Patrinia scabiosaefolia L. Modulates the Intestinal Microecology to Treat DSS-Induced Ulcerative Colitis: UHPLC-OE-MS/MS, Network Pharmacology, and Experimental Validation
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of P. scabiosaefolia
2.2. UHPLC-OE-MS/MS
2.3. Network Pharmacology
2.4. Molecular Docking
2.5. Animal Experiment
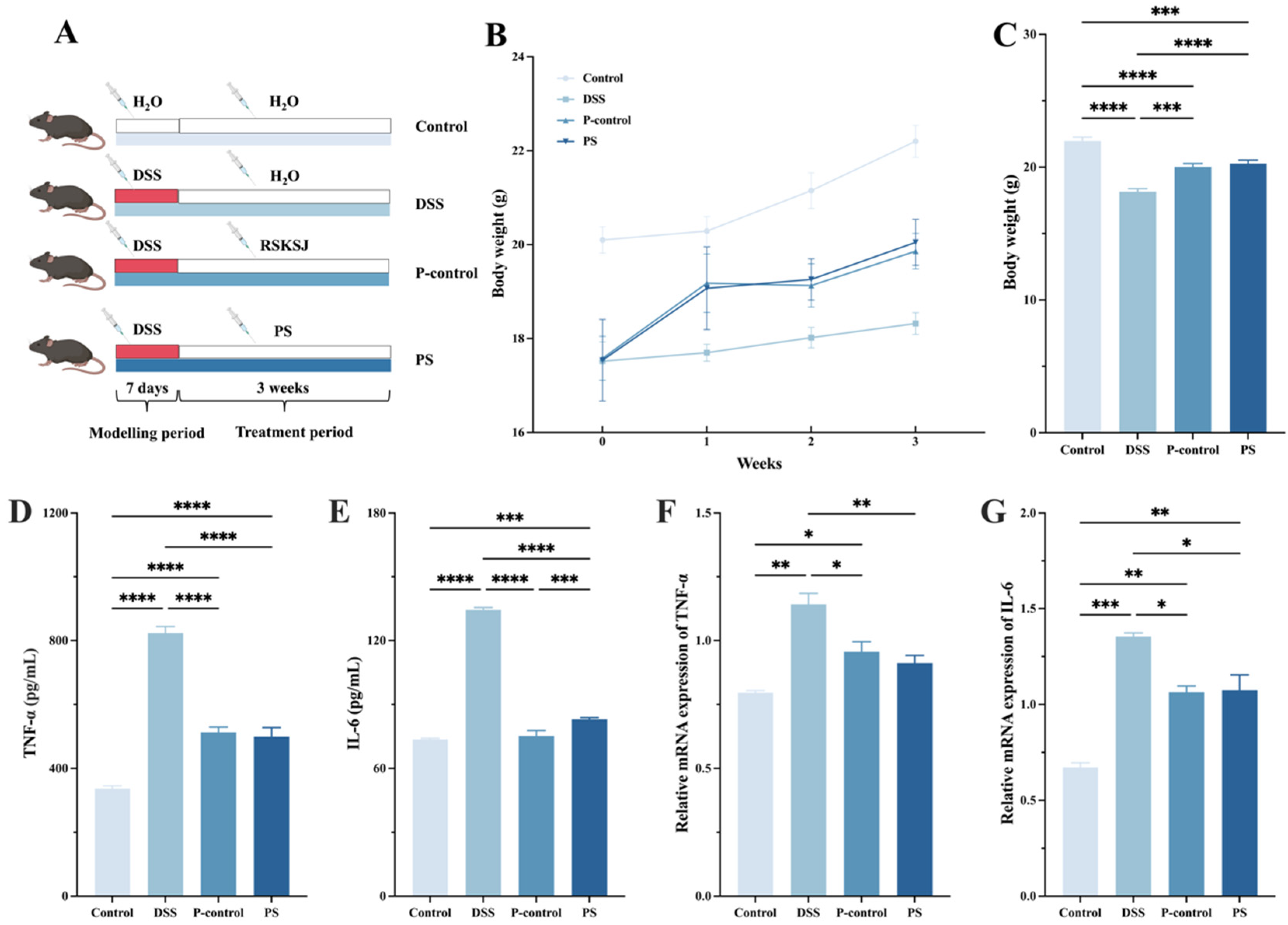
2.5.1. Experiment Design
2.5.2. Histopathological Examination
2.5.3. ELISA Assay
2.5.4. Quantitative Real-Time PCR Analysis
2.5.5. High-Throughput Sequencing of Gut Microbiota
2.6. Statistical Analysis
3. Results
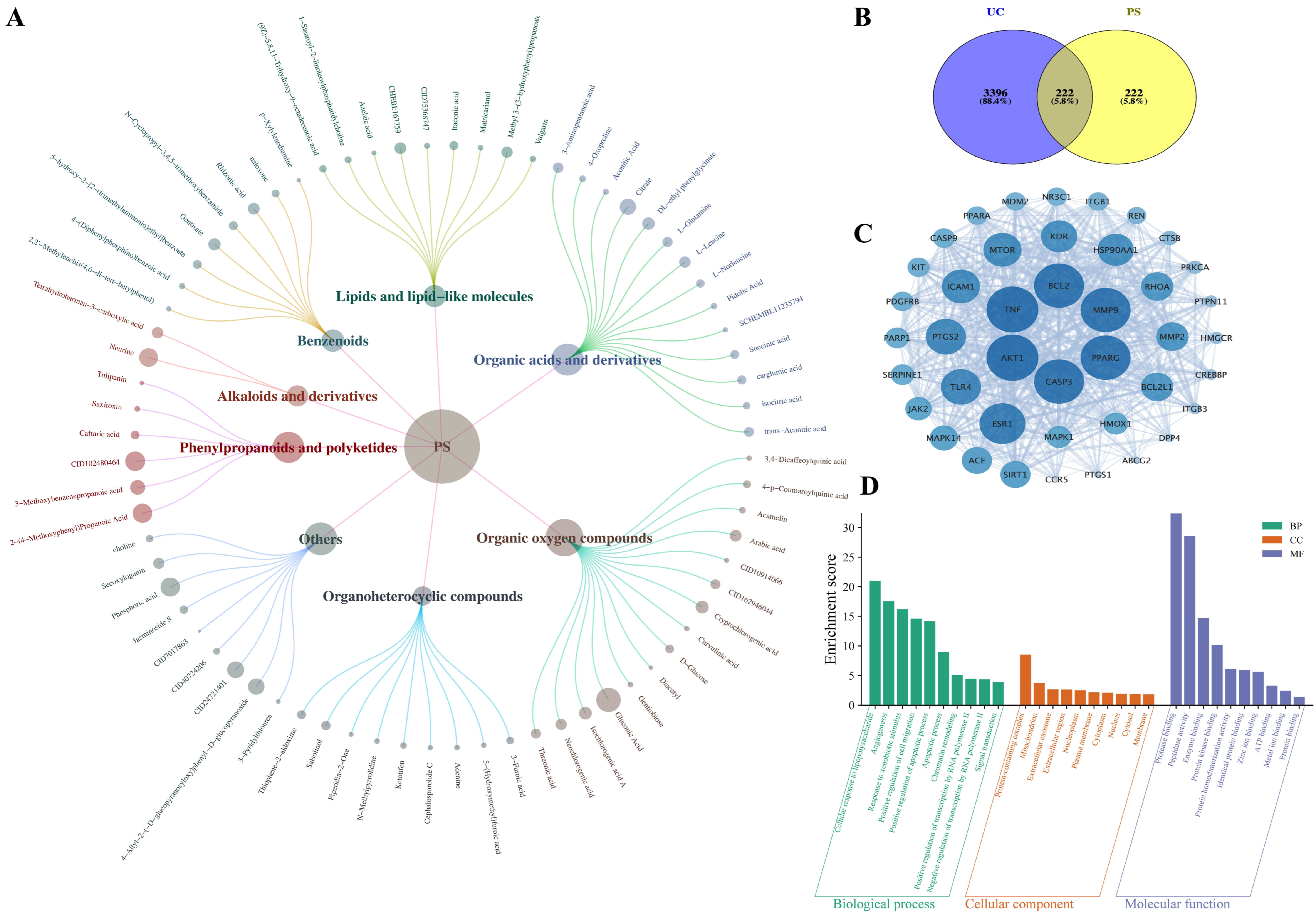
3.1. Identification of P. scabiosaefolia Components
3.2. Network Pharmacology Analysis
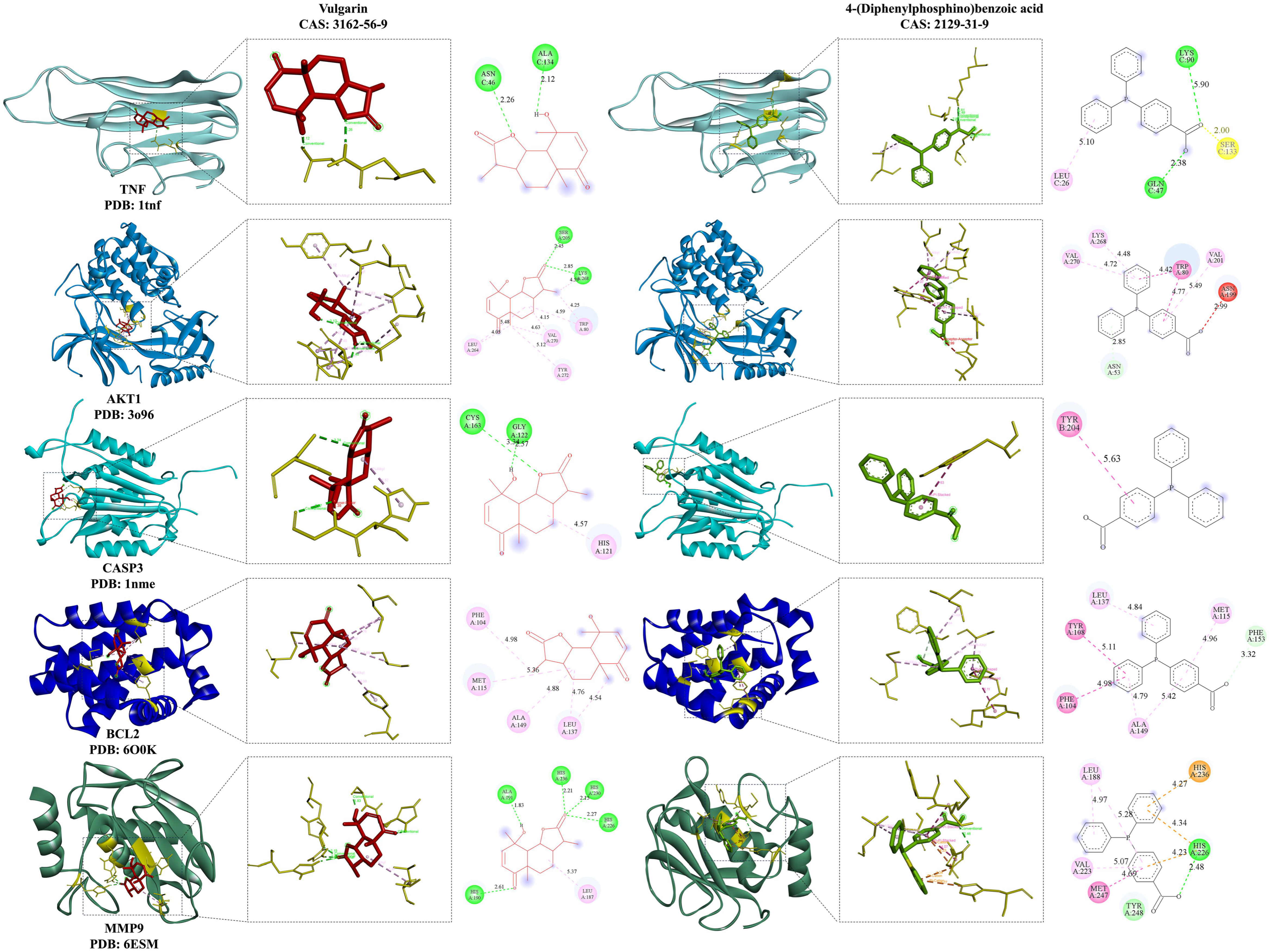
3.3. Molecular Docking Analysis
3.4. P. scabiosaefolia Alleviated the Symptoms of UC Mice
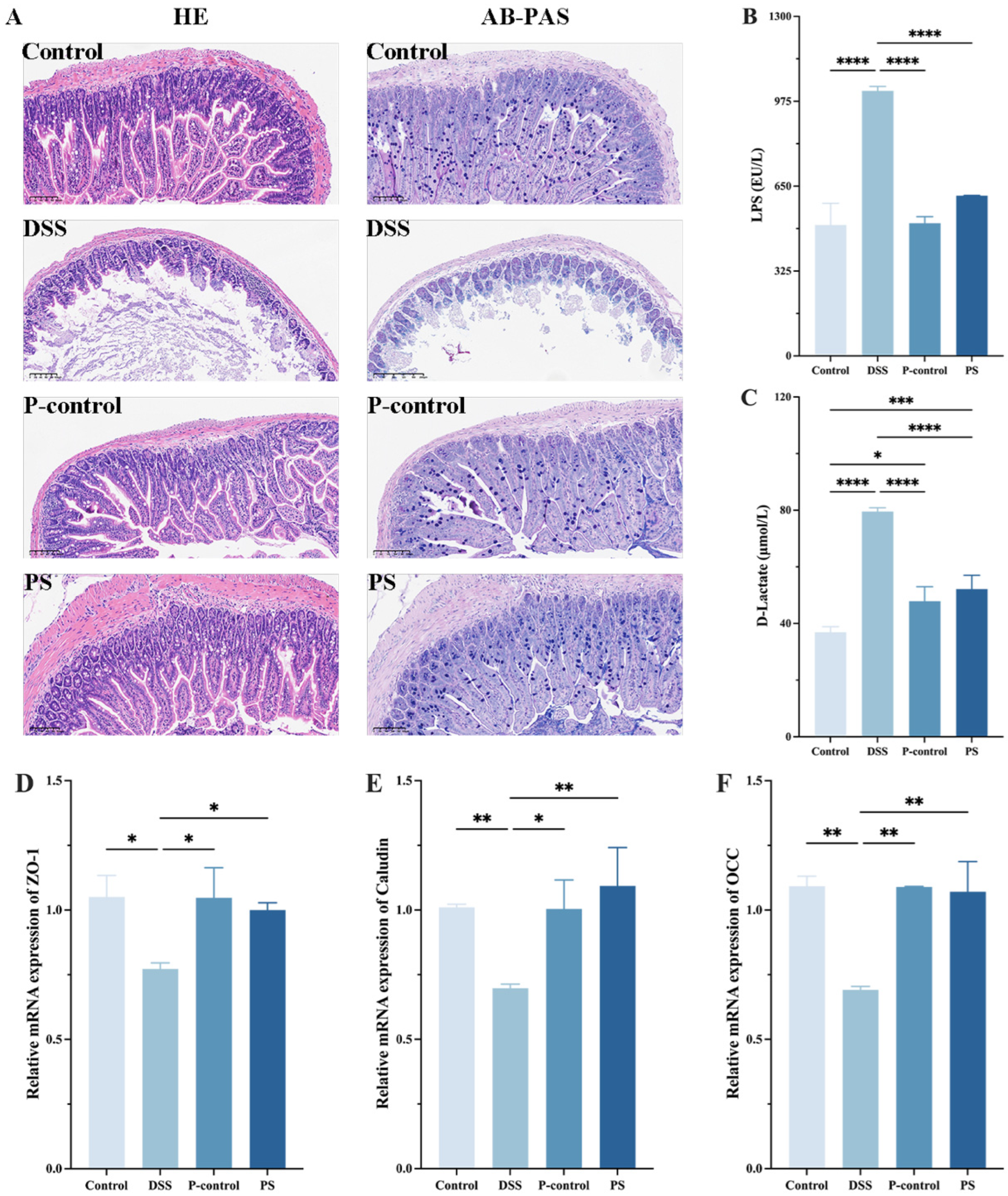
3.5. P. scabiosaefolia Modulates the Intestinal Barrier in the Colon of UC Mice
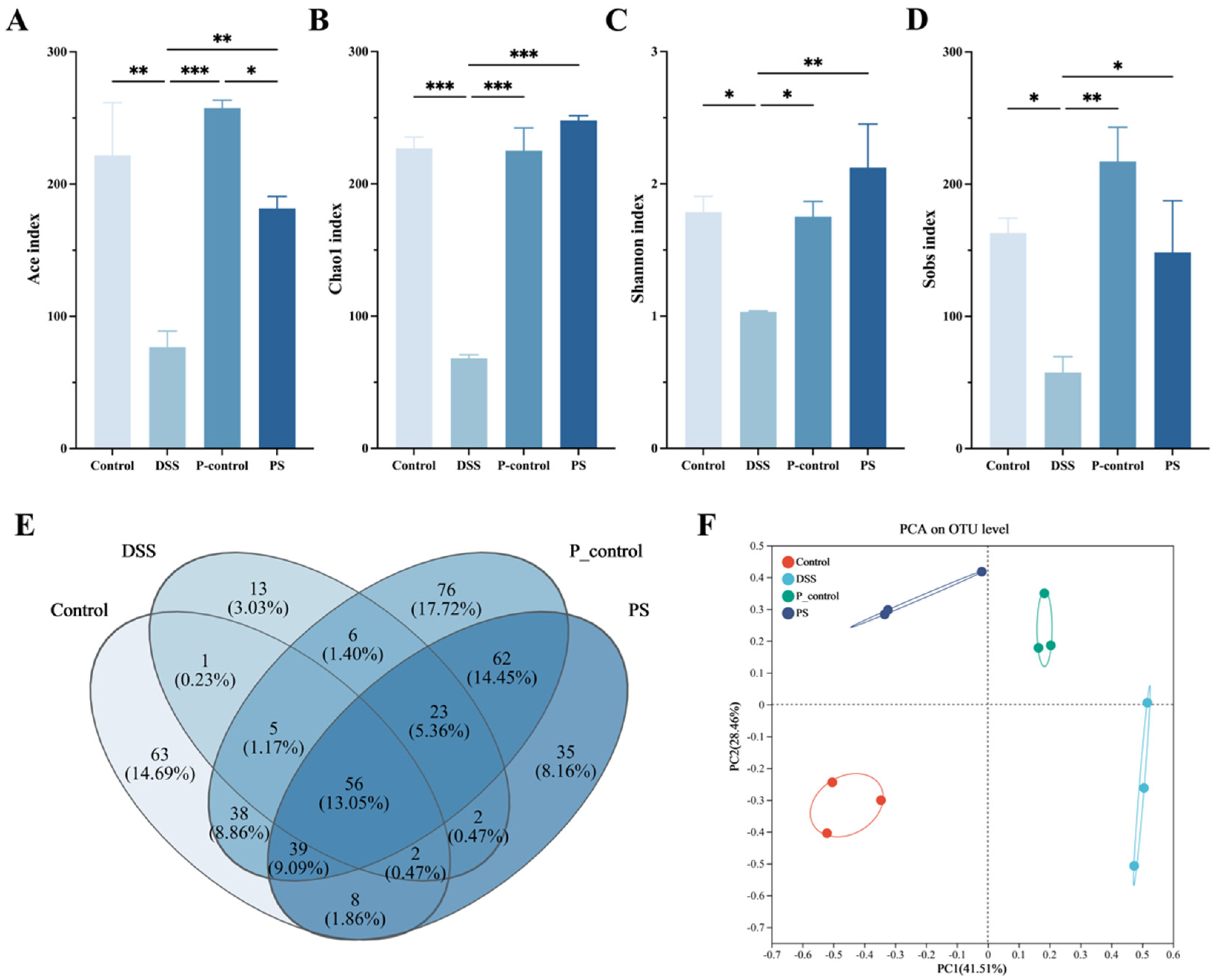
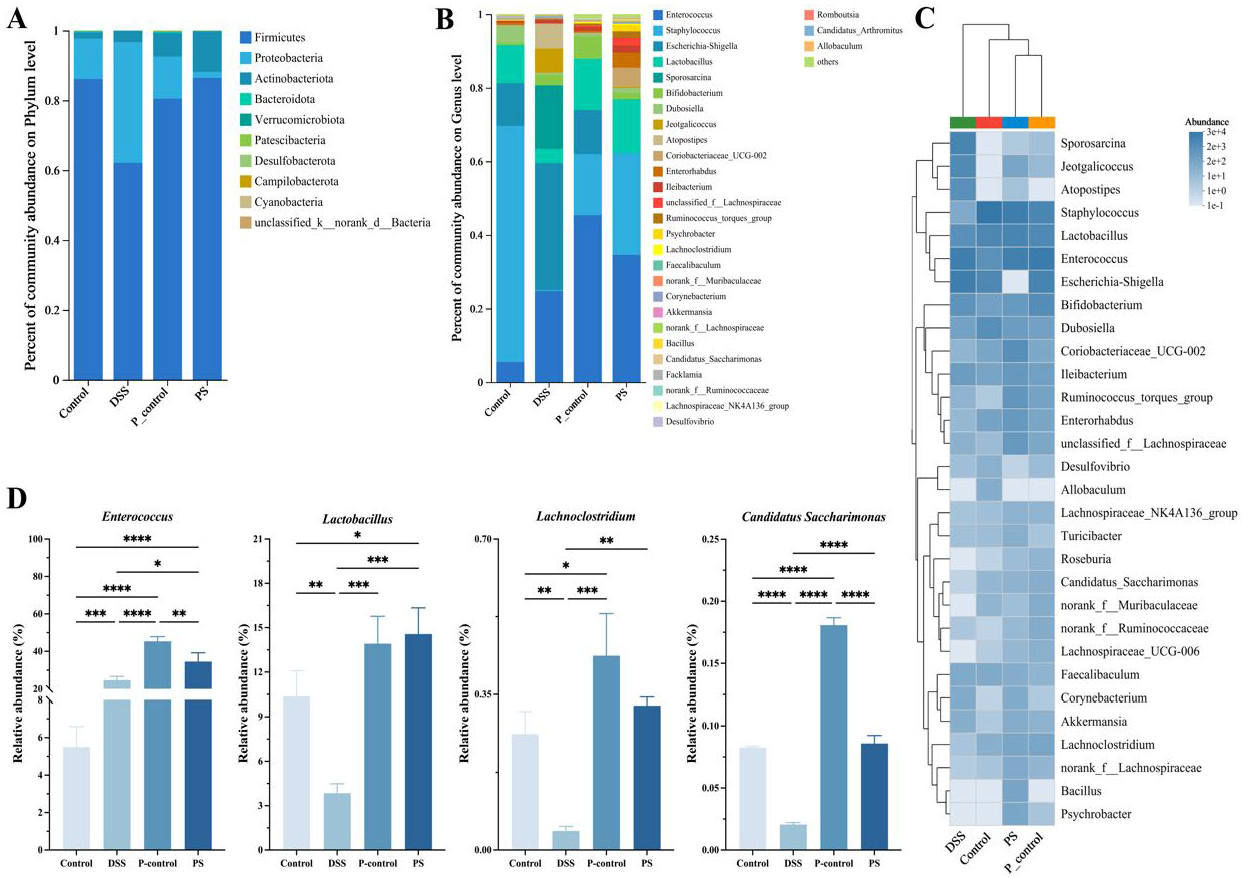
3.6. P. scabiosaefolia Modulates the Gut Microbiota in UC Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qu, Y.F.; Li, X.Y.; Xu, F.Y.; Zhao, S.M.; Wu, X.M.; Wang, Y.Z.; Xie, J.M. Kaempferol Alleviates Murine Experimental Colitis by Restoring Gut Microbiota and Inhibiting the LPS-TLR4-NF-κB Axis. Front. Immunol. 2021, 12, 679897. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Deng, H.; Zheng, Y.; Wang, Q.; Peng, J.; Bai, W.; Tian, L.; He, Z.; Jiao, R. In vitro gastrointestinal digestion and colonic fermentation of Mangiferin: Impacts on gut microbiota and metabolite profiles. J. Funct. Foods 2025, 124, 106667. [Google Scholar] [CrossRef]
- Qu, L.; Liu, C.; Ke, C.; Zhan, X.; Li, L.; Xu, H.; Xu, K.; Liu, Y. Atractylodes lancea Rhizoma Attenuates DSS-Induced Colitis by Regulating Intestinal Flora and Metabolites. Am. J. Chin. Med. 2022, 50, 525–552. [Google Scholar] [CrossRef]
- Oliyaei, N.; Zekri, S.; Iraji, A.; Oliyaei, A.; Tanideh, R.; Mussin, N.M.; Tamadon, A.; Tanideh, N. Health benefits of algae and marine-derived bioactive metabolites for modulating ulcerative colitis symptoms. J. Funct. Foods 2025, 125, 106690. [Google Scholar] [CrossRef]
- Chang, S.; Murphy, M.; Malter, L. A Review of Available Medical Therapies to Treat Moderate-to-Severe Inflammatory Bowel Disease. Am. J. Gastroenterol. 2024, 119, 55–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Meng, L.; Wang, M.; Wang, L.; Liu, Y.; Hou, G.; Li, S.; Kang, W. New iridoids from Patrinia scabiosaefolia and their hypoglycemic effects by activating PI3K/Akt signaling pathway. Fitoterapia 2023, 165, 105423. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Luan, F.; Zhao, Z.; Ning, N.; Li, M.; Jin, L.; Chang, Y.; Zhang, Q.; Wu, N.; Huang, L. The Genus Patrinia: A Review of Traditional Uses, Phytochemical and Pharmacological Studies. Am. J. Chin. Med. 2017, 45, 637–666. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; An, L.; Zhou, Y.; Peng, W.; Huang, C. Antibacterial Mechanism of Patrinia scabiosaefolia Against Methicillin Resistant Staphylococcus epidermidis. Infect. Drug Resist. 2023, 16, 1345–1355. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Liu, Y.; Ren, M.; Xi, X.; Li, S.; Kang, W. Patrinoside and Patrinoside A from Patrinia scabiosaefoli a Improve Insulin Resistance by Inhibiting NF-κB, MAPK Pathways and Oxidative Stress in RAW264.7 and 3T3-L1 Cells. Oxidative Med. Cell. Longev. 2023, 2023, 9069645. [Google Scholar] [CrossRef]
- Gong, L.; Zou, W.; Zheng, K.; Shi, B.; Liu, M. The Herba Patriniae (Caprifoliaceae): A review on traditional uses, phytochemistry, pharmacology and quality control. J. Ethnopharmacol. 2021, 265, 113264. [Google Scholar] [CrossRef]
- Yan, B.; Chen, X.; Wang, Y.; Yuan, M.; Xian, J.; Lu, D.; Shao, Z.; Qiu, M.; Fu, T.; Zheng, X. Chlorella pyrenoidosa ameliorates ulcerative colitis by tuning intestinal microecology: Butyric acid is a crucial player. J. Funct. Foods 2024, 121, 106414. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Edelblum, K.L.; Turner, J.R. The tight junction in inflammatory disease: Communication breakdown. Curr. Opin. Pharmacol. 2009, 9, 715–720. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Chen, Y.; Zhang, Q.; Li, B.; Zhong, Y.; Tu, Y.; Zhang, W.; Xu, G.; Jiang, L. Simultaneous Determination of Ten Bioactive Components from Shenling Baizhu San in Rat Plasma by UHPLC-MS/MS: Application to a Comparative Pharmacokinetic Study in Normal and Two Models of Ulcerative Colitis Rats. Evid. Based Complement. Altern. Med. eCAM 2021, 2021, 3518241. [Google Scholar] [CrossRef]
- Michiels, J.; Truffin, D.; Majdeddin, M.; Poucke, M.V.; Liefferinge, E.V.; Noten, N.V.; Vandaele, M.; Kerschaver, C.V.; Degroote, J.; Peelman, L. Gluconic acid improves performance of newly weaned piglets associated with alterations in gut microbiome and fermentation. Porc. Health Manag. 2023, 9, 10. [Google Scholar] [CrossRef]
- Wittwer, A.E.; Lee, S.G.; Ranadheera, C.S. Potential associations between organic dairy products, gut microbiome, and gut health: A review. Food Res. Int. 2023, 172, 113195. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Liu, S.; Xu, E.; Wang, Z. The traditional herb Sargentodoxa cuneata alleviates DSS-induced colitis by attenuating epithelial barrier damage via blocking necroptotic signaling. J. Ethnopharmacol. 2024, 319, 117761. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Han, X.; Jiang, H.; Wang, J.; Wang, M.Y.; Zhang, X.; Zhang, L.; Hu, J.; Fu, Z.H. Qingchang Wenzhong Decoction ameliorates intestinal inflammation and intestinal barrier dysfunction in ulcerative colitis via the GC-C signaling pathway. J. Ethnopharmacol. 2024, 322, 117503. [Google Scholar] [CrossRef]
- Ma, X.; Hu, Q.; Jiang, T.; Chen, Y.; Zhang, W.; Gao, P.; Zeng, J.; Efferth, T. Dehydroevodiamine Alleviates Ulcerative Colitis by Inhibiting the PI3K/AKT/NF-κB Signaling Pathway via Targeting AKT1 and Regulating Gut Microbes and Serum Metabolism. Molecules 2024, 29, 4031. [Google Scholar] [CrossRef]
- Althurwi, H.N.; Soliman, G.A.; Abdel-Rahman, R.F.; Abd-Elsalam, R.M.; Ogaly, H.A.; Alqarni, M.H.; Albaqami, F.F.; Abdel-Kader, M.S. Vulgarin, a Sesquiterpene Lactone from Artemisia judaica, Improves the Antidiabetic Effectiveness of Glibenclamide in Streptozotocin-Induced Diabetic Rats via Modulation of PEPCK and G6Pase Genes Expression. Int. J. Mol. Sci. 2022, 23, 15856. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.-F.; Xu, G.-B.; Wang, Z.; Wang, A.-M.; Guan, H.-Y.; Li, J.; He, X.; Liu, J.-H.; Zhou, M.; Li, Y.-J.; et al. Identification of anti-inflammatory constituents from Kalimeris indica with UHPLC-ESI-Q-TOF-MS/MS and GC-MS. J. Ethnopharmacol. 2015, 165, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, W.B.; Wang, H.Y.; Ma, Y.M.; Zhao, X.H.; Zhang, X.D.; Yang, H.; Qian, J.M.; Li, J.N. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-α and IL-6 levels and functions and by rebalancing intestinal microbiota. Bmc Microbiol. 2019, 19, 246. [Google Scholar] [CrossRef]
| Key Compounds | Dgree Centrality |
|---|---|
| 4-Oxo-4-[(3-oxo-2-decanyl)amino]butanoic acid | 18 |
| (9Z)-5,8,11-Trihydroxy-9-octadecenoic acid | 16 |
| Vulgarin | 14 |
| 4-(Diphenylphosphino)benzoic acid | 13 |
| 1-[(4-Bromo-3,5-dimethyl-1H-pyrazol-1-yl)methyl]-3,5-dimethyl-1H-pyrazol-4-amine | 7 |
| Ketotifen | 6 |
| Methyl 3-(3-hydroxyphenyl)propanoate | 6 |
| 2-(4-Methoxyphenyl)Propanoic Acid | 5 |
| 3-Methoxybenzenepropanoic acid | 4 |
| 5-hydroxy-2-[2-(trimethylammonio)ethyl]benzoate | 4 |
| Pidolic Acid | 4 |
| 1-methyl-1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid | 3 |
| Azelaic acid | 3 |
| N-Cyclopropyl-3,4,5-trimethoxybenzamide | 3 |
| 4-Oxoproline | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, X.; Xu, M.; Cheng, X.; Li, N.; Xu, H.; Feng, Y.; Guan, T.; Xiao, L. Patrinia scabiosaefolia L. Modulates the Intestinal Microecology to Treat DSS-Induced Ulcerative Colitis: UHPLC-OE-MS/MS, Network Pharmacology, and Experimental Validation. Foods 2025, 14, 1145. https://doi.org/10.3390/foods14071145
Zhang L, Liu X, Xu M, Cheng X, Li N, Xu H, Feng Y, Guan T, Xiao L. Patrinia scabiosaefolia L. Modulates the Intestinal Microecology to Treat DSS-Induced Ulcerative Colitis: UHPLC-OE-MS/MS, Network Pharmacology, and Experimental Validation. Foods. 2025; 14(7):1145. https://doi.org/10.3390/foods14071145
Chicago/Turabian StyleZhang, Longfei, Xiaoxiao Liu, Mingze Xu, Xinyi Cheng, Ning Li, Haiyan Xu, Yining Feng, Tianzhu Guan, and Lixia Xiao. 2025. "Patrinia scabiosaefolia L. Modulates the Intestinal Microecology to Treat DSS-Induced Ulcerative Colitis: UHPLC-OE-MS/MS, Network Pharmacology, and Experimental Validation" Foods 14, no. 7: 1145. https://doi.org/10.3390/foods14071145
APA StyleZhang, L., Liu, X., Xu, M., Cheng, X., Li, N., Xu, H., Feng, Y., Guan, T., & Xiao, L. (2025). Patrinia scabiosaefolia L. Modulates the Intestinal Microecology to Treat DSS-Induced Ulcerative Colitis: UHPLC-OE-MS/MS, Network Pharmacology, and Experimental Validation. Foods, 14(7), 1145. https://doi.org/10.3390/foods14071145






