Physicochemical Properties and Stability of Antioxidant Peptides from Swim Bladder of Grass Carp (Ctenopharyngodon idella)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Purification of GCPs
2.3. Determination of Amino Acid Composition
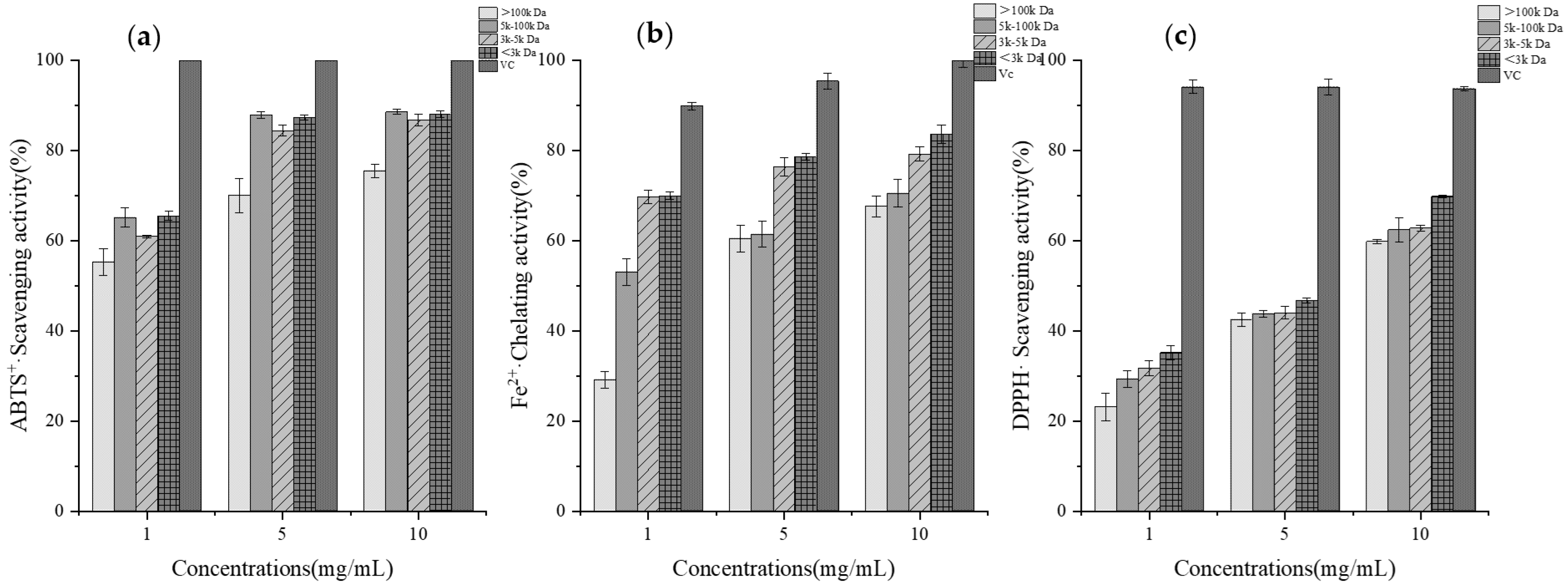
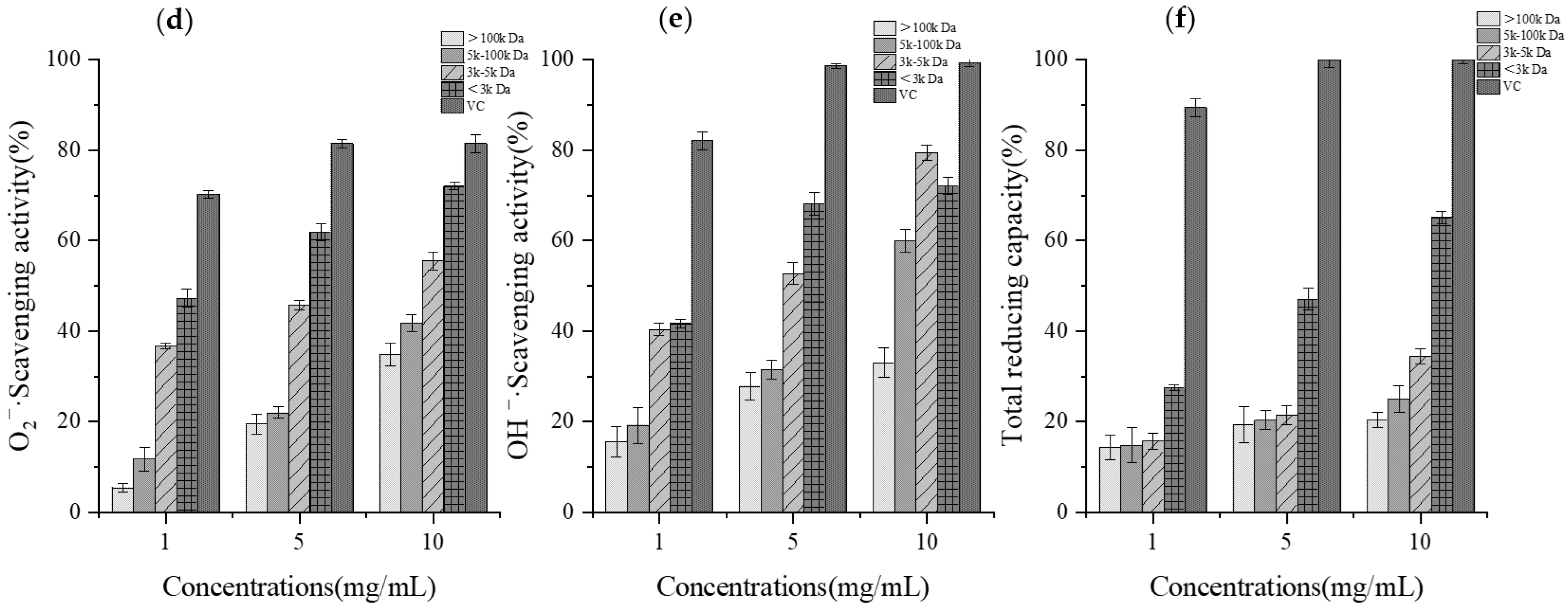
2.4. Determination of In Vitro Antioxidant Activity of GCPs
2.5. In Vitro Simulated Gastrointestinal Digestion Experiments
2.5.1. In Vitro Simulated Oral Digestion
2.5.2. In Vitro Simulated Gastric Digestion
2.5.3. In Vitro Simulated Intestinal Digestion
2.6. Circular Dichroism Analysis
2.7. Amino Acid Composition Analysis
2.8. Determination of Antioxidant Stability of GCPs
2.8.1. Effect of Temperature on the Antioxidant Stability of GCPs
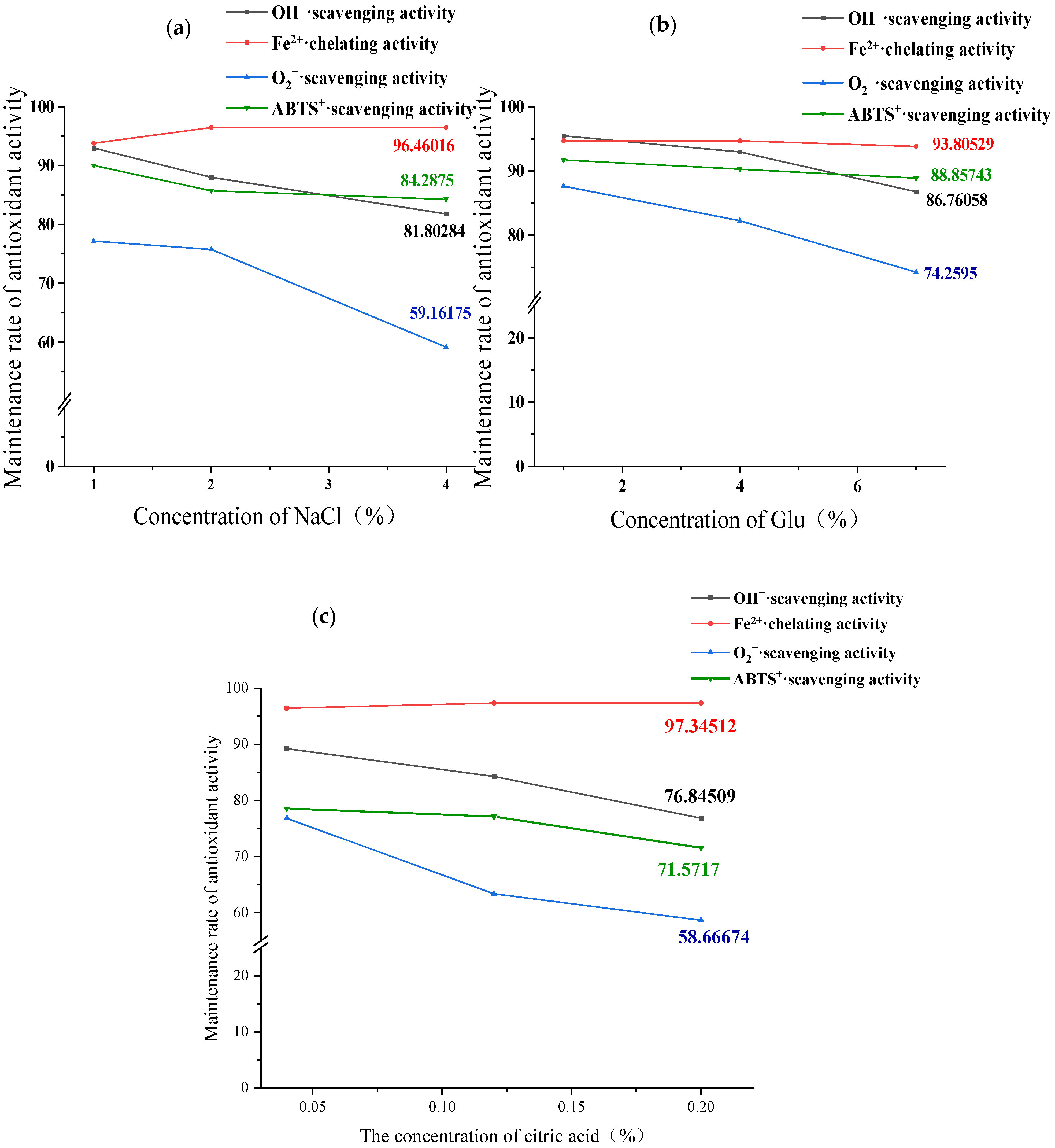
2.8.2. Effect of NaCl, Glucose, and Citric Acid on Antioxidant Stability of GCPs
2.8.3. Study of Antioxidant Stability of Digestive Products of GCPs
2.9. Structural Identification of GCPs
2.10. Solid-Phase Synthesis of Peptides
2.11. Prediction of Physicochemical Properties of Peptides
2.12. Verification of Antioxidant Activity of Peptides
2.13. Determination of Synergistic Effects of Peptides
3. Results and Discussion
3.1. Amino Acid Composition Analysis of GCPs
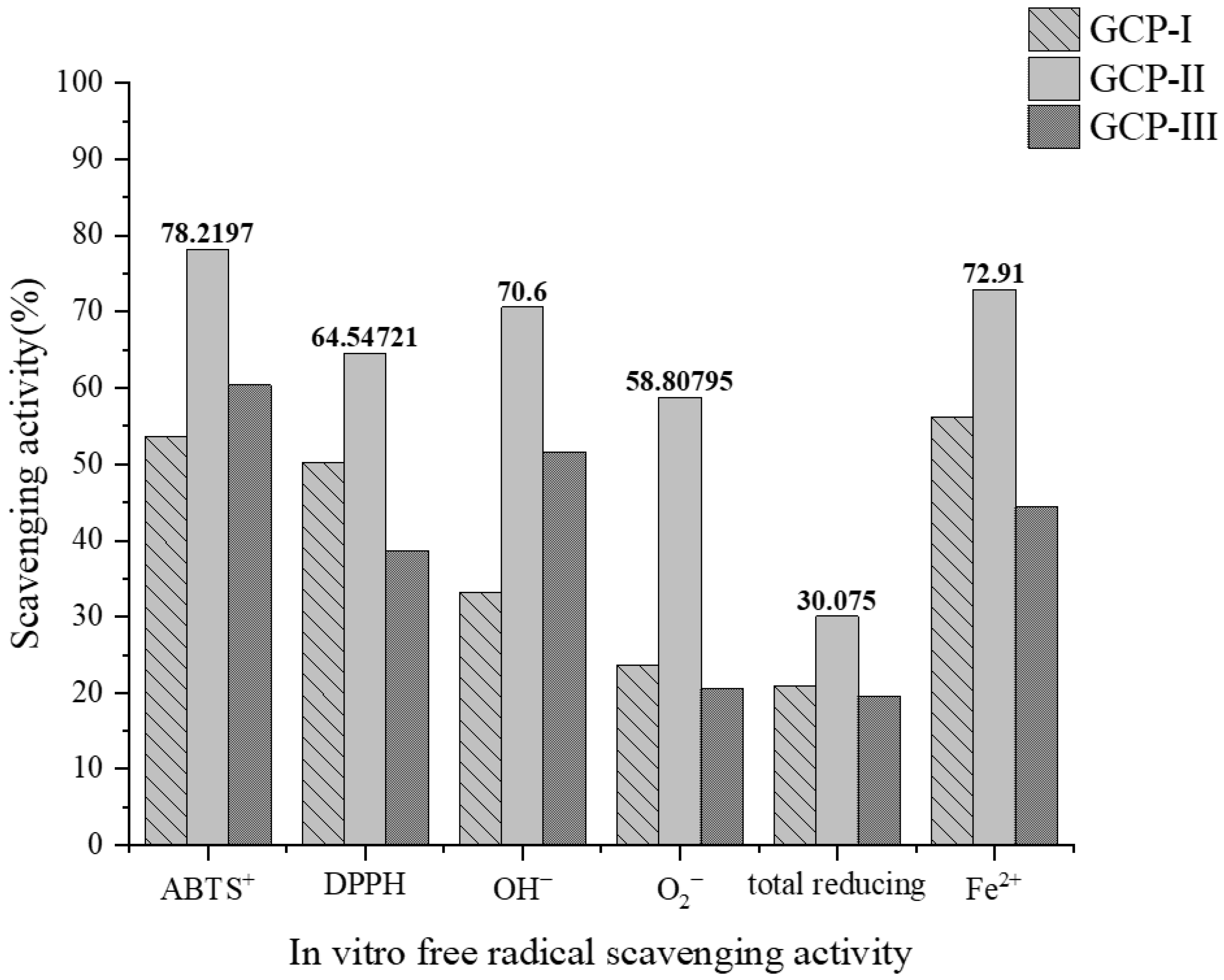
3.2. In Vitro Assessment of Antioxidant Activity of GCPs
3.3. Secondary Structure Analysis of GCPs and Digestive Products
3.4. Analysis of Amino Acid Composition of Digestive Products of GCPs
3.5. Impact of Simulated In Vitro Digestion on Antioxidant Activity of GCPs
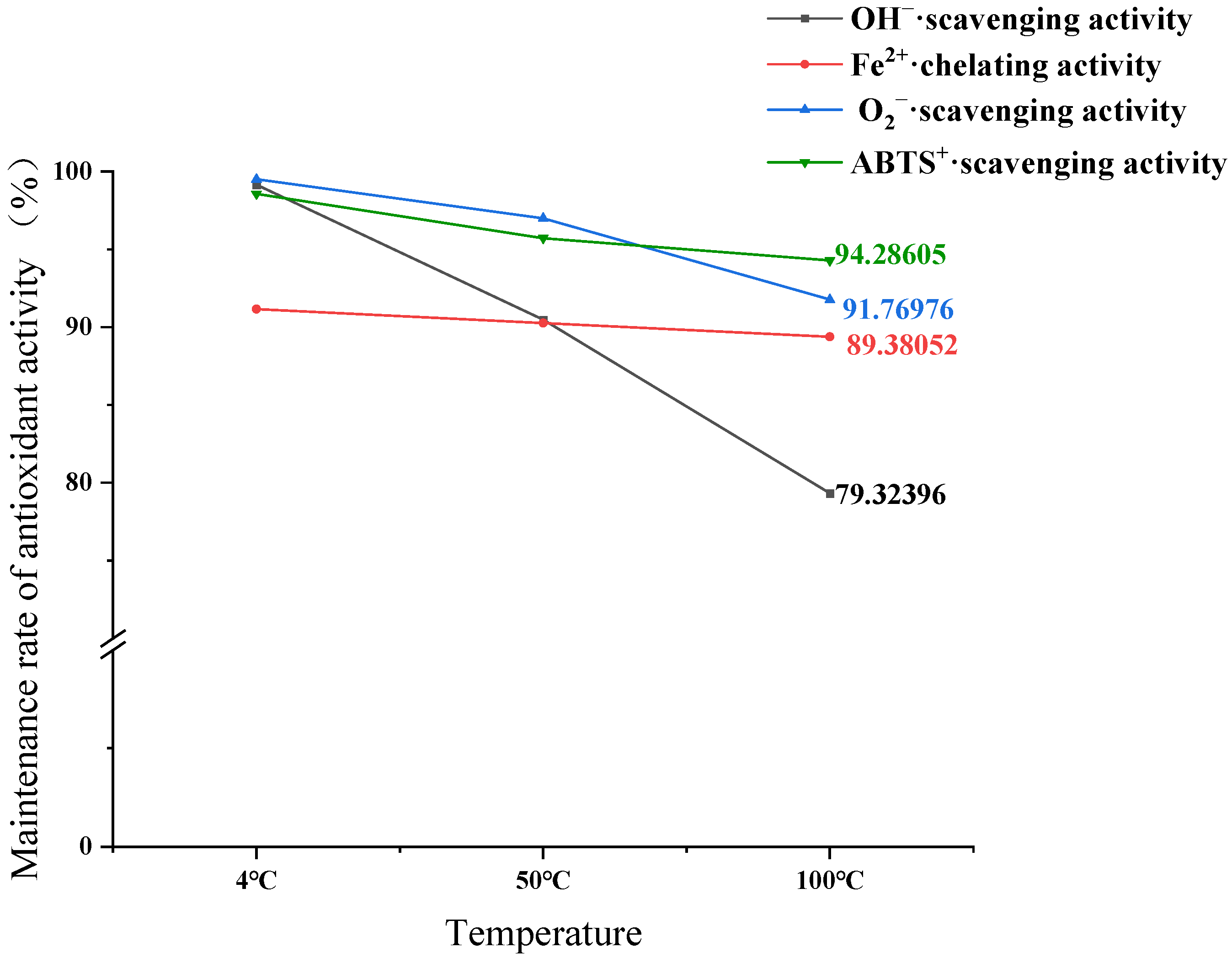
3.6. Antioxidant Stability Assessment Results of GCP-II
3.6.1. Impact of Temperature on Antioxidant Stability of GCP-II
3.6.2. Impact of Food Ingredient Components on Antioxidant Stability of GCP-II
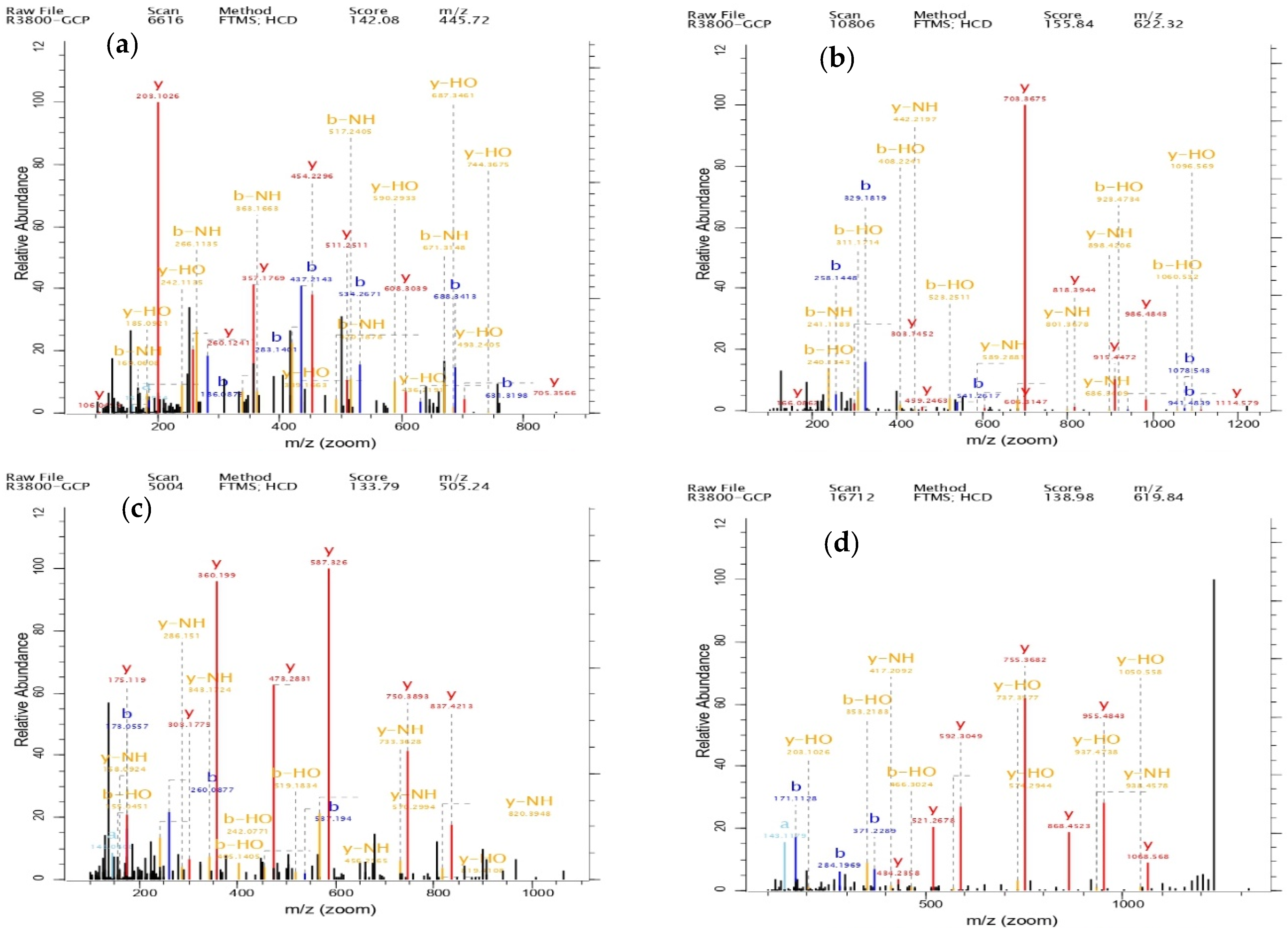
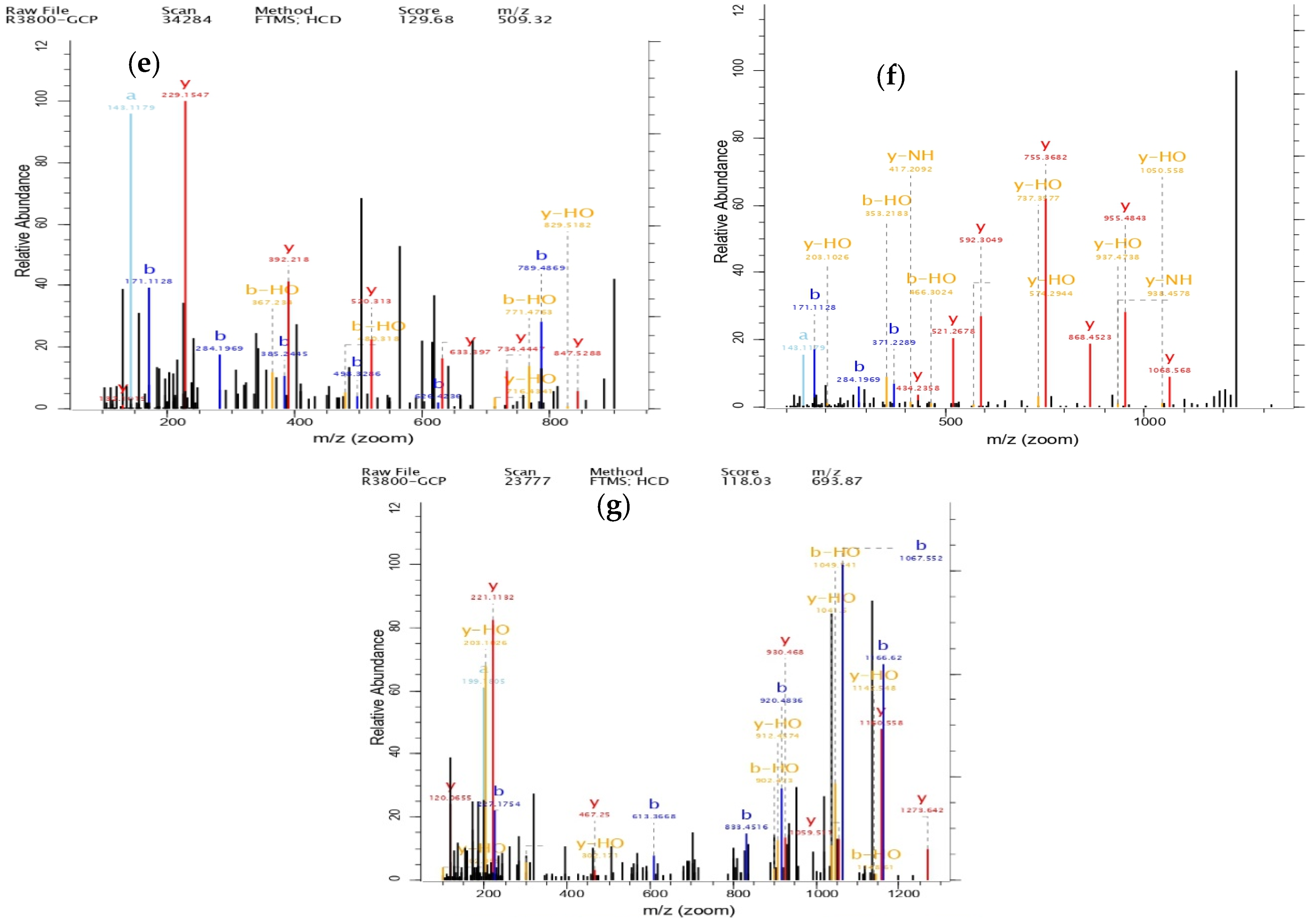
3.7. Structural Identification of GCP-II
3.8. Peptide Selection and Prediction of Physicochemical Properties
3.9. Verification of Physicochemical Properties of Synthesized Peptides
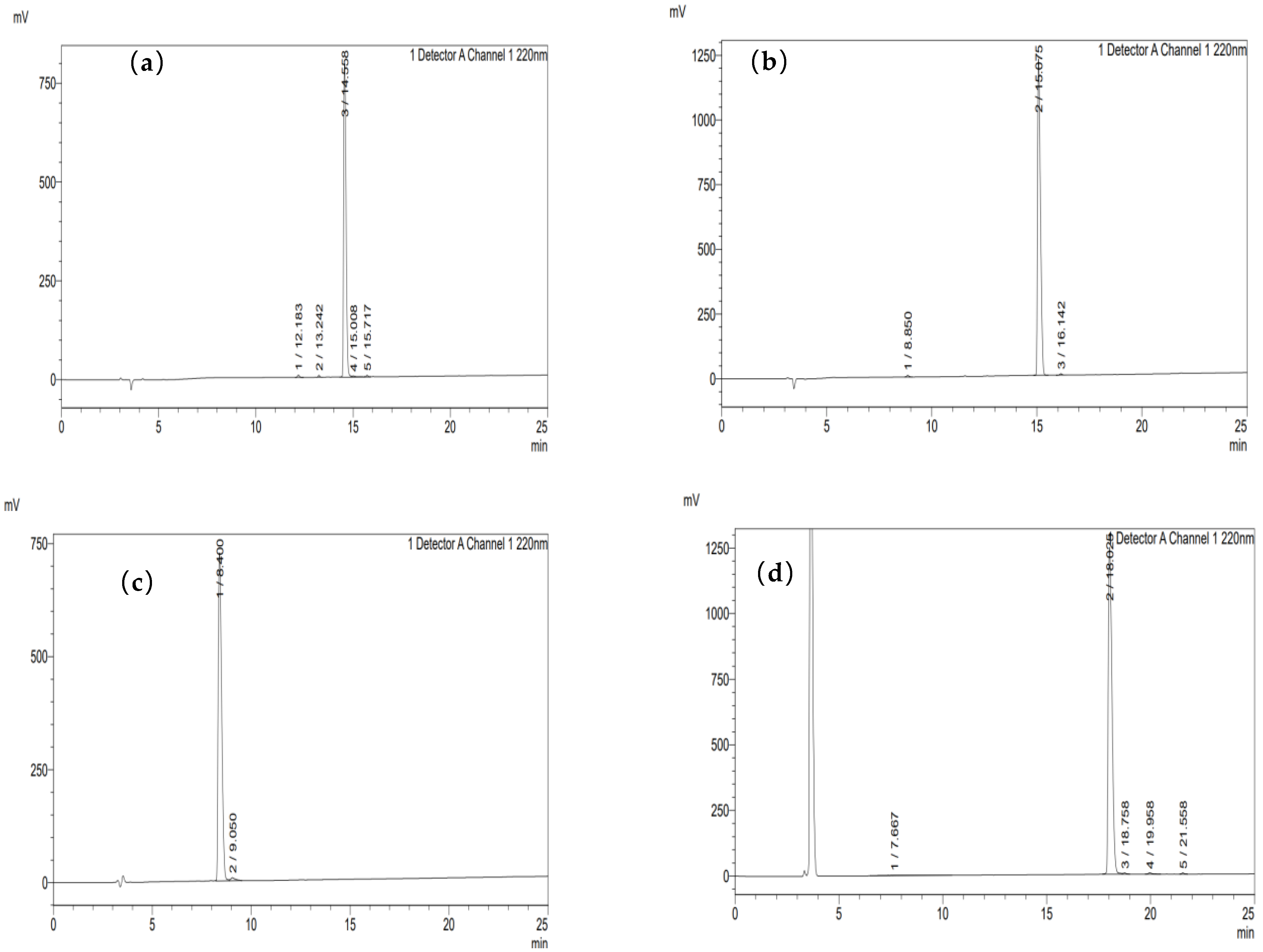
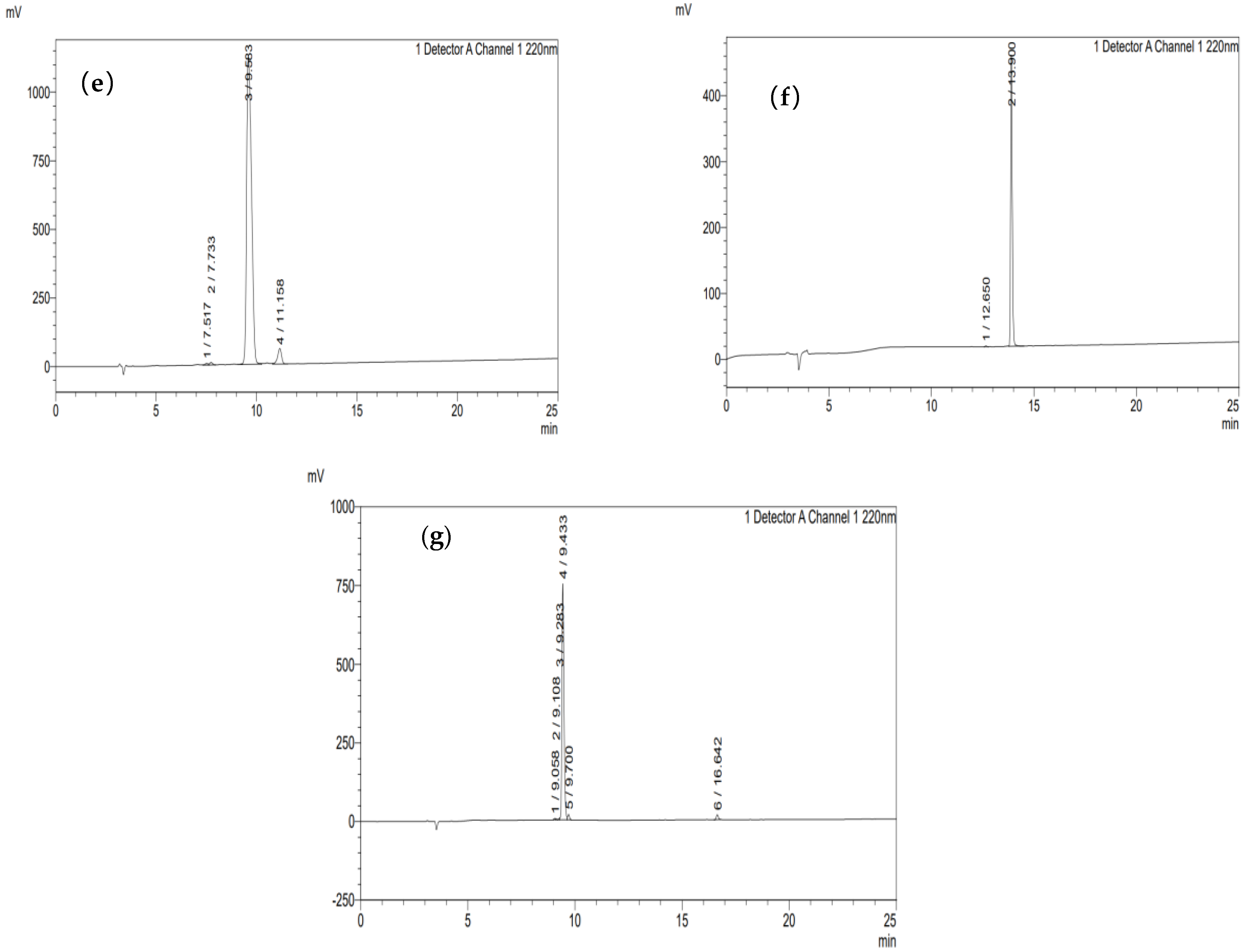
3.9.1. Purity Information of Synthesized Peptides
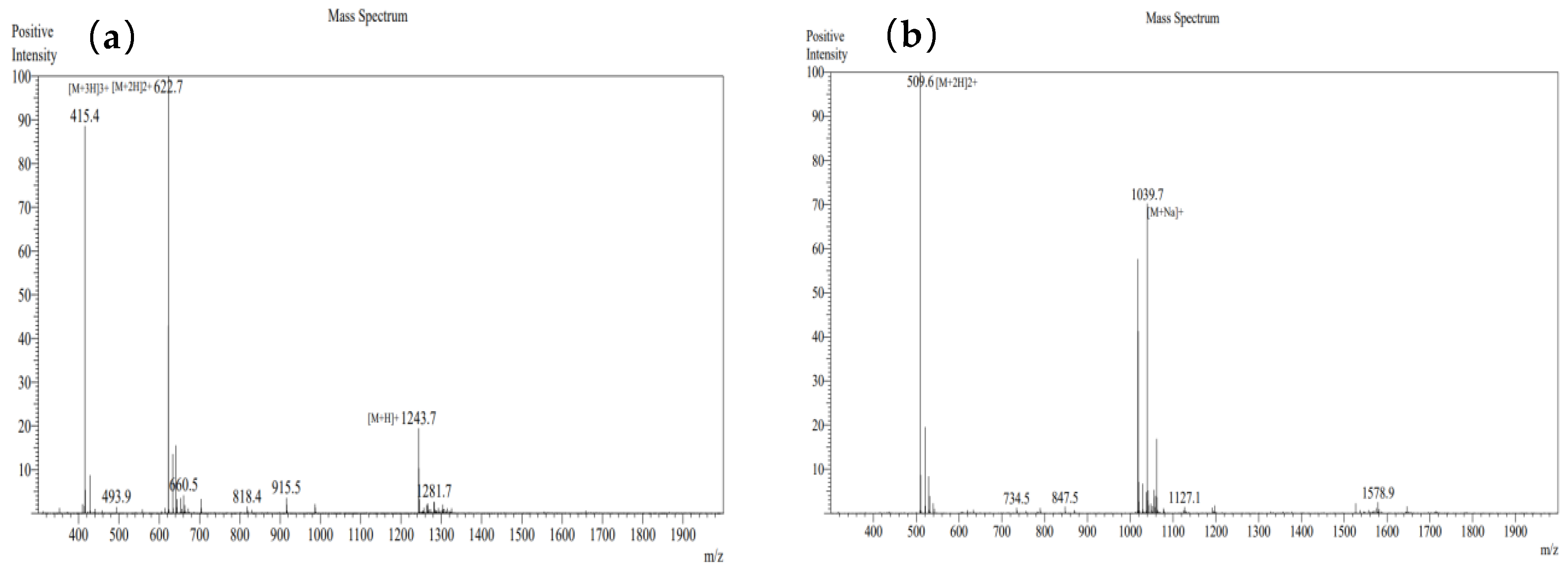
3.9.2. Verification of Mass Spectrometry Information for Synthesized Peptides
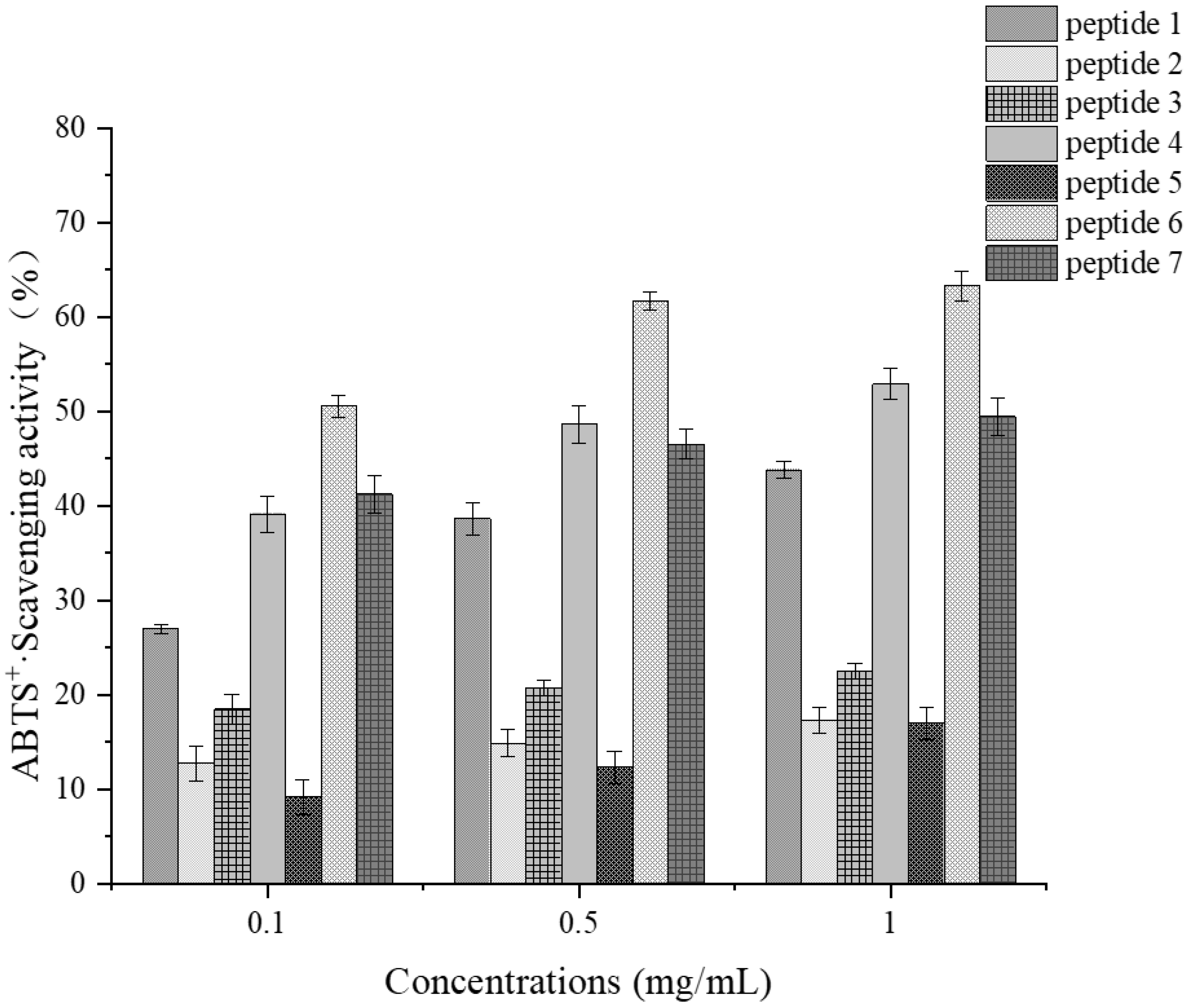
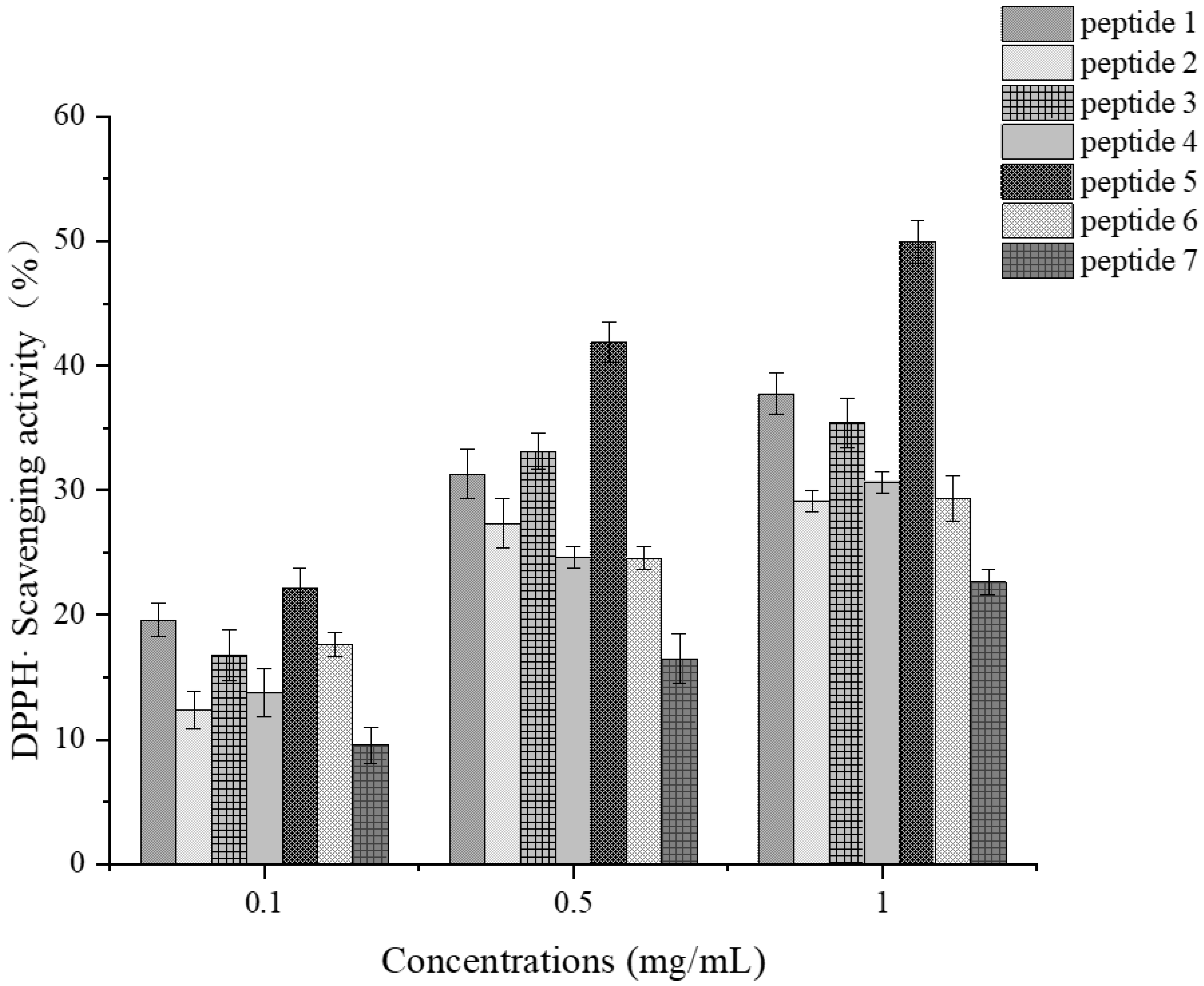
3.9.3. Verification of Antioxidant Activity of Synthetic Peptides
Determination of ABTS Radical Scavenging Activity
Determination of DPPH Radical Scavenging Activity
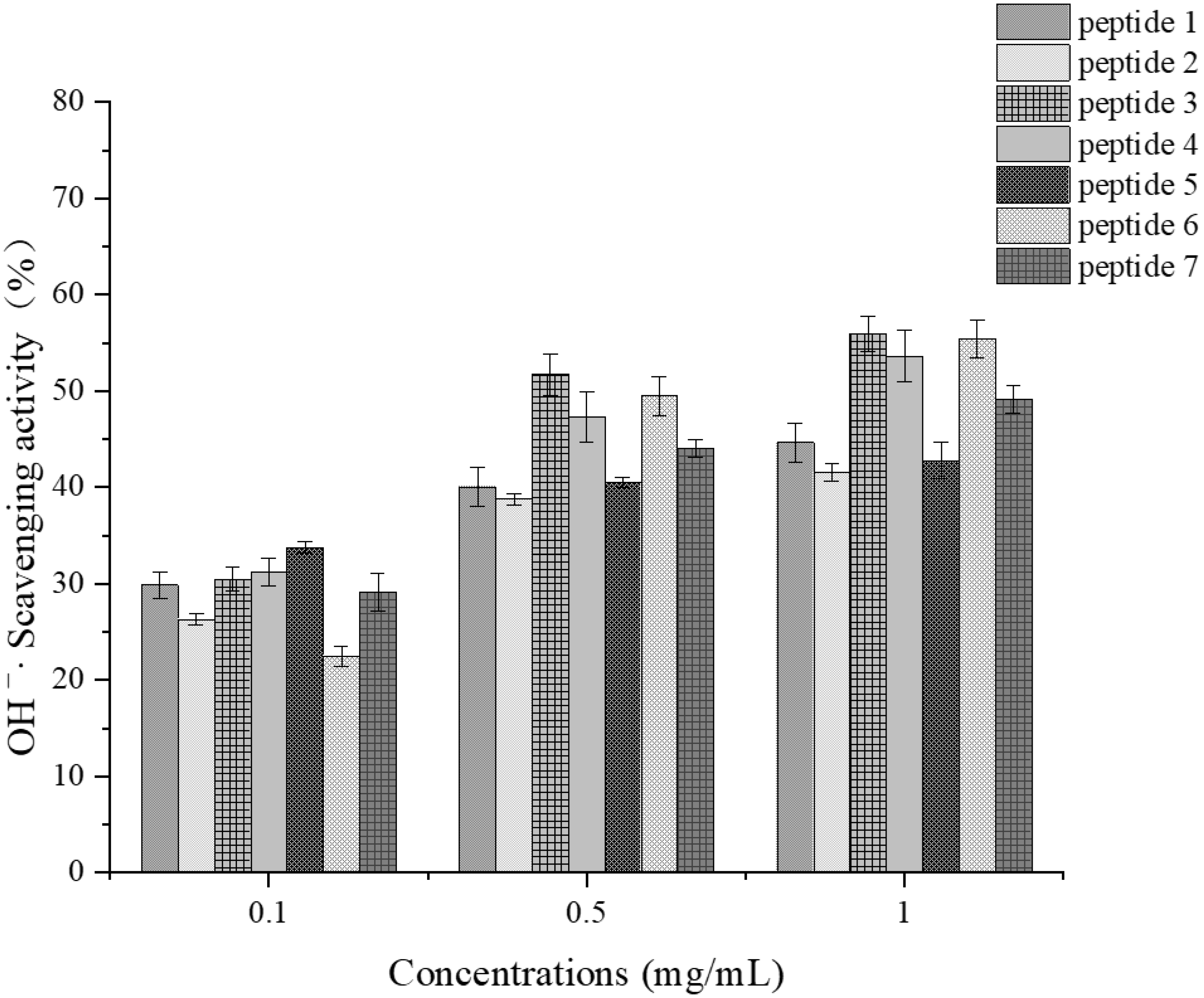
Determination of Hydroxyl Radical Scavenging Activity
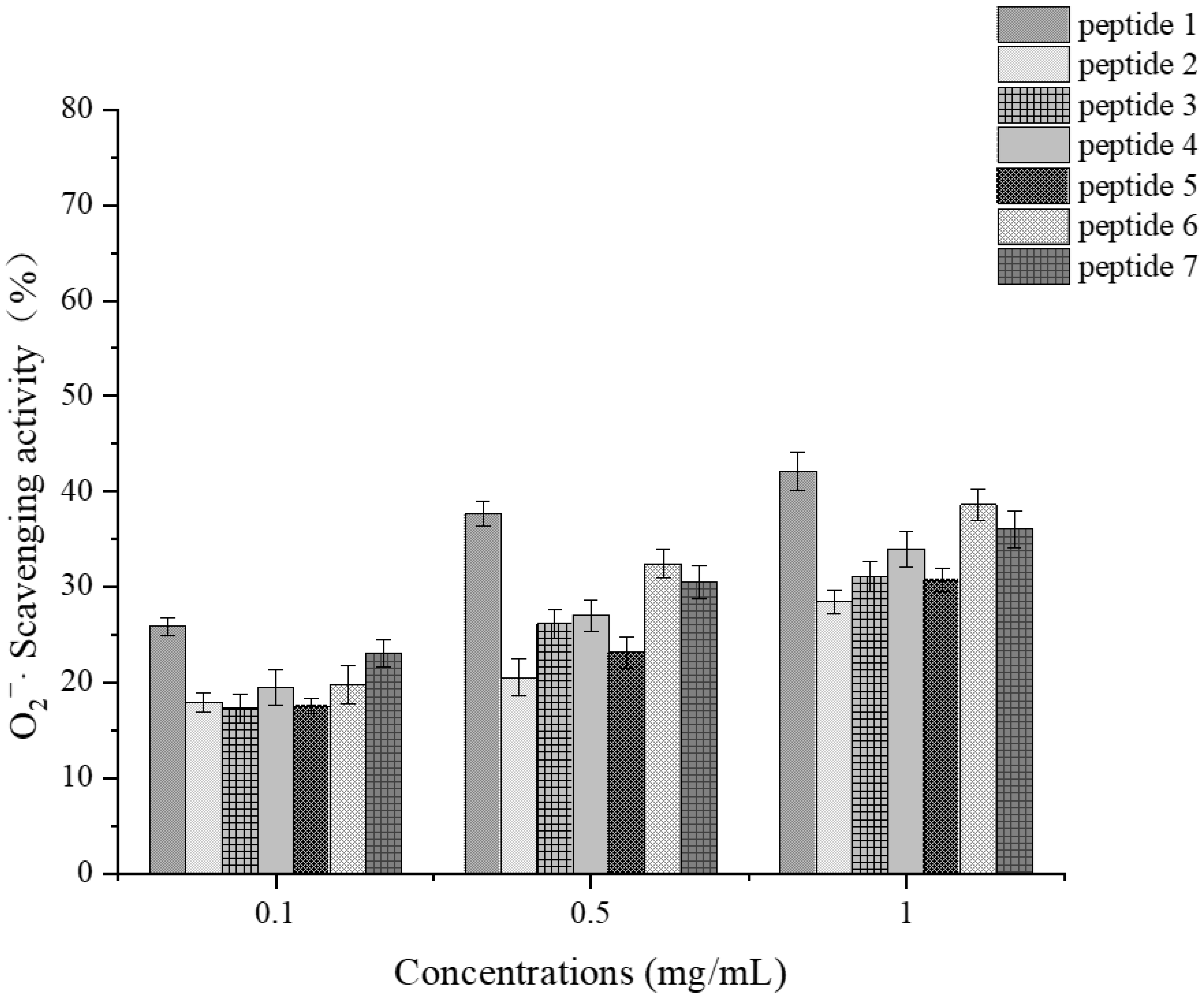
Determination of Superoxide Anion Scavenging Activity
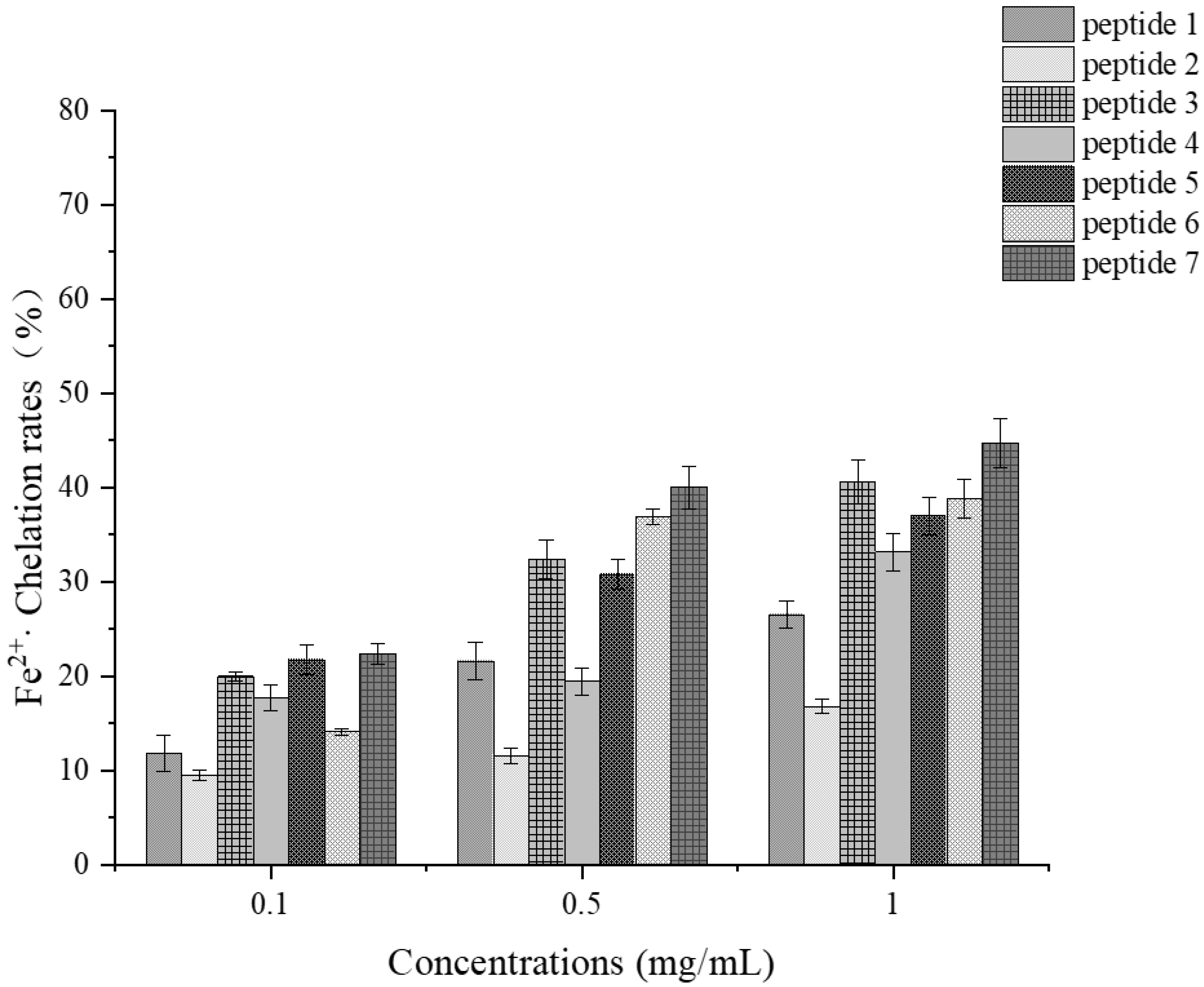
Determination of Ferrous Ion Chelating Ability
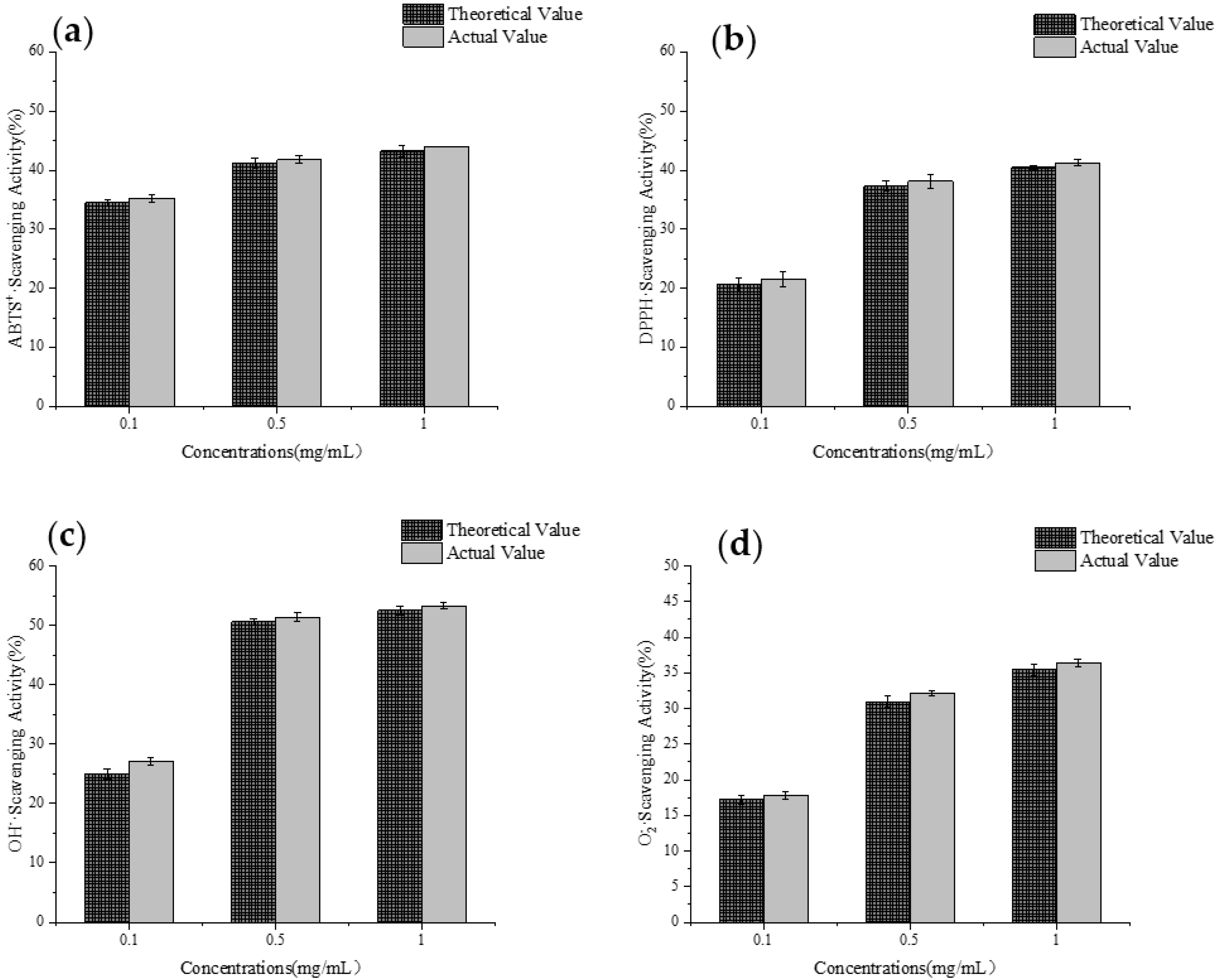
3.10. Analysis of Synergistic Interactions in Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Chen, W.; Xu, T.; Liu, H.; Jin, J.; Yang, Y.; Zhu, X.; Han, D.; Xie, S. Exploring the Indicator Gut Microbiota Taxa in Grass Carp (Ctenopharyngodon idella): Correlations with Growth Rates. Aquaculture 2025, 599, 742080. [Google Scholar] [CrossRef]
- Xie, X.-D.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Ren, H.-M.; Jin, X.-W.; Zhang, R.-N.; Zhou, X.-Q. From Antioxidant to Muscle Enhancer: Resveratrol’s Role in Grass Carp (Ctenopharyngodon idella) Nutrition. Aquac. Rep. 2024, 39, 102499. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, L.; Farag, M.A.; Cai, X.; Wang, S. Identification of novel antioxidant peptides from Lateolabrax japonicus and the underlying molecular mechanisms against oxidative stress injury in Caco-2 cells. Food Biosci. 2024, 62, 105538. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, H.; Li, X.; Lai, F.; Xiao, X. Purification and characterization of high antioxidant peptides from duck egg white protein hydrolysates. Biochem. Biophys. Res. Commun. 2014, 452, 888–894. [Google Scholar] [CrossRef]
- Rosti, G.; Romano, F.; Secondino, S.; Caccialanza, R.; Lobascio, F.; Carminati, O.; Pedrazzoli, P.; Tralongo, P. The Role of Nutritional Support in Cured/Chronic Patients. Nutrients 2020, 12, 3167. [Google Scholar] [CrossRef] [PubMed]
- Abeynayake, R.; Zhang, S.; Yang, W.; Chen, L. Development of antioxidant peptides from brewers’ spent grain proteins. LWT 2022, 158, 113162. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhang, H.; Liu, S.; Yang, M.; Cui, M.; Zhang, T.; Yu, Y.; Xiao, H.; Du, Z. Fabrication, characterization and functional attributes of zein-egg white derived peptides (EWDP)-chitosan ternary nanoparticles for encapsulation of curcumin: Role of EWDP. Food Chem. 2022, 372, 131266. [Google Scholar] [CrossRef]
- Hazam, P.K.; Selvaraj, S.P.; Negi, A.; Lin, W.-C.; Chen, J.-Y. Use of natural peptide TP4 as a food preservative prevents contamination by fungal pathogens. Food Chem. 2024, 455, 139874. [Google Scholar] [CrossRef]
- Lammi, C.; Aiello, G.; Boschin, G.; Arnoldi, A. Multifunctional peptides for the prevention of cardiovascular disease: A new concept in the area of bioactive food-derived peptides. J. Funct. Foods 2019, 55, 135–145. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef]
- Liang, L.L.; Cai, S.Y.; Gao, M.; Chu, X.M.; Pan, X.Y.; Gong, K.K.; Xiao, C.W.; Chen, Y.; Zhao, Y.Q.; Wang, B.; et al. Purification of antioxidant peptides of Moringa oleifera seeds and their protective effects on H₂O₂ oxidative damaged Chang liver cells. J. Funct. Foods 2020, 64, 103698. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, X.-Q.; Jiang, W.-D.; Wu, P.; Liu, Y.; Ma, Y.-B.; Tang, L.; Li, S.-W.; Kuang, S.-Y.; Feng, L. Histidine Promotes Muscle Growth and Protein Deposition in Grass Carp (Ctenopharyngodon idella): Evidence from in vivo and in vitro Models. Food Biosci. 2024, 62, 105537. [Google Scholar] [CrossRef]
- Li, S.; Gu, J.; Zhong, B.; Feng, R.; Pan, H.; Liu, Y.; Shi, W. Physicochemical Properties and Stability of Antioxidant Peptides from Swim Bladder of Grass Carp (Ctenopharyngodon idella). Aquac. Fish. 2023, 8. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Contreras, M.M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and Characterization of Antioxidant Peptides from Chickpea Protein Hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef]
- Shen, C.H.; Yang, J.; Zhang, Y.H.; Zeng, X.-F. Optimization of Double-Enzyme Hydrolysis Process of Pigeon Breast Meat and Evaluation of Its Antioxidant Activity. Food Mach. 2023, 39, 163–169. [Google Scholar] [CrossRef]
- Mohottige, M.W.J.; Juhász, A.; Nye-Wood, M.G.; Farquharson, K.A.; Bose, U.; Colgrave, M.L. Beyond Nutrition: Exploring Immune Proteins, Bioactive Peptides, and Allergens in Cow and Arabian Camel Milk. Food Chem. 2025, 467, 142471. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Zhao, Z. Construction of Lemongrass Essential Oil Microemulsion and Its Antioxidant Activity Analysis. Mod. Food Sci. Technol. 2018, 34, 156–164. [Google Scholar] [CrossRef]
- Sihag, S.; Pal, A.; Ravikant; Saharan, V. Antioxidant Properties and Free Radicals Scavenging Activities of Pomegranate (Punica granatum L.) Peels: An In Vitro Study. Biocatal. Agric. Biotechnol. 2022, 42, 102368. [Google Scholar] [CrossRef]
- Li, N.; Shen, X.R.; Liu, Y.M.; Zhang, J.; He, Y.; Liu, Q.; Jiang, D.; Zong, J.; Li, J.; Hou, D.; et al. Isolation, Characterization, and Radiation Protection of Sipunculus nudus L. Polysaccharide. Int. J. Biol. Macromol. 2016, 83, 288–296. [Google Scholar] [CrossRef]
- Xiang, A.; Xu, S.; Ju, H.; Zhao, S.; Yue, T.; Yuan, Y. Purification, Structural Characterization and Antioxidant Activity of Selenoprotein from Cyanobacteria chinensis. J. Northwest Agric. Sci. 2019, 31, 299–309. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Duan, X.Y.; Tang, T.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Liu, Y. Characterization and Antioxidant Activities of Polysaccharides from Thirteen Boletus Mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static In Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wu, Y.; Li, L. Relationship between Primary Structure or Spatial Conformation and Functional Activity of Antioxidant Peptides from Pinctada fucata. Food Chem. 2018, 264, 108–117. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.Y.; Fan, X.; Zhang, T.; Wang, H.; Ye, K.P. Novel antioxidant peptides from bovine blood: Purification, identification and mechanism of action. LWT 2024, 205, 116499. [Google Scholar] [CrossRef]
- Ren, L.K.; Yang, Y.; Ma, C.M.; Fan, J.; Bian, X.; Liu, B.X.; Wang, D.F.; Zhu, P.Y.; Fu, Y.; Zhang, N. Identification and in silico analysis of novel antioxidant peptides in broken rice protein hydrolysate and its cytoprotective effect against H₂O₂-induced 2BS cell model. Food Res. Int. 2022, 162 Pt B, 112108. [Google Scholar] [CrossRef]
- Mi, C.; Yang, L.; Yang, H.; Liu, Y.; Yu, X.; Hu, Z.; Cai, Y.; Li, X.; Zhou, H.; Wu, L. Tilapia skin antioxidant peptide structure-activity parsing and microcapsule steady research. Sci. Technol. Food Ind. 2024, 1–28. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Lin, S.; Cheng, S. Contribution of Specific Amino Acid and Secondary Structure to the Antioxidant Property of Corn Gluten Proteins. Food Res. Int. 2018, 105, 836–844. [Google Scholar] [CrossRef]
- Zhang, S.; Shan, Y.; Zhang, S.; Sui, Z.; Zhang, L.; Liang, Z.; Zhang, Y. NIPTL-Novo: Non-Isobaric Peptide Termini Labeling Assisted Peptide De Novo Sequencing. J. Proteom. 2017, 154, 40–48. [Google Scholar] [CrossRef]
- Fang, S.; Ren, Q.; Zhou, Z.; Ji, Z.; Zhou, J.; Xu, Y.; Mao, J. The Separation and Purification of Rice Wine Peptide: Research Progress and Its Functions. Food Ferment. Ind. 2024, 50. [Google Scholar] [CrossRef]
- Orsini Delgado, M.C.; Nardo, A.; Pavlovic, M.; Rogniaux, H.; Añón, M.C.; Tironi, V.A. Identification and characterization of antioxidant peptides obtained by gastrointestinal digestion of amaranth proteins. Food Chem. 2016, 197, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Study on Memory Improving Effect, Absorption Metabolism and Mechanism of Action of Walnut Peptide. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2023. [Google Scholar]
- Özyürek, M.; Bektaşoğlu, B.; Güçlü, K.; Apak, R. Hydroxyl radical scavenging assay of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method using catalase for hydrogen peroxide degradation. Anal. Chim. Acta 2008, 616, 196–206. [Google Scholar] [CrossRef]
- Yoshida, H.; Komiya, A.; Ohtsuki, R.; Kusaka-Kikushima, A.; Sakai, S.; Kawabata, K.; Kobayashi, M.; Nakamura, S.; Nagaoka, A.; Sayo, T.; et al. Relationship of hyaluronan and HYBID (KIAA1199) expression with roughness parameters of photoaged skin in Caucasian women. Skin Res. Technol. 2018, 24, 562–569. [Google Scholar] [CrossRef]
- Liu, H.; Liu, F. Study on the whitening effect and antioxidant effect of iso-astilbe. Daily Chem. Ind. (Chin. Engl.) 2024, 54, 1368–1374. [Google Scholar]
- Hernández-Ledesma, B.; Dávalos, A.; Bartolomé, B.; Amigo, L. Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulin. Identification of active peptides by HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 588–593. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Podio, N.S.; Wang, X.-Y.; Jiang, S.; Xu, S.; Qiu, X.; Zeng, Z.; Gong, W.; Wang, S.; et al. Untargeted metabolomics profiling of purple rice phenolics and their antioxidant activities. LWT 2024, 214, 117127. [Google Scholar] [CrossRef]
- Sunde, H.; Ryder, K.; Bekhit, A.E.-D.A.; Carne, A. Analysis of peptides in a sheep beta lactoglobulin hydrolysate as a model to evaluate the effect of peptide amino acid sequence on bioactivity. Food Chem. 2021, 365, 130346. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, Y.; Wang, C. Extraction, compositional analysis, in vitro antioxidant and antiproliferative activities of dandelion (Taraxacum officinale) seed oil. Food Chem. 2025, 476, 143435. [Google Scholar] [CrossRef]
- Shabestarian, H.; Asoodeh, A.; Homayouni-Tabrizi, M.; Hossein-Nejad-Ariani, H. Antioxidant and Angiotensin I Converting Enzyme (ACE) Inhibitory Properties of GL-9 Peptide. J. Food Process. Preserv. 2017, 41, e12838. [Google Scholar] [CrossRef]
- Aursuwanna, T.; Noitang, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Puthong, S.; Reamtong, O.; Karnchanatat, A. Investigating the cellular antioxidant and anti-inflammatory effects of the novel peptides in lingzhi mushrooms. Heliyon 2022, 8, e11067. [Google Scholar] [CrossRef]
- Rodríguez, M.; Tironi, V.A. Chemical and cell antioxidant activity of amaranth flour and beverage after simulated gastrointestinal digestion. Role of peptides. Food Res. Int. 2023, 173 Pt 2, 113410. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Ou, Y.; Chen, H.; Xiao, S.; Liu, G.; Cao, Y.; Huang, Q. Cellular antioxidant activities of polyphenols isolated from Eucalyptus leaves (Eucalyptus grandis × Eucalyptus urophylla GL9). J. Funct. Foods 2014, 7, 737–745. [Google Scholar] [CrossRef]
- Song, R.; Wei, R.-B.; Ruan, G.-Q.; Luo, H.-Y. Isolation and identification of antioxidative peptides from peptic hydrolysates of half-fin anchovy (Setipinna taty). LWT-Food Sci. Technol. 2015, 60, 221–229. [Google Scholar] [CrossRef]
- Yang, W. Evaluation of the antioxidant activity and identification of potential antioxidant peptides in commercially available probiotic Cheddar cheese. LWT 2024, 205, 116486. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Khammuang, S.; Sarnthima, R.; Sanachai, K. Purification and Identification of Novel Antioxidant Peptides from Silkworm Pupae (Bombyx mori) Protein Hydrolysate and Molecular Docking Study. Biocatal. Agric. Biotechnol. 2022, 42, 102367. [Google Scholar] [CrossRef]
- Wang, J.N.; Yang, G.; Li, H.F.; Zhang, T.; Sun, D.; Lu, W.P.; Zhang, W.; Wang, Y.; Ma, M.; Cao, X.; et al. Preparation and Identification of Novel Antioxidant Peptides from Camel Bone Protein. Food Chem. 2023, 424, 136253. [Google Scholar] [CrossRef]
- Shanmugam, V.P.; Kapila, S.; Sonfack, T.K.; Kapila, R. Antioxidative Peptide Derived from Enzymatic Digestion of Buffalo Casein. Int. Dairy J. 2015, 42, 1–5. [Google Scholar] [CrossRef]
- Shi, Y.N.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Antioxidant Activity of Enzymatic Hydrolysates from Eggshell Membrane Proteins and Its Protective Capacity in Human Intestinal Epithelial Caco-2 Cells. J. Funct. Foods 2014, 10, 35–45. [Google Scholar] [CrossRef]
- Krobthong, S.; Yingchutrakul, Y. Identification and Enhancement of Antioxidant P1-Peptide Isolated from Ganoderma lucidum Hydrolysate. Food Biotechnol. 2020, 34, 338–351. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, B.; Xu, C.; Wang, J.; Wang, Z.; Huang, Y.; Ma, C. Impact of enzymatic fermentation on taste, chemical compositions and in vitro antioxidant activities in Chinese teas using E-tongue, HPLC and amino acid analyzer. LWT 2022, 163, 113549. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, Y.; Huo, X.; Long, P.; Zheng, Y.; Guo, X.; Liu, J.; Zhang, Y.; Niu, Y. Multifunctional ACE-Inhibitory Peptides with Antioxidant and Ferrous-Chelating Capacities from Ginkgo Kernel Glutelin-2 Hydrolysates: Identification, Virtual Screening, Inhibition Mechanism, and Gastrointestinal Stability Studies. LWT 2025, 217, 117461. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Z.; Shao, J.; Wang, C.; Zhan, C. Effect of Fermentation on the Peptide Content, Phenolics and Antioxidant Activity of Defatted Wheat Germ. Food Biosci. 2017, 20, 141–148. [Google Scholar] [CrossRef]
- Wang, J.; Guo, M.; Wang, Q.; Dong, J.; Lu, S.; Lyv, B.; Ma, X. Antioxidant Activities of Peptides Derived from Mutton Ham, Xuanwei Ham and Jinhua Ham. Food Res. Int. 2021, 142, 110195. [Google Scholar] [CrossRef] [PubMed]




| Items | Manufacturer | Country and City |
|---|---|---|
| Fresh grass carp swim bladder | Shanghai Pudong New Area Nanhui new town aquatic products shop | Shanghai, China |
| Alkaline protease (200 μ/mg) | Shanghai Yuanye Bio-Technology Co., Ltd. | Shanghai, China |
| Neutral protease (50 μ/mg) | Shanghai Aladdin Biochemical Technology Co., Ltd. | Shanghai, China |
| NaOH (AR) | Sinopharm Chemical Reagent Co., Ltd. | Shanghai, China |
| HCl (AR) | Sinopharm Chemical Reagent Co., Ltd. | Shanghai, China |
| NaCl | Sinopharm Chemical Reagent Co., Ltd. | Shanghai, China |
| Glucose | Sinopharm Chemical Reagent Co., Ltd. | Shanghai, China |
| Citric acid | Sinopharm Chemical Reagent Co., Ltd. | Shanghai, China |
| pH meter | Mettler Toledo International Inc. | Columbus, OH, USA |
| 1,1-Diphenyl-2-picrylhydrazyl | FTY. Phygene Life Sciences Co., Ltd. | Fuzhou, China |
| ABTS | FTY. Phygene Life Sciences Co., Ltd. | Fuzhou, China |
| H2O2 | Guangdong Hengjian Pharmaceutical Co., Ltd. | Jiangmen, China |
| Pyrogallol | Sinopharm Chemical Reagent Co., Ltd. | Shanghai, China |
| Potassium ferricyanide | Sinopharm Chemical Reagent Co., Ltd. | Shanghai, China |
| FeCl3 | Sinopharm Chemical Reagent Co., Ltd. | Shanghai, China |
| FeCl2 | Sinopharm Chemical Reagent Co., Ltd. | Shanghai, China |
| 1,10-Phenanthroline | Shanghai Yien Chemical Technology Co., Ltd. | Shanghai, China |
| Freeze dryer XY-FD-L1 | Shanghai XinYU Instrument Co., Ltd. | Shanghai, China |
| Ultrafiltration membrane | Sartorius AG | Göttingen, Germany |
| Sephadex G-15 | Sigma-Aldrich (Shanghai) Trading Co., Ltd. | Shanghai, China |
| Total amino acid analyzer LA8080 | Hitachi Limited | Hitachi, Japan |
| Circular dichroism spectrometer | Applied Photophysics Ltd. | Leatherhead, UK |
| H1750R High-speed refrigerated centrifuge | Xiangyi centrifuge Instrument Co., Ltd | Changsha, China |
| Visible spectrophotometer | Shanghai Metash Instruments Co., Ltd. | Shanghai, China |
| LC-MS/MS | Science Compass | Wenzhou, China |
| Synthetic peptide | Jiangsu Jinsilui Biotechnology Co., Ltd | Yangzhou, China |
| Items | Forecasting Methods |
|---|---|
| Water solubility | Innovagen |
| Toxicity assessment | ToxinPred |
| Molecular weight and isoelectric point | Expasy-compute |
| Net charge and hydrophobicity | Pepdraw |
| Items | Content (g/100 g) |
|---|---|
| Gly | 19.632 |
| Phe *# | 8.584 |
| Lys * | 7.157 |
| Arg | 5.370 |
| Leu *# | 4.454 |
| Glu | 4.429 |
| Tyr | 4.298 |
| Ala # | 2.965 |
| Asp | 2.936 |
| Ser | 2.084 |
| Ile *# | 2.012 |
| Thr | 1.815 |
| Val *# | 1.533 |
| Pro # | 1.078 |
| His | 0.391 |
| Met *# | 0.344 |
| Cys | 0.010 |
| Items | GCP-II | GCP-II After Digestion |
|---|---|---|
| Gly | 7.032 | 0.169 |
| Lys * | 5.459 | 0.058 |
| Arg | 2.819 | 0.311 |
| Ser | 1.797 | 0.098 |
| Asp | 1.135 | 0.129 |
| Glu | 0.855 | 0.153 |
| Phe *# | 0.815 | 0.104 |
| Thr | 0.641 | 0.071 |
| Val *# | 0.639 | 0.075 |
| Leu *# | 0.487 | 0.094 |
| Gcu # | 0.419 | 0.068 |
| Ile *# | 0.304 | 0.049 |
| Met *# | 0.207 | 0.011 |
| His | 0.125 | 0.019 |
| Tyr | 0.025 | 0.038 |
| Pro # | 0.009 | 0.001 |
| Toatal | 22.768 | 1.448 |
| Sequence | Water Solubility | Hydroph-Obicity kcal/mol | Toxicity Assessment | Isoelectric Point | MW (Molecular Weight) | Net Charge |
|---|---|---|---|---|---|---|
| EKAPDPFRHF | High | 19.47 | Non-toxic | 6.85 | 1243.39 | 0 |
| QGPPGPPGPS | High | 13.28 | / | 5.52 | 889.96 | 0 |
| GERGPPGPM | High | 16.54 | Non-toxic | 6.00 | 897.02 | 0 |
| DGSYNIGQR | High | 15.90 | Non-toxic | 6.84 | 1009.04 | 0 |
| GILTLKYPI | Lower | 6.79 | Non-toxic | 8.59 | 1017.28 | 1 |
| VLSLYASGRTT | Lower | 9.11 | Non-toxic | 8.72 | 1167.33 | 1 |
| ILTERGYSFVTT | Lower | 10.45 | Non-toxic | 6.00 | 1386.57 | 0 |
| Items | Sequence | Length | Score | Frequency of Detection | Peak Intensity |
|---|---|---|---|---|---|
| Peptide 1 | EKAPDPFRHF | 10 | 155.84 | 1 | 3,260,300,000 |
| Peptide 2 | GILTLKYPI | 9 | 129.68 | 2 | 1,151,900,000 |
| Peptide 3 | GERGPPGPM | 9 | 139.14 | 1 | 804,920,000 |
| Peptide 4 | ILTERGYSFVTT | 12 | 118.03 | 1 | 581,870,000 |
| Peptide 5 | QGPPGPPGPS | 10 | 142.08 | 1 | 359,000,000 |
| Peptide 6 | VLSLYASGRTT | 11 | 122.13 | 3 | 347,310,000 |
| Peptide 7 | DGSYNIGQR | 9 | 133.79 | 1 | 313,090,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Gu, J.; Liu, Y.; Qiu, W.; Shi, W. Physicochemical Properties and Stability of Antioxidant Peptides from Swim Bladder of Grass Carp (Ctenopharyngodon idella). Foods 2025, 14, 1216. https://doi.org/10.3390/foods14071216
Li S, Gu J, Liu Y, Qiu W, Shi W. Physicochemical Properties and Stability of Antioxidant Peptides from Swim Bladder of Grass Carp (Ctenopharyngodon idella). Foods. 2025; 14(7):1216. https://doi.org/10.3390/foods14071216
Chicago/Turabian StyleLi, Suxin, Jinhui Gu, Yiyi Liu, Weiqiang Qiu, and Wenzheng Shi. 2025. "Physicochemical Properties and Stability of Antioxidant Peptides from Swim Bladder of Grass Carp (Ctenopharyngodon idella)" Foods 14, no. 7: 1216. https://doi.org/10.3390/foods14071216
APA StyleLi, S., Gu, J., Liu, Y., Qiu, W., & Shi, W. (2025). Physicochemical Properties and Stability of Antioxidant Peptides from Swim Bladder of Grass Carp (Ctenopharyngodon idella). Foods, 14(7), 1216. https://doi.org/10.3390/foods14071216






