Pre-Administration of Saccharomyces boulardii-Derived Postbiotics Effectively Prevents Dextran Sulfate Sodium-Induced Colitis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Major Reagents
2.2. Preparation of Postbiotics
2.3. Characterization of Postbiotics
2.4. Animals
2.5. Animals Experiment
2.6. Histopathological Analysis and Immunohistochemical Analysis
2.7. Detection of Inflammatory Factor Levels
2.8. Analysis of Gut Microbiota
2.9. Statistical Analysis
3. Results
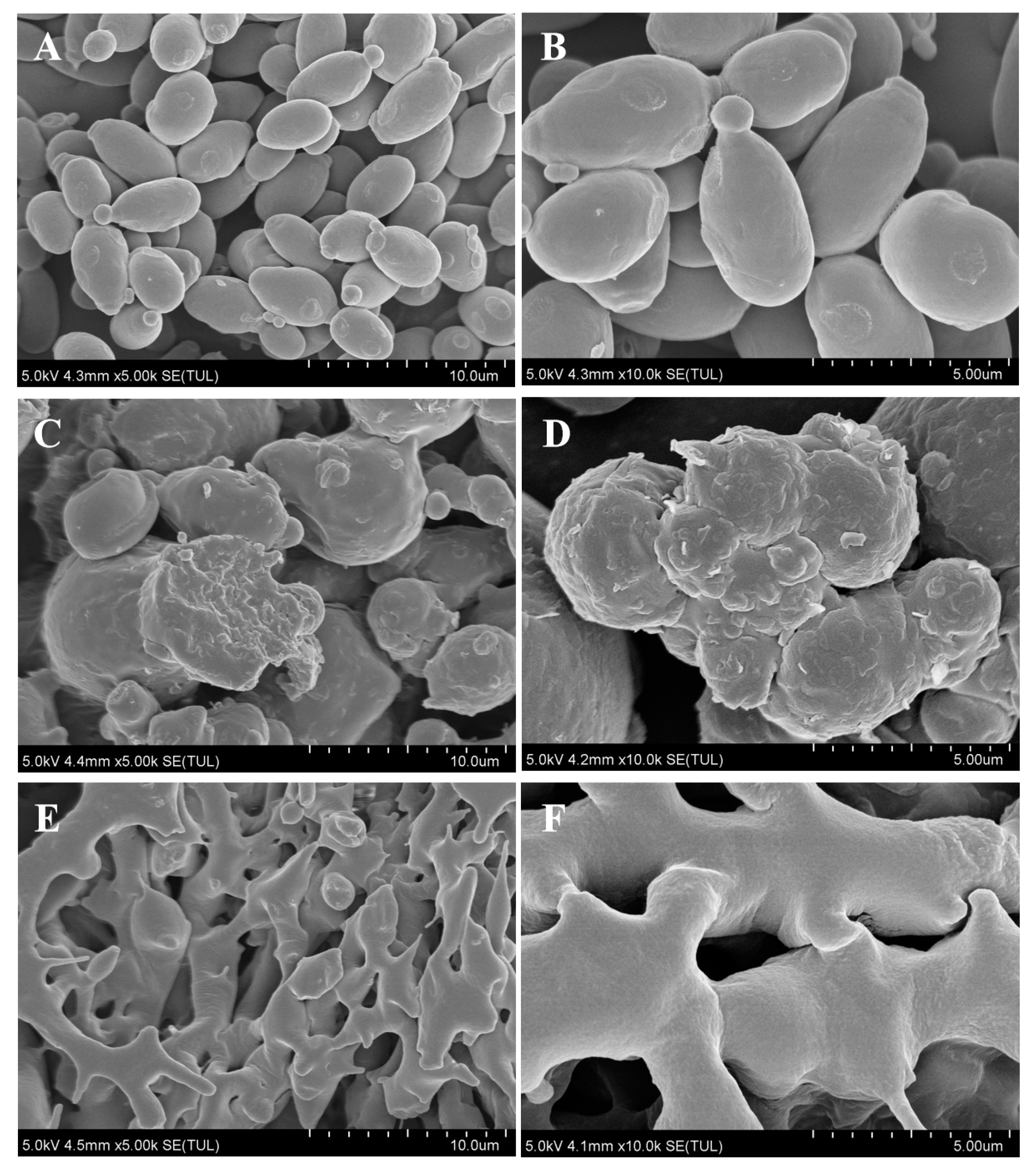
3.1. Morphological Characterization of the Postbiotic of S. boulardii
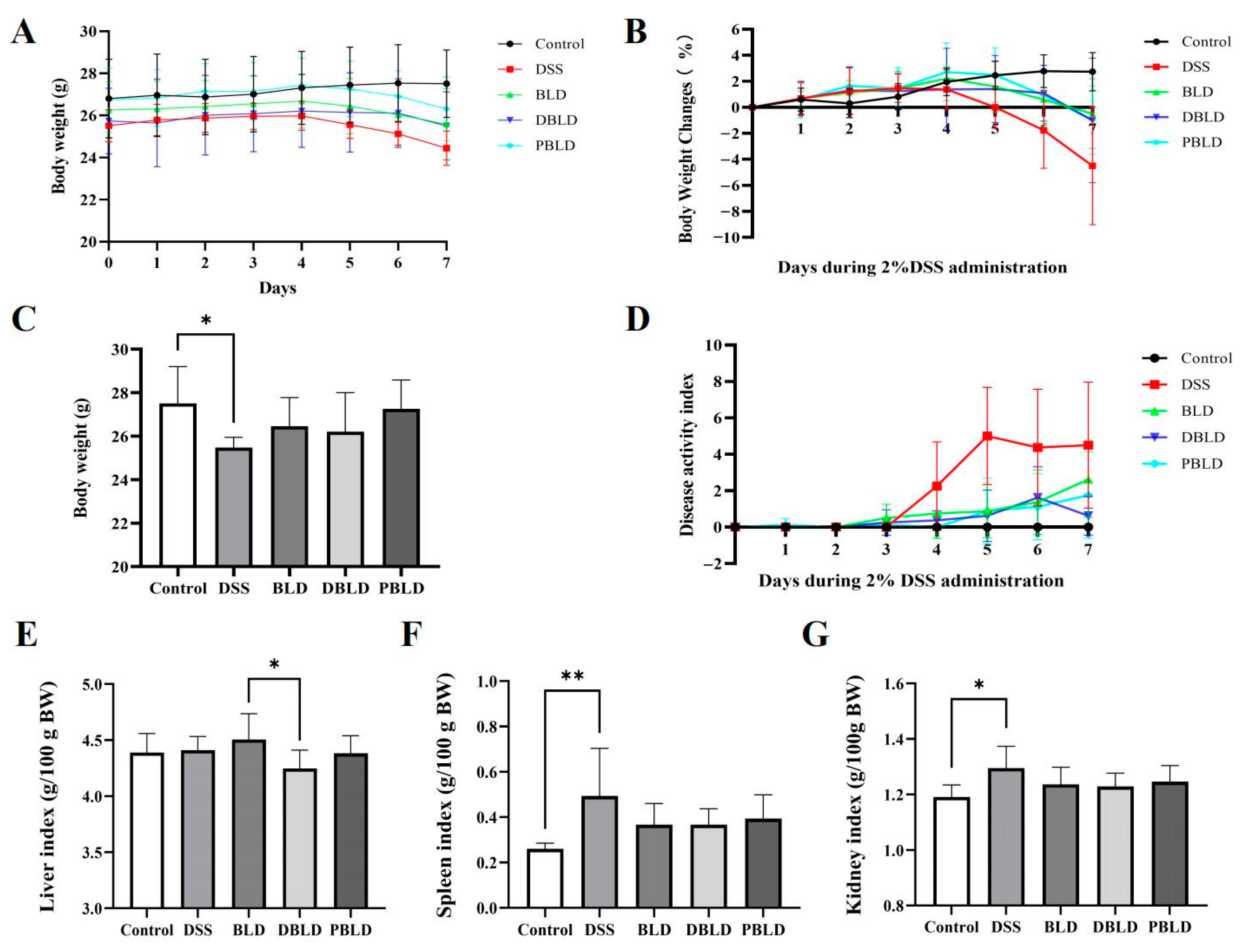
3.2. The Intervention of S. boulardii Postbiotics Attenuated DSS-Induced Changes in Growth Performance
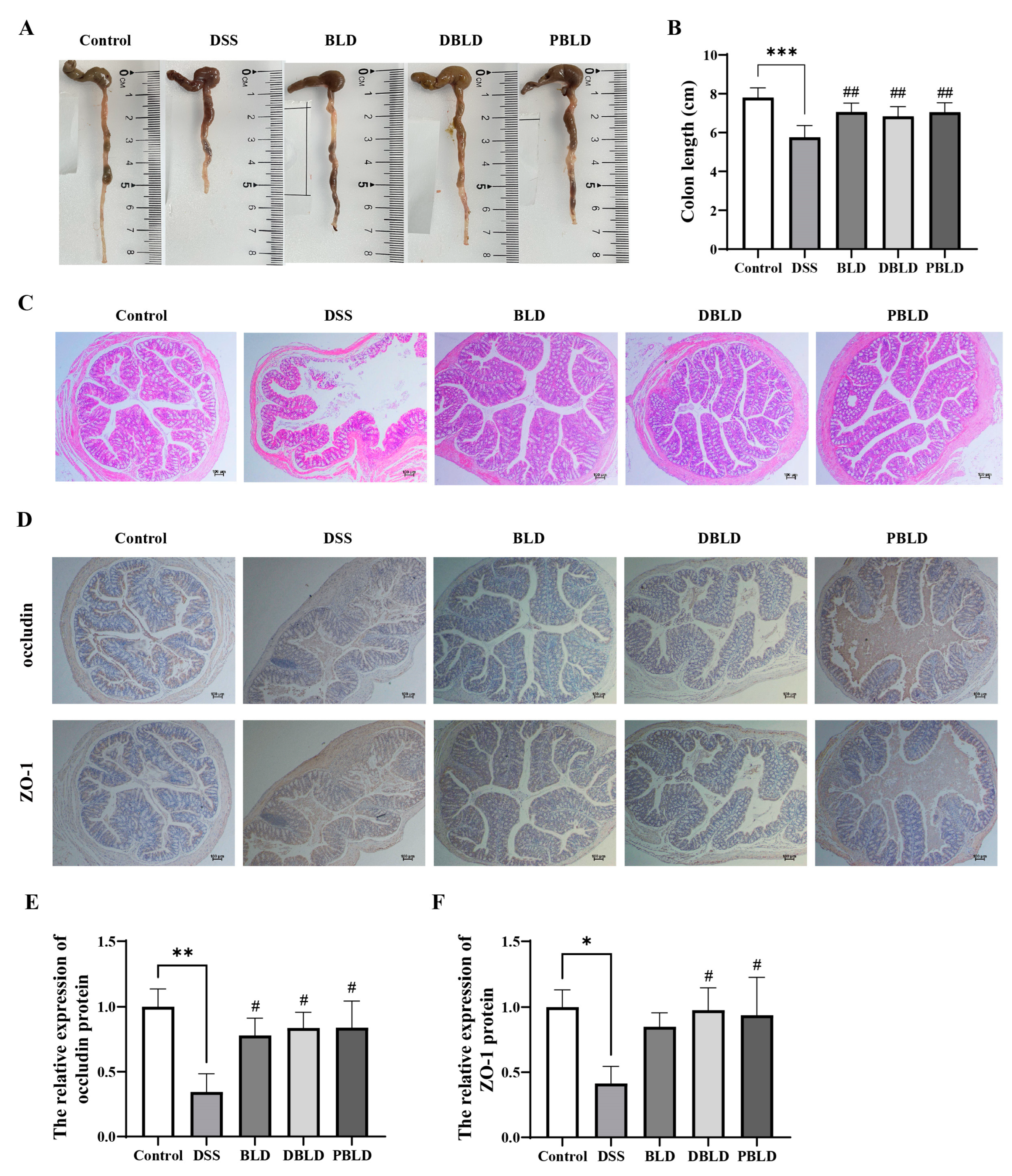
3.3. S. boulardii Postbiotics Mitigated DSS-Induced Alterations in Colon Tissue Structure
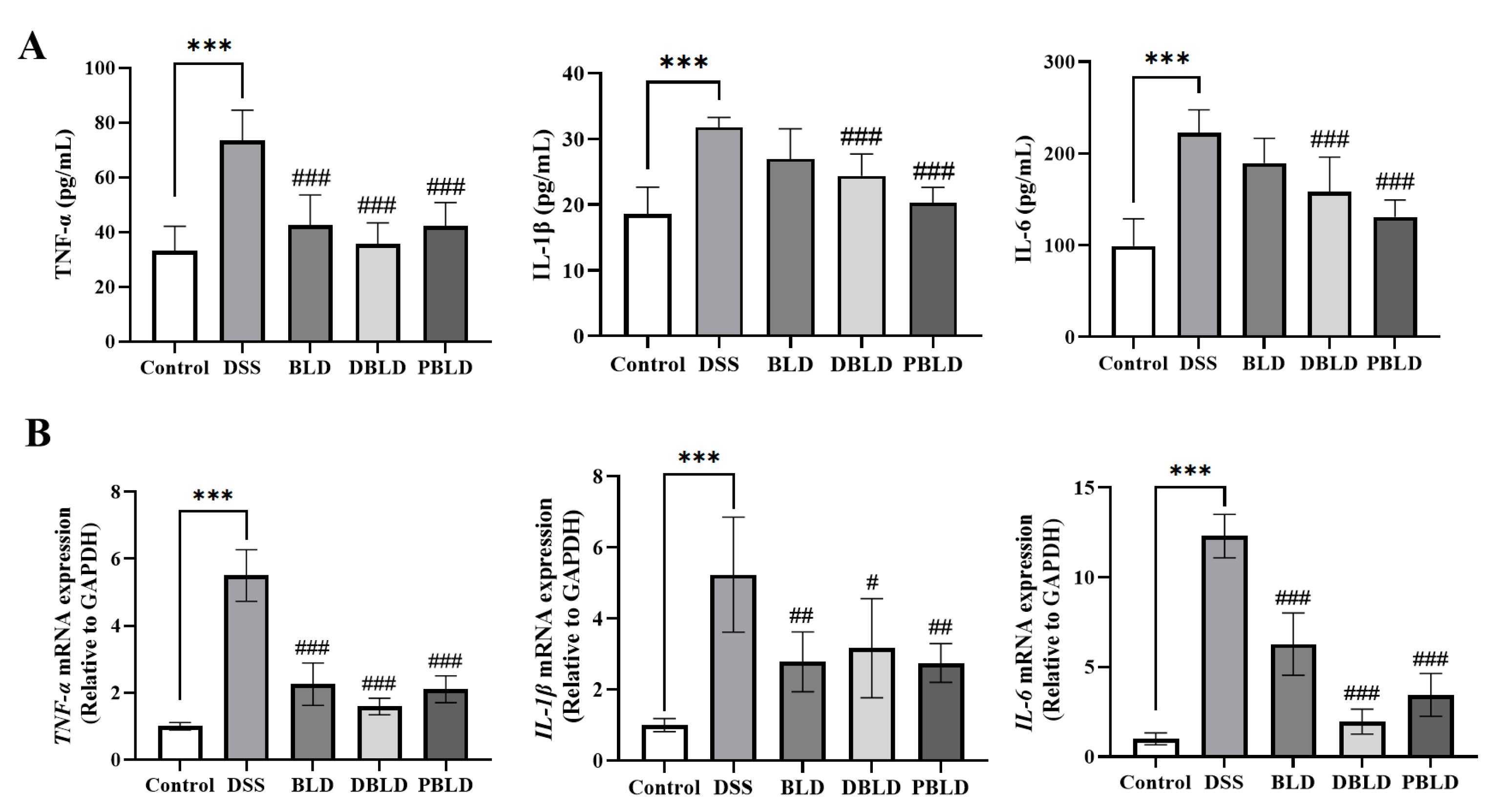
3.4. S. boulardii Postbiotics Inhibit the Increase of Pro-Inflammatory Cytokines Induced by DSS
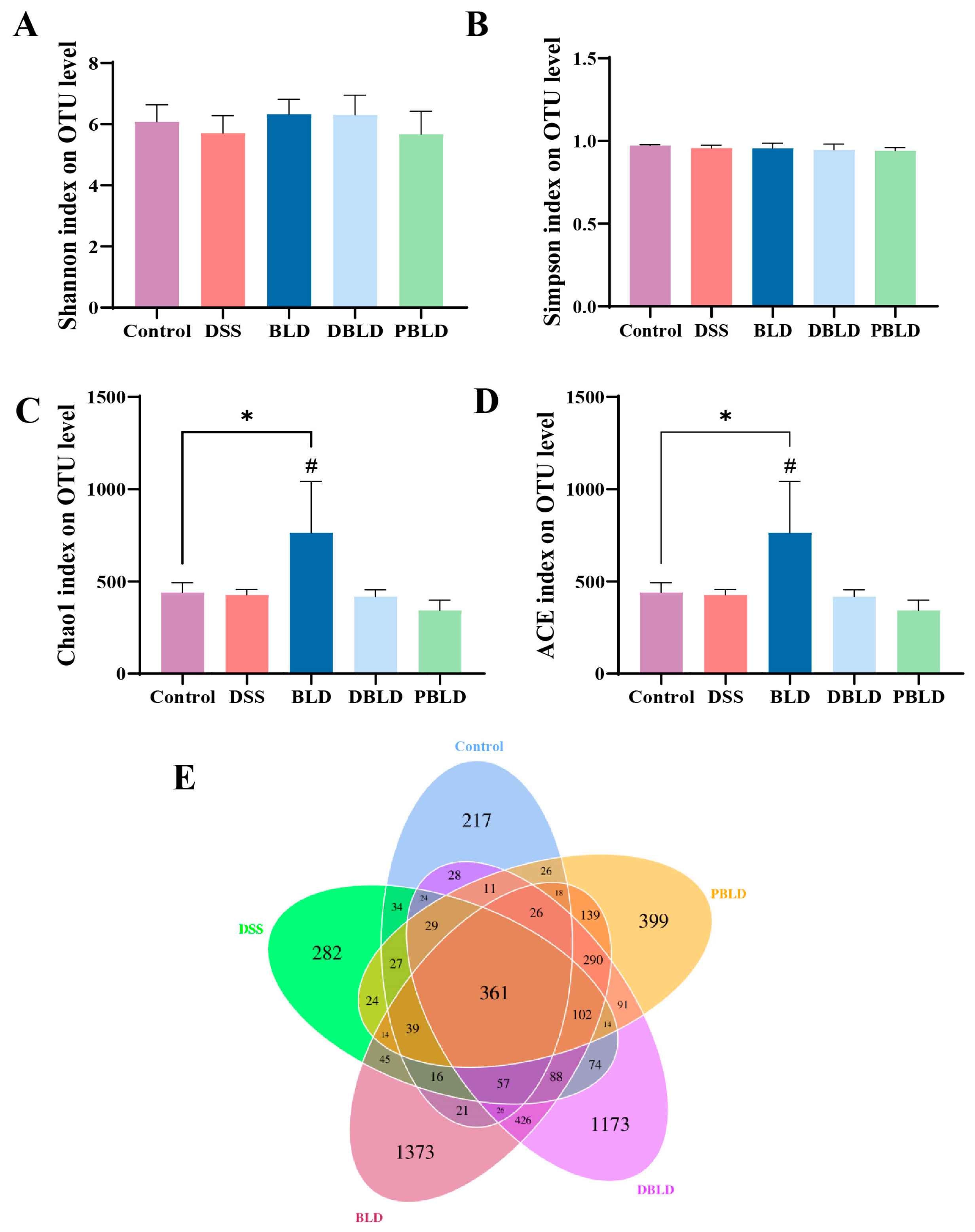
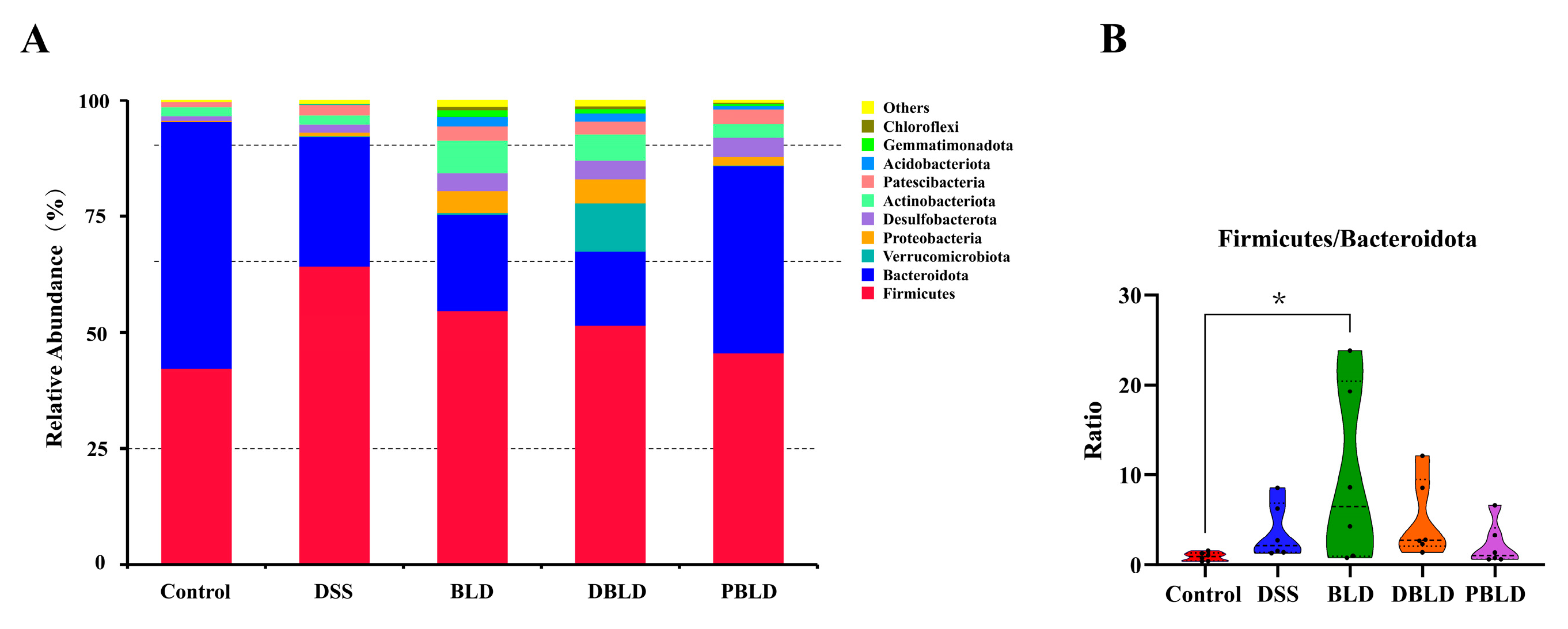
3.5. S. boulardii Postbiotics Regulated the Diversity of the Gut Microbiota
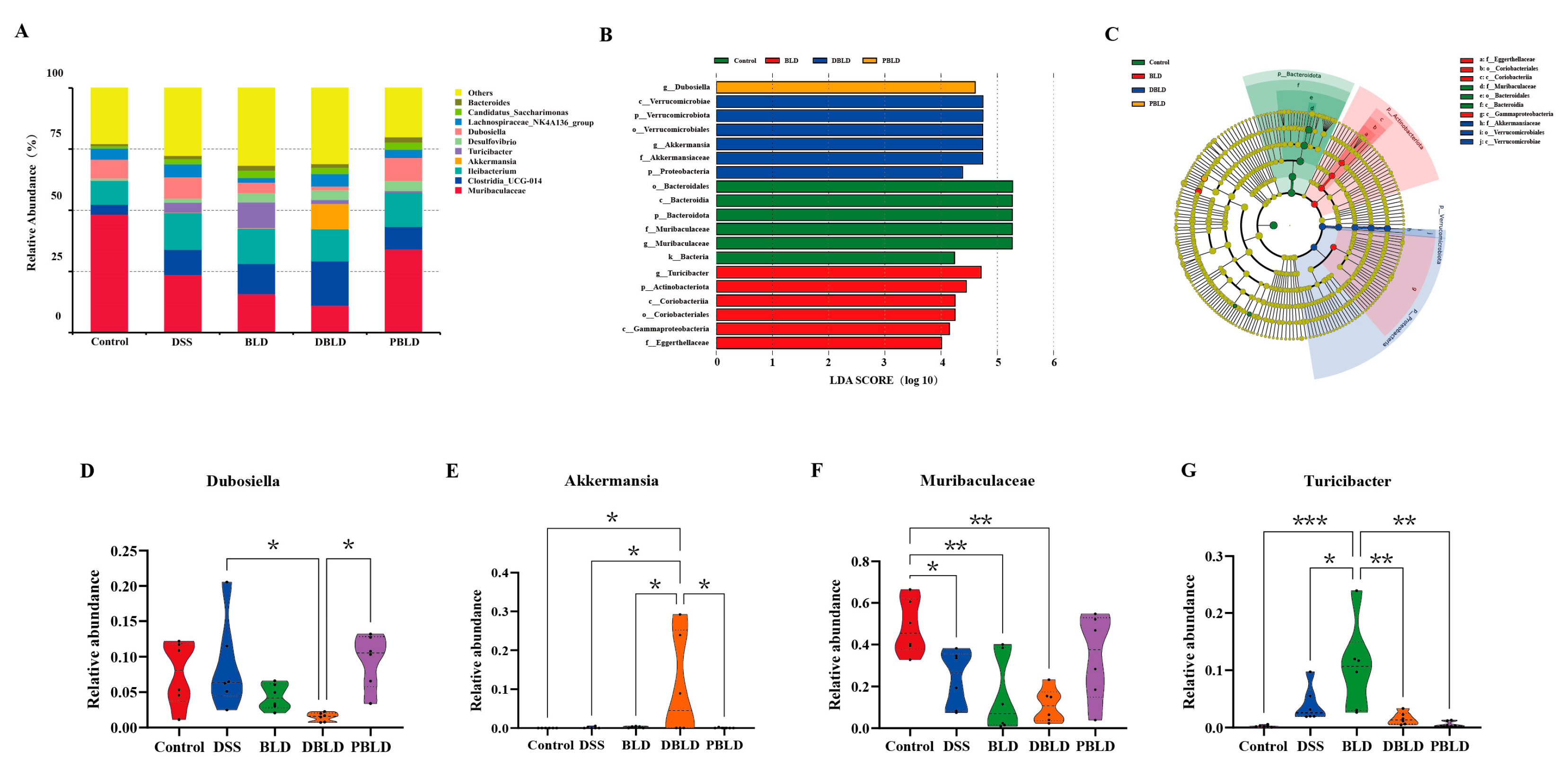
3.6. S. boulardii Postbiotics Altered the Composition of the Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory Bowel Disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative Colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-C.; Sollano, J.; Hui, Y.T.; Yu, W.; Santos Estrella, P.V.; Llamado, L.J.Q.; Koram, N. Epidemiology, Burden of Disease, and Unmet Needs in the Treatment of Ulcerative Colitis in Asia. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 275–289. [Google Scholar]
- Hoentjen, F.; Seinen, M.L.; Hanauer, S.B.; de Boer, N.K.H.; Rubin, D.T.; Bouma, G.; Harrell, L.E.; van Bodegraven, A.A. Safety and Effectiveness of Long-Term Allopurinol–Thiopurine Maintenance Treatment in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 363–369. [Google Scholar]
- Hvas, C.L.; Bendix, M.; Dige, A.; Dahlerup, J.F.; Agnholt, J. Current, Experimental, and Future Treatments in Inflammatory Bowel Disease: A Clinical Review. Immunopharmacol. Immunotoxicol. 2018, 40, 446–460. [Google Scholar] [PubMed]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar]
- Round, J.L.; Mazmanian, S.K. The Gut Microbiota Shapes Intestinal Immune Responses during Health and Disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [PubMed]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.-P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal Microbiota Dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar]
- Wombwell, E.; Patterson, M.E.; Bransteitter, B.; Gillen, L.R. The Effect of Saccharomyces boulardii Primary Prevention on Risk of Hospital-Onset Clostridioides difficile Infection in Hospitalized Patients Administered Antibiotics Frequently Associated With C. difficile Infection. Clin. Infect. Dis. 2021, 73, e2512–e2518. [Google Scholar] [CrossRef]
- Constante, M.; De Palma, G.; Lu, J.; Jury, J.; Rondeau, L.; Caminero, A.; Collins, S.M.; Verdu, E.F.; Bercik, P. Saccharomyces boulardii CNCM I-745 Modulates the Microbiota-Gut-Brain Axis in a Humanized Mouse Model of Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2021, 33, e13985. [Google Scholar]
- Sivananthan, K.; Petersen, A.M. Review of Saccharomyces boulardii as a Treatment Option in IBD. Immunopharmacol. Immunotoxicol. 2018, 40, 465–475. [Google Scholar]
- Martin, I.W.; Tonner, R.; Trivedi, J.; Miller, H.; Lee, R.; Liang, X.; Rotello, L.; Isenbergh, E.; Anderson, J.; Perl, T.; et al. Saccharomyces boulardii Probiotic-Associated Fungemia: Questioning the Safety of This Preventive Probiotic’s Use. Diagn. Microbiol. Infect. Dis. 2017, 87, 286–288. [Google Scholar] [CrossRef]
- Lenka, S.; Singh, D.; Paul, S.; Gayen, A.; Chandra, M. S. boulardii Fails to Hold Its Cell Wall Integrity against Nonpathogenic E. coli: Are Probiotic Yeasts Losing the Battle? ACS Infect. Dis. 2021, 7, 733–745. [Google Scholar] [PubMed]
- Wang, S.; Ahmadi, S.; Nagpal, R.; Jain, S.; Mishra, S.P.; Kavanagh, K.; Zhu, X.; Wang, Z.; McClain, D.A.; Kritchevsky, S.B.; et al. Lipoteichoic Acid from the Cell Wall of a Heat Killed Lactobacillus paracasei D3-5 Ameliorates Aging-Related Leaky Gut, Inflammation and Improves Physical and Cognitive Functions: From C. elegans to Mice. Geroscience 2020, 42, 333–352. [Google Scholar]
- Feng, C.; Zhang, W.; Zhang, T.; He, Q.; Kwok, L.-Y.; Tan, Y.; Zhang, H. Heat-Killed Bifidobacterium Bifidum B1628 May Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, and the Anti-Inflammatory Effect Is Associated with Gut Microbiota Modulation. Nutrients 2022, 14, 5233. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Yilmaz, Y. Postbiotics as Antiinflammatory and Immune-Modulating Bioactive Compounds in Metabolic Dysfunction-Associated Steatotic Liver Disease. Mol. Nutr. Food Res. 2024, 68, 2400754. [Google Scholar]
- Yolmeh, M.; Xavier-Santos, D.; Sant’Ana, A.S. Modulating Gut Microbiota by Paraprobiotics: Mechanisms, Advantages, and Challenges. Food Biosci. 2024, 60, 104305. [Google Scholar]
- Xu, X.; Wu, J.; Jin, Y.; Huang, K.; Zhang, Y.; Liang, Z. Both Saccharomyces boulardii and Its Postbiotics Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, Association with Modulating Inflammation and Intestinal Microbiota. Nutrients 2023, 15, 1484. [Google Scholar] [CrossRef]
- Ye, M.; Joosse, M.E.; Liu, L.; Sun, Y.; Dong, Y.; Cai, C.; Song, Z.; Zhang, J.; Brant, S.R.; Lazarev, M.; et al. Deletion of IL-6 Exacerbates Colitis and Induces Systemic Inflammation in IL-10-Deficient Mice. J. Crohns Colitis 2020, 14, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lin, Q.; Yang, T.; Zeng, L.; Shi, L.; Chen, Y.; Luo, F. Oat β-Glucan Ameliorates Dextran Sulfate Sodium (DSS)-Induced Ulcerative Colitis in Mice. Food Funct. 2015, 6, 3454–3463. [Google Scholar] [CrossRef] [PubMed]
- Chapman, T.P.; Frias Gomes, C.; Louis, E.; Colombel, J.-F.; Satsangi, J. Review Article: Withdrawal of 5-Aminosalicylates in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2020, 52, 73–84. [Google Scholar] [CrossRef]
- Gao, H.; Li, Y.; Xu, J.; Zuo, X.; Yue, T.; Xu, H.; Sun, J.; Wang, M.; Ye, T.; Yu, Y.; et al. Saccharomyces boulardii Protects against Murine Experimental Colitis by Reshaping the Gut Microbiome and Its Metabolic Profile. Front. Microbiol. 2023, 14, 1204122. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H.; Shi, L.; Li, R.; Luo, Y.; Deng, Y.; Li, S.; Li, R.; Liu, Z. Saccharomyces boulardii Alleviates DSS-Induced Intestinal Barrier Dysfunction and Inflammation in Humanized Mice. Food Funct. 2022, 13, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Jounai, K.; Sakamoto, A.; Tomita, Y.; Sugihara, Y.; Suzuki, H.; Ohshio, K.; Otake, M.; Fujiwara, D.; Kanauchi, O.; et al. Long-Term Intake of Lactobacillus paracasei KW3110 Prevents Age-Related Chronic Inflammation and Retinal Cell Loss in Physiologically Aged Mice. Aging 2018, 10, 2723–2740. [Google Scholar] [CrossRef]
- Li, Y.; Zhen, S.; Cao, L.; Sun, F.; Wang, L. Effects of Lactobacillus Plantarum Postbiotics on Growth Performance, Immune Status, and Intestinal Microflora of Growing Minks. Animals 2023, 13, 2958. [Google Scholar] [CrossRef]
- Pradhan, D.; Gulati, G.; Avadhani, R.; HM, R.; Soumya, K.; Kumari, A.; Gupta, A.; Dwivedi, D.; Kaushik, J.K.; Grover, S. Postbiotic Lipoteichoic Acid of Probiotic Lactobacillus Origin Ameliorates Inflammation in HT-29 Cells and Colitis Mice. Int. J. Biol. Macromol. 2023, 236, 123962. [Google Scholar] [CrossRef]
- Liu, W.; Wang, C.; Tang, L.; Yang, H. Associations between Gene Polymorphisms in Pro-Inflammatory Cytokines and the Risk of Inflammatory Bowel Disease: A Meta-Analysis. Immunol. Investig. 2021, 50, 869–883. [Google Scholar] [CrossRef]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; García, F.; Rodríguez-Cabezas, M.E.; Gálvez, J. Intestinal Anti-Inflammatory Effect of the Probiotic Saccharomyces boulardii in DSS-Induced Colitis in Mice: Impact on microRNAs Expression and Gut Microbiota Composition. J. Nutr. Biochem. 2018, 61, 129–139. [Google Scholar] [CrossRef]
- Xu, C.-L.; Sun, R.; Qiao, X.-J.; Xu, C.-C.; Shang, X.-Y.; Niu, W.-N. Protective Effect of Glutamine on Intestinal Injury and Bacterial Community in Rats Exposed to Hypobaric Hypoxia Environment. World J. Gastroenterol. 2014, 20, 4662–4674. [Google Scholar] [CrossRef]
- Kang, S.-J.; Yang, J.; Lee, N.-Y.; Lee, C.-H.; Park, I.-B.; Park, S.-W.; Lee, H.J.; Park, H.-W.; Yun, H.S.; Chun, T. Monitoring Cellular Immune Responses after Consumption of Selected Probiotics in Immunocompromised Mice. Food Sci. Anim. Resour. 2022, 42, 903–914. [Google Scholar] [PubMed]
- Knights, D.; Lassen, K.G.; Xavier, R.J. Advances in Inflammatory Bowel Disease Pathogenesis: Linking Host Genetics and the Microbiome. Gut 2013, 62, 1505–1510. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The Microbiome in Inflammatory Bowel Disease: Current Status and the Future Ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [PubMed]
- Hirata, Y.; Ihara, S.; Koike, K. Targeting the Complex Interactions between Microbiota, Host Epithelial and Immune Cells in Inflammatory Bowel Disease. Pharmacol. Res. 2016, 113, 574–584. [Google Scholar] [PubMed]
- Kim, J.; Lee, H.K. Potential Role of the Gut Microbiome In Colorectal Cancer Progression. Front. Immunol. 2022, 12, 807648. [Google Scholar]
- Han, B.; Lv, X.; Liu, G.; Li, S.; Fan, J.; Chen, L.; Huang, Z.; Lin, G.; Xu, X.; Huang, Z.; et al. Gut Microbiota-Related Bile Acid Metabolism-FXR/TGR5 Axis Impacts the Response to Anti-A4β7-Integrin Therapy in Humanized Mice with Colitis. Gut Microbes 2023, 15, 2232143. [Google Scholar]
- Kim, K.-Y.; Son, J.D.; Hwang, S.-J.; Lee, J.K.; Park, J.Y.; Park, K.I.; Oh, T.W. Fermented Glutinous Rice Extract Mitigates DSS-Induced Ulcerative Colitis by Alleviating Intestinal Barrier Function and Improving Gut Microbiota and Inflammation. Antioxidants 2023, 12, 336. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Grigor’eva, I.N. Gallstone Disease, Obesity and the Firmicutes/Bacteroidetes Ratio as a Possible Biomarker of Gut Dysbiosis. J. Pers. Med. 2021, 11, 13. [Google Scholar] [CrossRef]
- Cui, H.; Cai, Y.; Wang, L.; Jia, B.; Li, J.; Zhao, S.; Chu, X.; Lin, J.; Zhang, X.; Bian, Y.; et al. Berberine Regulates Treg/Th17 Balance to Treat Ulcerative Colitis Through Modulating the Gut Microbiota in the Colon. Front. Pharmacol. 2018, 9, 571. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Hong, G.; Huang, C.; Qian, W.; Bai, T.; Song, J.; Song, Y.; Hou, X. Probiotic Mixtures with Aerobic Constituent Promoted the Recovery of Multi-Barriers in DSS-Induced Chronic Colitis. Life Sci. 2020, 240, 117089. [Google Scholar] [CrossRef] [PubMed]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and Cultivation Study of Muribaculaceae Reveals Novel Species, Host Preference, and Functional Potential of This yet Undescribed Family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef]
- Sibai, M.; Altuntaş, E.; Yıldırım, B.; Öztürk, G.; Yıldırım, S.; Demircan, T. Microbiome and Longevity: High Abundance of Longevity-Linked Muribaculaceae in the Gut of the Long-Living Rodent Spalax Leucodon. OMICS 2020, 24, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Li, S.; Liu, F.; Li, Q.; Pang, D.; Wang, H.; Liao, S.; Zou, Y. Analysis of Akkermansia Muciniphila in Mulberry Galacto-Oligosaccharide Medium via Comparative Transcriptomics. Foods 2023, 12, 440. [Google Scholar] [CrossRef]
- Jian, H.; Liu, Y.; Wang, X.; Dong, X.; Zou, X. Akkermansia Muciniphila as a Next-Generation Probiotic in Modulating Human Metabolic Homeostasis and Disease Progression: A Role Mediated by Gut-Liver-Brain Axes? Int. J. Mol. Sci. 2023, 24, 3900. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, L.; Zhang, C.; Li, X.; Wu, T.; Dai, Y.; Sheng, Y.; Ren, Y.; Xue, Y. Gut Microbial Evidence Chain in High-Salt Diet Exacerbates Intestinal Aging Process. Front. Nutr. 2022, 9, 1046833. [Google Scholar] [CrossRef]
- Liu, T.-H.; Wang, J.; Zhang, C.-Y.; Zhao, L.; Sheng, Y.-Y.; Tao, G.-S.; Xue, Y.-Z. Gut Microbial Characteristical Comparison Reveals Potential Anti-Aging Function of Dubosiella Newyorkensis in Mice. Front. Endocrinol. 2023, 14, 1133167. [Google Scholar] [CrossRef]
- Fung, T.C.; Vuong, H.E.; Luna, C.D.G.; Pronovost, G.N.; Aleksandrova, A.A.; Riley, N.G.; Vavilina, A.; McGinn, J.; Rendon, T.; Forrest, L.R.; et al. Intestinal Serotonin and Fluoxetine Exposure Modulate Bacterial Colonization in the Gut. Nat. Microbiol. 2019, 4, 2064–2073. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, B.; Tian, P.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Daily Intake of Lactobacillus Alleviates Autistic-like Behaviors by Ameliorating the 5-Hydroxytryptamine Metabolic Disorder in VPA-Treated Rats during Weaning and Sexual Maturation. Food Funct. 2021, 12, 2591–2604. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Xu, X.; Huang, K.; Liang, Z. Pre-Administration of Saccharomyces boulardii-Derived Postbiotics Effectively Prevents Dextran Sulfate Sodium-Induced Colitis in Mice. Foods 2025, 14, 1109. https://doi.org/10.3390/foods14071109
Jin Y, Xu X, Huang K, Liang Z. Pre-Administration of Saccharomyces boulardii-Derived Postbiotics Effectively Prevents Dextran Sulfate Sodium-Induced Colitis in Mice. Foods. 2025; 14(7):1109. https://doi.org/10.3390/foods14071109
Chicago/Turabian StyleJin, Yuxin, Xinge Xu, Kunlun Huang, and Zhihong Liang. 2025. "Pre-Administration of Saccharomyces boulardii-Derived Postbiotics Effectively Prevents Dextran Sulfate Sodium-Induced Colitis in Mice" Foods 14, no. 7: 1109. https://doi.org/10.3390/foods14071109
APA StyleJin, Y., Xu, X., Huang, K., & Liang, Z. (2025). Pre-Administration of Saccharomyces boulardii-Derived Postbiotics Effectively Prevents Dextran Sulfate Sodium-Induced Colitis in Mice. Foods, 14(7), 1109. https://doi.org/10.3390/foods14071109





