Grifola frondosa Polysaccharide F2 Ameliorates Disordered Glucose and Lipid Metabolism in Prediabetic Mice by Modulating Bile Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of G. frondosa Polysaccharide F2
2.3. Animals and Drug Administration
2.4. Biochemical Parameters
2.5. UPLC-MS/MS Analysis of Fecal Samples
2.6. Fecal Microbiota Composition Analysis [27]
2.7. Correlation Analysis
2.8. Statistical Analysis
3. Results
3.1. F2 Ameliorated Lipid and Glucose Metabolism in Pre-DM Mice
3.2. Hypoglycemic Effect of F2 Featured with Changed Bile Acids
3.2.1. Principal Component Analysis (PCA) for Metabolomics
3.2.2. The Characteristics of Metabolic Disorder in Pre-DM Mice
3.2.3. Regulated Bile Acids in Pre-DM Mice May Be the Targets of F2 in the Alleviation of Pre-DM
3.2.4. Correlation Between Bile Acids and Physiological Traits
3.3. Structural Characteristics of the Fecal Microbiome in Pre-DM Mice and F2-Treated Pre-DM Mice
3.4. Correlations Between Physiological Traits and BSH-Producing Bacteria
3.5. Correlations Between Bile Acids and BSH-Producing Bacteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, R.M.M.; Chua, J.C.; Tan, Z.J.Y.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.D.; Sathish, T.; Zimmet, P.Z.; Thankappan, K.R.; Oldenburg, B.; Owens, D.R.; Shaw, J.E.; Tapp, R.J. Benefit of lifestyle-based T2DM prevention is influenced by prediabetes phenotype. Nat. Rev. Endocrinol. 2020, 16, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, S.M.; Carson, T.; Lowe, J.; Ramaraj, T.; Leff, J.W.; Luo, L.; Bell, C.J.; Shah, V.O. Composition, Diversity and Abundance of Gut Microbiome in Prediabetes and Type 2 Diabetes. J. Diabetes Obes. 2015, 2, 1–7. [Google Scholar]
- Narayan, K.M.V.; Kondal, D.; Chang, H.H.; Mohan, D.; Gujral, U.P.; Anjana, R.M.; Staimez, L.R.; Patel, S.A.; Ali, M.K.; Prabhakaran, D.; et al. Natural history of type 2 diabetes in Indians: Time to progression. Diabetes Care 2024, 47, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Echouffo-Tcheugui, J.B.; Perreault, L.; Ji, L.; Dagogo-Jack, S. Diagnosis and management of prediabetes: A review. JAMA 2023, 329, 1206–1216. [Google Scholar] [CrossRef]
- Allin, K.H.; Tremaroli, V.; Caesar, R.; Jensen, B.A.H.; Damgaard, M.T.F.; Bahl, M.I.; Licht, T.R.; Hansen, T.H.; Nielsen, T.; Dantoft, T.M.; et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia 2018, 61, 810–820. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut microbiota: An important player in type 2 diabetes mellitus. Front. Cell Infect. Microbiol. 2022, 12, 834485. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, G.; Chen, D.; Ye, H.; Zeng, X. The antidiabetic effect and potential mechanisms of natural polysaccharides based on the regulation of gut microbiota. J. Funct. Foods 2020, 75, 104222. [Google Scholar] [CrossRef]
- Sanna, S.; Van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Martin, A.M.; Yabut, J.M.; Choo, J.M.; Page, A.J.; Keating, D.J. The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc. Natl. Acad. Sci. USA 2019, 116, 201909311. [Google Scholar] [CrossRef]
- Ren, B.; Wei, S.X.; Huang, H. Recent advances in Grifola frondosa polysaccharides: Production, properties, and bioactivities. Curr. Opin. Food Sci. 2023, 49, 100946. [Google Scholar] [CrossRef]
- Wu, J.Y.; Siu, K.C.; Geng, P. Bioactive ingredients and medicinal values of Grifola frondosa (Maitake). Foods 2021, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wu, Q.; Xie, Y.; Zhang, J.; Tan, J. Hypoglycemic effects of Grifola frondosa (Maitake) polysaccharides F2 and F3 through improvement of insulin resistance in diabetic rats. Food Funct. 2015, 6, 3567–3575. [Google Scholar] [CrossRef]
- Lei, H.; Ma, X.; Wu, W.T. Anti-diabetic effect of an alpha-glucan from fruit body of maitake (Grifola frondosa) on KK-Ay mice. J. Pharm. Pharmacol. 2007, 59, 575–582. [Google Scholar]
- Lei, H.; Zhang, M.; Wang, Q.; Guo, S.; Han, J.; Sun, H.; Wu, W. MT-alpha-glucan from the fruit body of the maitake medicinal mushroom Grifola frondosa (higher Basidiomyetes) shows protective effects for hypoglycemic pancreatic beta-cells. Int. J. Med. Mushrooms. 2013, 15, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Guo, S.; Han, J.; Wang, Q.; Zhang, X.; Wu, W. Hypoglycemic and hypolipidemic activities of MT-α-glucan and its effect on immune function of diabetic mice. Carbohydr. Polym. 2012, 89, 245–250. [Google Scholar] [CrossRef]
- Kubo, K.; Aoki, H.; Nanba, H. Anti-diabetic Activity Present in the Fruit Body of Grifola frondosa (Maitake). I. Biol. Pharm. Bull. 1994, 17, 1106–1110. [Google Scholar] [CrossRef]
- Manohar, V.; Talpur, N.A.; Echard, B.W.; Lieberman, S.; Preuss, H.G. Effects of a water-soluble extract of maitake mushroom on circulating glucose/insulin concentrations in KK mice. Diabetes. Obes. Metab. 2002, 4, 43–48. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Bagchi, D.; Perricone, N.V.; Zhuang, C. Enhanced insulin-hypoglycemic activity in rats consuming a specific glycoprotein extracted from maitake mushroom. Mol. Cell. Biochem. 2007, 306, 105–113. [Google Scholar] [CrossRef]
- Konno, S.; Alexander, B.; Zade, J.; Choudhury, M. Possible hypoglycemic action of SX fraction targeting insulin signal transduction pathway. Int. J. Gen. Med. 2013, 6, 181–187. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, D.; Wang, D.; Lai, S.; Zhong, R.; Liu, Y.; Yang, C.; Liu, B.; Sarker, M.R.; Zhao, C. Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food Chem. Toxicol. 2019, 126, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, L. The positive effects of Grifola frondosa heteropolysaccharide on NAFLD and regulation of the gut microbiota. Int. J. Mol. Sci. 2019, 20, 5302. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Deng, J.C.; Pan, Y.Y.; Xu, J.X.; Hong, J.L.; Shi, F.F.; Liu, G.L.; Qian, M.; Bai, W.D.; Zhang, W.; et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020, 153, 1231–1240. [Google Scholar] [CrossRef]
- Gao, X.X.; Liu, D.; Gao, L.Y.; Ouyang, Y.Z.; Wen, Y.X.; Ai, C.; Chen, Y.Q.; Zhao, C. Health benefits of polysaccharide on intestinal microbiota in type 2 diabetic mice. Food Sci. Hum. Wellness 2022, 11, 68–73. [Google Scholar] [CrossRef]
- Xiao, C.; Jiao, C.W.; Xie, Y.Z.; Ye, L.H.; Li, Q.Q.; Wu, Q.P. Grifola frondosa GF5000 improves insulin resistance by modulation the composition of gut microbiota in diabetic rats. J. Funct. Foods 2021, 77, 104313. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Z.; Fan, Y.; Liu, X.; Yi, R.; Gan, Y.; Yang, Z.; Liu, S. Integrated gut microbiome and UHPLC-MS metabolomics to reveal the prevention mechanism of pidanjiangtang granules on IGT Rats. Phytomedicine 2024, 135, 156201. [Google Scholar] [CrossRef]
- Jiao, J.; Yong, T.Q.; Huang, L.H.; Chen, S.D.; Xiao, C.; Wu, Q.P.; Hu, H.P.; Xie, Y.Z.; Li, X.M.; Liu, Y.C.; et al. A Ganoderma lucidum polysaccharide F31 alleviates hyperglycemia through kidney protection and adipocyte apoptosis. Int. J. Biol. Macromol. 2023, 226, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T., Jr. Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. 2015, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for investigating physiological and pathophysiological processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Kotlowska, A. Application of steroid hormone metabolomics in search of biomarkers in clinical research. Drug Develop. Res. 2012, 73, 381–389. [Google Scholar] [CrossRef]
- Shah, N.J.; Sureshkumar, S.; Shewade, D.G. Metabolomics: A tool ahead for understanding molecular mechanisms of drugs and diseases. Indian J. Clin. Biochem. 2015, 30, 247–254. [Google Scholar] [CrossRef]
- Zaborska, K.E.; Cummings, B.P. Rethinking bile acid metabolism and signaling for type 2 diabetes treatment. Curr. Diab. Rep. 2018, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acids as metabolic regulators and nutrient sensors. Annu. Rev. Nutr. 2019, 39, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Cai, Y.; Lao, X.; Wang, X.; Lin, X.; Cui, Y.; Kalavagunta, P.K.; Liao, J.; Jin, L.; Shang, J.; et al. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome 2019, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B.; et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Kir, S.; Beddow, S.A.; Samuel, V.T.; Miller, P.; Previs, S.F.; Suino-Powell, K.; Xu, H.E.; Shulman, G.I.; Kliewer, S.A.; Mangelsdorf, D.J. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 2011, 331, 1621–1624. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Li, R.J.W.; Lim, Y.M.; Batchuluun, B.; Liu, H.Y.; Waise, T.M.Z.; Lam, T.K.T. FXR in the dorsal vagal complex is sufficient and necessary for upper small intestinal microbiome-mediated changes of TCDCA to alter insulin action in. Gut 2021, 70, 1675–1683. [Google Scholar] [CrossRef]
- Duboc, H.; Taché, Y.; Hofmann, A.F. The bile acid TGR5 membrane receptor: From basic research to clinical application. Dig. Liver Dis. 2014, 46, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Xie, C.; Nichols, R.G.; Ferrell, J.M.; Boehme, S.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J.; Chiang, J.Y.L. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 2018, 68, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
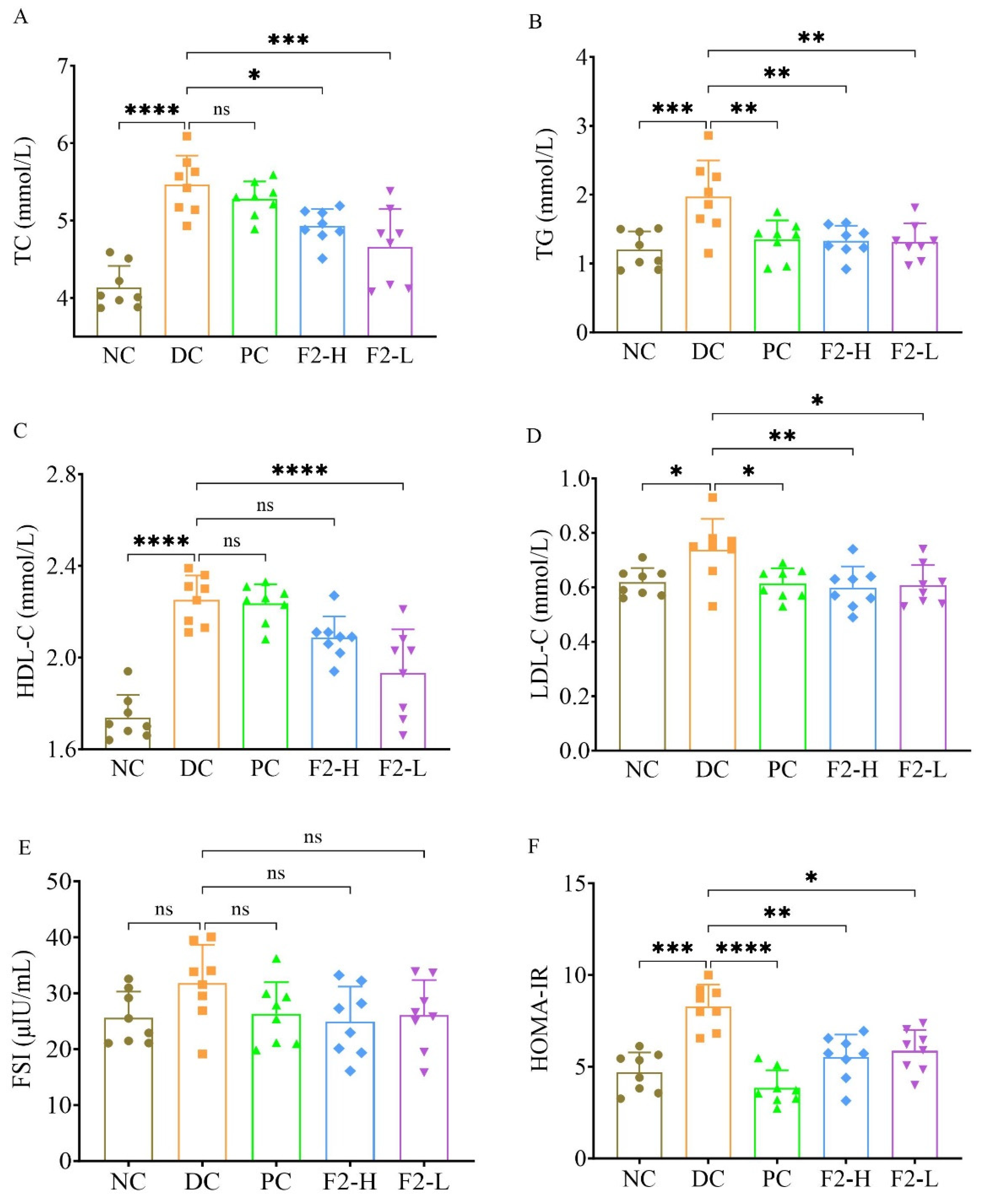
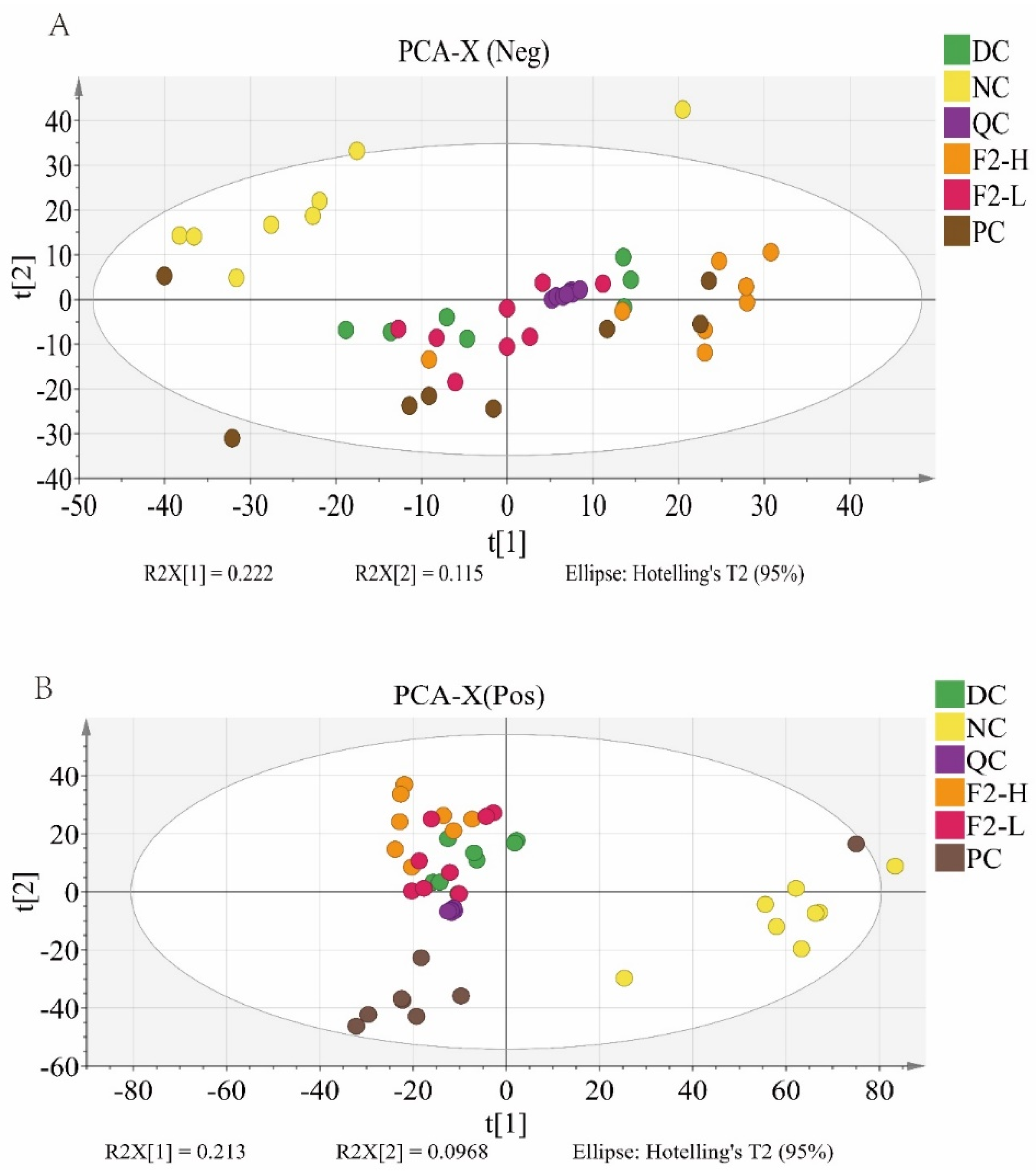
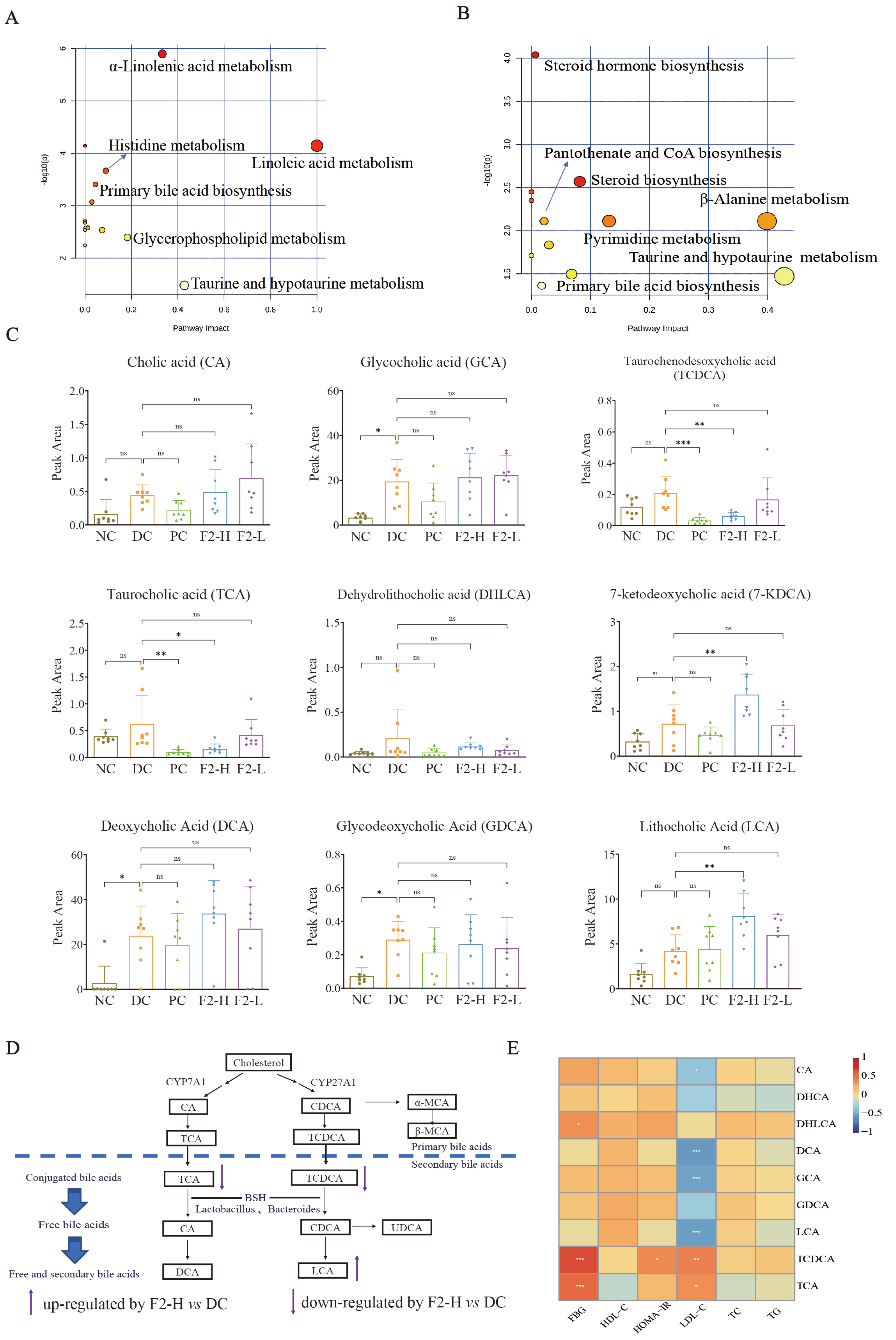


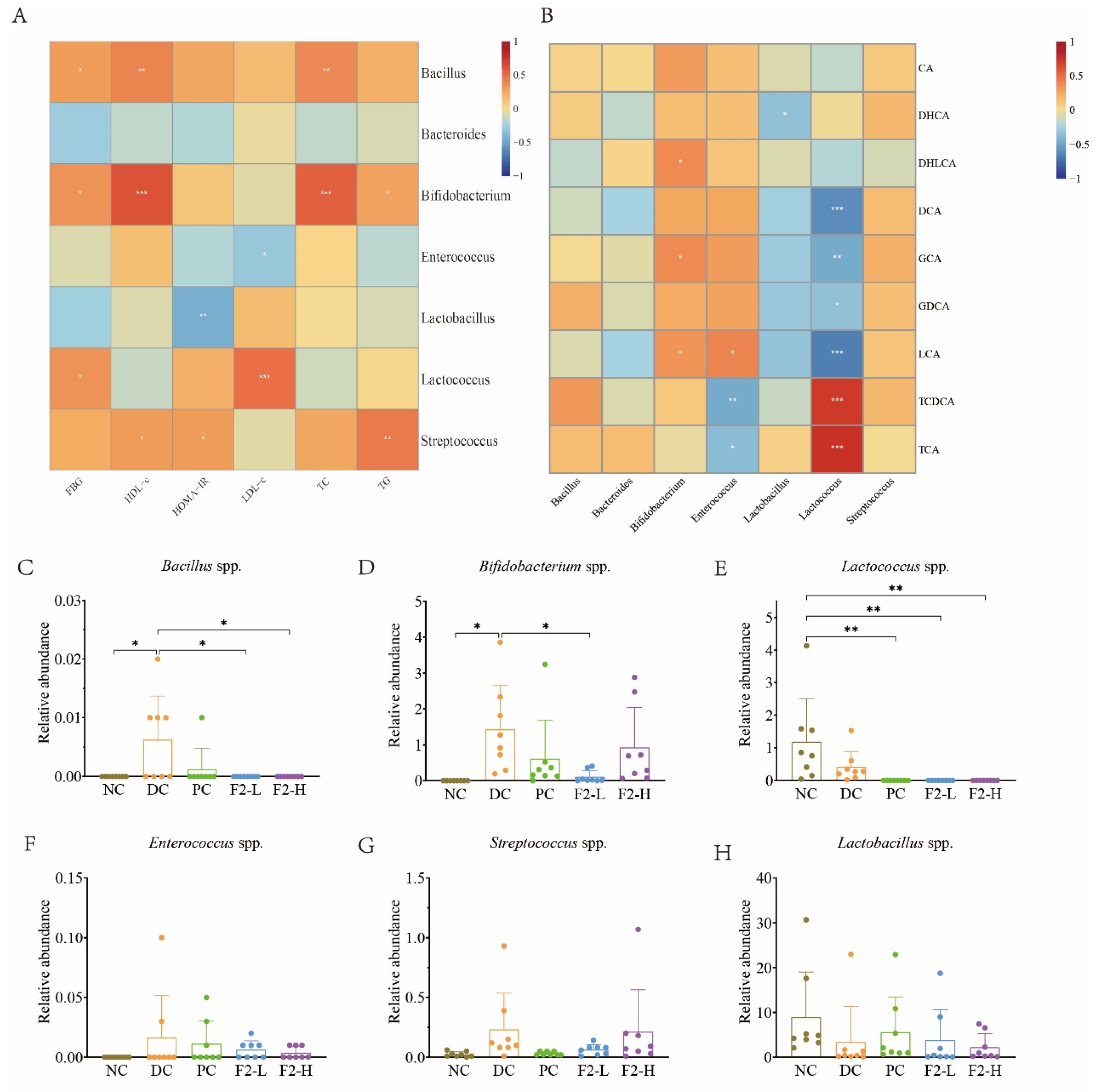
| Group | Fasting Blood Glucose (FBG, mmol/L) | ||||
|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |
| NC | 7.27 ± 1.00 ** | 6.61 ± 0.71 * | 6.88 ± 0.64 | 6.49 ± 0.71 ** | 6.13 ± 0.61 **** |
| DC | 8.33 ± 0.62 | 7.35 ± 0.54 | 6.67 ± 0.73 | 7.27 ± 0.77 | 7.34 ± 0.67 |
| PC | 7.86 ± 0.63 | 6.84 ± 0.58 | 6.69 ± 0.78 | 6.67 ± 0.55 * | 6.42 ± 0.8 ** |
| F2-L | 7.77 ± 0.96 | 6.20 ± 1.11 ** | 6.53 ± 0.74 | 6.49 ± 0.67 ** | 6.12 ± 0.55 **** |
| F2-H | 7.80 ± 0.55 | 6.98 ± 0.97 | 6.38 ± 0.99 | 6.62 ± 0.45 * | 6.37 ± 0.57 ** |
| Group | Blood Glucose (mmol/L) | ||||
|---|---|---|---|---|---|
| 0 h | 0.5 h | 1 h | 1.5 h | 2 h | |
| NC | 4.00 ±0.41 **** | 13.25 ± 1.98 | 7.13 ± 1.00 | 5.07 ± 0.36 **** | 4.58 ± 0.39 **** |
| DC | 5.53 ± 0.64 | 12.24 ± 2.17 | 8.51 ± 1.25 | 6.77 ± 1.43 | 5.94 ± 0.89 |
| PC | 3.44 ± 0.40 **** | 11.37 ± 3.26 | 8.00 ± 2.09 | 5.54 ± 0.84 ** | 4.83 ± 0.71 **** |
| F2-L | 4.81 ± 0.62 ** | 13.27 ± 2.43 | 8.73 ± 1.24 | 6.31 ± 0.65 | 5.51 ± 0.70 |
| F2-H | 4.87 ± 0.67 ** | 15.46 ± 3.65 * | 9.91 ± 2.19 | 6.28 ± 0.70 | 5.73 ± 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Xiao, C.; Yong, T.; Huang, L.; Hu, H.; Xie, Y.; Wu, Q. Grifola frondosa Polysaccharide F2 Ameliorates Disordered Glucose and Lipid Metabolism in Prediabetic Mice by Modulating Bile Acids. Foods 2025, 14, 955. https://doi.org/10.3390/foods14060955
Zhang R, Xiao C, Yong T, Huang L, Hu H, Xie Y, Wu Q. Grifola frondosa Polysaccharide F2 Ameliorates Disordered Glucose and Lipid Metabolism in Prediabetic Mice by Modulating Bile Acids. Foods. 2025; 14(6):955. https://doi.org/10.3390/foods14060955
Chicago/Turabian StyleZhang, Ruifang, Chun Xiao, Tianqiao Yong, Longhua Huang, Huiping Hu, Yizhen Xie, and Qingping Wu. 2025. "Grifola frondosa Polysaccharide F2 Ameliorates Disordered Glucose and Lipid Metabolism in Prediabetic Mice by Modulating Bile Acids" Foods 14, no. 6: 955. https://doi.org/10.3390/foods14060955
APA StyleZhang, R., Xiao, C., Yong, T., Huang, L., Hu, H., Xie, Y., & Wu, Q. (2025). Grifola frondosa Polysaccharide F2 Ameliorates Disordered Glucose and Lipid Metabolism in Prediabetic Mice by Modulating Bile Acids. Foods, 14(6), 955. https://doi.org/10.3390/foods14060955






