities of Pediococcus pentosaceus LWQ1 and Lactiplantibacillus plantarum LWQ17 isolated from sourdough and their efficacies on preventing spoilage of Chinese steamed bread. Food Control 2025, 168, 110940. [Google Scholar] [CrossRef]
- Pontonio, E.; Dingeo, C.; Di Cagno, R.; Blandino, M.; Gobbetti, M.; Rizzello, C.G. Brans from hull-less barley, emmer and pigmented wheat varieties: From by-products to bread nutritional improvers using selected lactic acid bacteria and xylanase. Int. J. Food Microbiol. 2020, 313, 108384. [Google Scholar] [CrossRef] [PubMed]
- Dopazo, V.; Musto, L.; Nazareth, T.D.M.; Lafuente, C.; Meca, G.; Luz, C. Revalorization of rice bran as a potential ingredient for reducing fungal contamination in bread by lactic acid bacterial fermentation. Food Biosci. 2024, 58, 103703. [Google Scholar] [CrossRef]
- Zhao, S.; Hao, X.; Yang, F.; Wang, Y.; Fan, X.; Wang, Y. Antifungal activity of Lactobacillus plantarum ZZUA493 and its application to extend the shelf life of Chinese steamed buns. Foods 2022, 11, 195. [Google Scholar] [CrossRef]
- Luz, C.; D’Opazo, V.; Quiles, J.M.; Romano, R.; Mañes, J.; Meca, G. Biopreservation of tomatoes using fermented media by lactic acid bacteria. LWT 2020, 130, 109618. [Google Scholar] [CrossRef]
- Tomić, J.; Dapčević-Hadnađev, T.; Škrobot, D.; Maravić, N.; Popović, N.; Stevanović, D.; Hadnađev, M. Spontaneously fermented ancient wheat sourdoughs in breadmaking: Impact of flour quality on sourdough and bread physico-chemical properties. LWT 2023, 175, 114482. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Z.; Zhang, X.; Wang, Z.; Yu, Y.; Shi, J.; Xu, Z. Metagenomics reveals flavour metabolic network of cereal vinegar microbiota. Food Microbiol. 2017, 62, 23–31. [Google Scholar] [CrossRef]
- Divyashree, S.; Ramu, R.; Sreenivasa, M.Y. Evaluation of new candidate probiotic lactobacillus strains isolated from a traditional fermented food- multigrain-millet dosa batter. Food Biosci. 2024, 57, 103450. [Google Scholar] [CrossRef]
- Liu, A.; Su, S.; Sun, Y.; Li, Q.; Li, J.; Hu, K.; Zhao, N.; He, L.; Chen, S.; Liu, S. Enhancing the highland barley-wheat dough network structure and bread quality using freeze-dried sourdough powder with inulin as a protectant. LWT 2024, 191, 115599. [Google Scholar] [CrossRef]
- Jin, J.; Nguyen, T.T.H.; Humayun, S.; Park, S.; Oh, H.; Lim, S.; Mok, I.; Li, Y.; Pal, K.; Kim, D. Characteristics of sourdough bread fermented with Pediococcus pentosaceus and Saccharomyces cerevisiae and its bio-preservative effect against Aspergillus flavus. Food Chem. 2021, 345, 128787. [Google Scholar] [CrossRef]
- Fekri, A.; Torbati, M.; Yari Khosrowshahi, A.; Bagherpour Shamloo, H.; Azadmard-Damirchi, S. Functional effects of phytate-degrading, probiotic lactic acid bacteria and yeast strains isolated from Iranian traditional sourdough on the technological and nutritional properties of whole wheat bread. Food Chem. 2020, 306, 125620. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Peng, Y.; Xi, J.; Zhao, Q.; Xu, D.; Jin, Z.; Xu, X. Effect of sourdough fermented with corn oil and lactic acid bacteria on bread flavor. LWT 2022, 155, 112935. [Google Scholar] [CrossRef]
- Sun, L.; Li, X.; Zhang, Y.; Yang, W.; Ma, G.; Ma, N.; Hu, Q.; Pei, F. A novel lactic acid bacterium for improving the quality and shelf life of whole wheat bread. Food Control 2020, 109, 106914. [Google Scholar] [CrossRef]
- De Simone, N.; López, L.; Ciudad, C.S.; Scauro, A.; Russo, P.; Rodríguez, J.; Spano, G.; Martínez, B. Antifungal activity of Lactiplantibacillus plantarum isolated from fruit and vegetables and detection of novel antifungal VOCs from fungal-LAB co-cultures. Food Biosci. 2024, 58, 103824. [Google Scholar] [CrossRef]
- De Vuyst, L.; Comasio, A.; And Kerrebroeck, S.V. Sourdough production: Fermentation strategies, microbial ecology, and use of non-flour ingredients. Crit. Rev. Food Sci. Nutr. 2023, 63, 2447–2479. [Google Scholar] [CrossRef]
- Boyaci Gunduz, C.P.; Agirman, B.; Gaglio, R.; Franciosi, E.; Francesca, N.; Settanni, L.; Erten, H. Evaluation of the variations in chemical and microbiological properties of the sourdoughs produced with selected lactic acid bacteria strains during fermentation. Food Chem. X 2022, 14, 100357. [Google Scholar] [CrossRef]
- Ooms, N.; Delcour, J.A. How to impact gluten protein network formation during wheat flour dough making. Curr. Opin. Food Sci. 2019, 25, 88–97. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Lyu, L.; Deng, Q.; Fan, H.; Xu, X.; Xu, D. Effects of sourdough on bread staling rate: From the perspective of starch retrogradation and gluten depolymerization. Food Biosci. 2024, 59, 103877. [Google Scholar] [CrossRef]
- Sun, X.; Wu, S.; Li, W.; Koksel, F.; Du, Y.; Sun, L.; Fang, Y.; Hu, Q.; Pei, F. The effects of cooperative fermentation by yeast and lactic acid bacteria on the dough rheology, retention and stabilization of gas cells in a whole wheat flour dough system—A review. Food Hydrocoll. 2023, 135, 108212. [Google Scholar] [CrossRef]
- Hong, T.; Xu, D.; Jin, Y.; Wu, F.; Huang, G.; Zhong, X.; Zhang, J.; Xu, X. Gluten protein transformations during bread processing: Molecular and microstructural analysis. LWT 2025, 217, 117341. [Google Scholar] [CrossRef]
- Marti, A.; Bock, J.E.; Pagani, M.A.; Ismail, B.; Seetharaman, K. Structural characterization of proteins in wheat flour doughs enriched with intermediate wheatgrass (Thinopyrum intermedium) flour. Food Chem. 2016, 194, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, Y.; Li, J.; Ju, X.; Wang, L. Impact of exopolysaccharides-producing lactic acid bacteria on the chemical, rheological properties of buckwheat sourdough and the quality of buckwheat bread. Food Chem. 2023, 425, 136369. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zhang, X.; Niu, M.; Xiang, X.; Chang, Y.; Zhao, Z.; Xiong, L.; Zhao, S.; Rong, J.; Tang, C.; et al. Gluten development and water distribution in bread dough influenced by bran components and glucose oxidase. LWT 2021, 137, 110427. [Google Scholar] [CrossRef]
- Diowksz, A.; Sadowska, A. Impact of sourdough and transglutaminase on gluten-free buckwheat bread quality. Food Biosci. 2021, 43, 101309. [Google Scholar] [CrossRef]
- Eraslan, H.; Wehbeh, J.; Ermis, E. Effect of sourdough prepared with the combination of chickpea and carob on bread properties. Int. J. Gastron. Food Sci. 2023, 32, 100753. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, J.; Li, Y.; Xie, Y.; Cui, M.; Hu, Y.; Yin, R.; Ma, X.; Niu, J.; Cheng, W.; et al. Enhancing physicochemical properties, organic acids, antioxidant capacity, amino acids and volatile compounds for ‘Summer Black’ grape juice by lactic acid bacteria fermentation. LWT 2024, 209, 116791. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.; Adhikari, K.; Shi, Y. Determination of volatile compounds in heat-treated straight-grade flours from normal and waxy wheats. J. Cereal Sci. 2017, 75, 77–83. [Google Scholar] [CrossRef]
- Kaseleht, K.; Paalme, T.; Mihhalevski, A.; Sarand, I. Analysis of volatile compounds produced by different species of lactobacilli in rye sourdough using multiple headspace extraction. Int. J. Food Sci. Technol. 2011, 46, 1940–1946. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Mortazavi, S.A.; Abedfar, A. Application of the selected antifungal LAB isolate as a protective starter culture in pan whole-wheat sourdough bread. Food Control 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Mani-López, E.; López-Malo, A. Antifungal capacity of poolish-type sourdough supplemented with Lactiplantibacillus plantarum and its aqueous extracts in vitro and bread. Antibiotics 2022, 11, 1813. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Mani-López, E.; Palou, E.; López-Malo, A. Sourdoughs as natural enhancers of bread quality and shelf life: A review. Fermentation 2024, 10, 7. [Google Scholar] [CrossRef]

| Bread | Wheat Flour (g) | Water (g) | Salt (g) | Yeast (g) | Sugar (g) | Butter (g) | Rye Bran/Rye Bran Sourdough (g) |
|---|---|---|---|---|---|---|---|
| RB | 80.0 | 52.0 | 1.0 | 1.5 | 6.0 | 4.0 | 8.0 |
| G8 | 80.0 | 40.0 | 1.0 | 1.5 | 6.0 | 4.0 | 20.0 |
| G12 | 80.0 | 40.0 | 1.0 | 1.5 | 6.0 | 4.0 | 20.0 |
| Strain | Antifungal Rate (%) | |||
|---|---|---|---|---|
| A. niger | P. chrysogenum | A. fumigatus | A. flavus | |
| Lpb. plantarum LXRR03 | 75.64 ± 0.04 c | 71.94 ± 0.04 cd | 89.13 ± 0.03 a | 69.89 ± 0.01 c |
| L. Lactis LXRR12 | 75.90 ± 0.07 c | 82.78 ± 0.04 abc | 84.79 ± 0.03 abc | 72.22 ± 0.09 bc |
| Leu. citreum LXRR09 | 95.90 ± 0.03 a | 72.22 ± 0.03 cd | 86.96 ± 0.00 ab | 70.76 ± 0.02 bc |
| Lpb. plantarum LXRR21 | 92.82 ± 0.01 ab | 83.62 ± 0.11 abc | 81.52 ± 0.05 abc | 71.64 ± 0.00 bc |
| Leu. mesenteroides LXRR29 | 72.82 ± 0.02 c | 63.89 ± 0.08 d | 32.61 ± 0.06 d | 30.12 ± 0.02 d |
| Leu. mesenteroides D3 | 82.54 ± 0.00 bc | 97.56 ± 0.01 a | 79.00 ± 0.03 abc | 94.71 ± 0.02 a |
| Lpb. plantarum LXRR04 | 75.90 ± 0.07 c | 87.50 ± 0.07 ab | 77.18 ± 0.08 bc | 77.49 ± 0.02 bc |
| Lpb. plantarum G8 | 96.22 ± 0.02 a | 95.82 ± 0.04 ab | 84.73 ± 0.02 abc | 84.75 ± 0.01 b |
| Lpb. plantarum LXRR16 | 80.77 ± 0.06 c | 79.17 ± 0.07 bc | 75.00 ± 0.02 c | 74.27 ± 0.03 bc |
| Lpb. plantarum LXRR13 | 83.08 ± 0.01 bc | 80.28 ± 0.00 bc | 78.26 ± 0.03 bc | 78.66 ± 0.01 b |
| Bread | Specific Volume (mL/g) | Baking Loss Rate (%) | Hardness (g) | Chewiness | Resilience | Gumminess |
|---|---|---|---|---|---|---|
| RB | 4.32 ± 0.17 a | 8.99 ± 0.12 a | 700.97 ± 12.34 a | 540.74 ± 10.48 a | 0.43 ± 0.01 a | 572.14 ± 1.61 a |
| G8 | 3.33 ± 0.05 c | 6.87 ± 0 c | 530.91 ± 10.29 b | 414.83 ± 25.53 c | 0.44 ± 0 a | 440.83 ± 10.73 c |
| G12 | 3.7 ± 0.08 b | 7.84 ± 0.31 b | 606.49 ± 38.48 b | 473.26 ± 13.36 b | 0.43 ± 0.01 a | 495.91 ± 23.29 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mou, T.; Xu, R.; Li, Q.; Li, J.; Liu, S.; Ao, X.; Chen, S.; Liu, A. Screening of Antifungal Lactic Acid Bacteria and Their Impact on the Quality and Shelf Life of Rye Bran Sourdough Bread. Foods 2025, 14, 1253. https://doi.org/10.3390/foods14071253
Mou T, Xu R, Li Q, Li J, Liu S, Ao X, Chen S, Liu A. Screening of Antifungal Lactic Acid Bacteria and Their Impact on the Quality and Shelf Life of Rye Bran Sourdough Bread. Foods. 2025; 14(7):1253. https://doi.org/10.3390/foods14071253
Chicago/Turabian StyleMou, Tianyu, Ruixia Xu, Qin Li, Jianlong Li, Shuliang Liu, Xiaolin Ao, Shujuan Chen, and Aiping Liu. 2025. "Screening of Antifungal Lactic Acid Bacteria and Their Impact on the Quality and Shelf Life of Rye Bran Sourdough Bread" Foods 14, no. 7: 1253. https://doi.org/10.3390/foods14071253
APA StyleMou, T., Xu, R., Li, Q., Li, J., Liu, S., Ao, X., Chen, S., & Liu, A. (2025). Screening of Antifungal Lactic Acid Bacteria and Their Impact on the Quality and Shelf Life of Rye Bran Sourdough Bread. Foods, 14(7), 1253. https://doi.org/10.3390/foods14071253





