Current Directions of Selected Plant-Origin Wastes’ Valorization in Biotechnology of Food Additives and Other Important Chemicals
Abstract
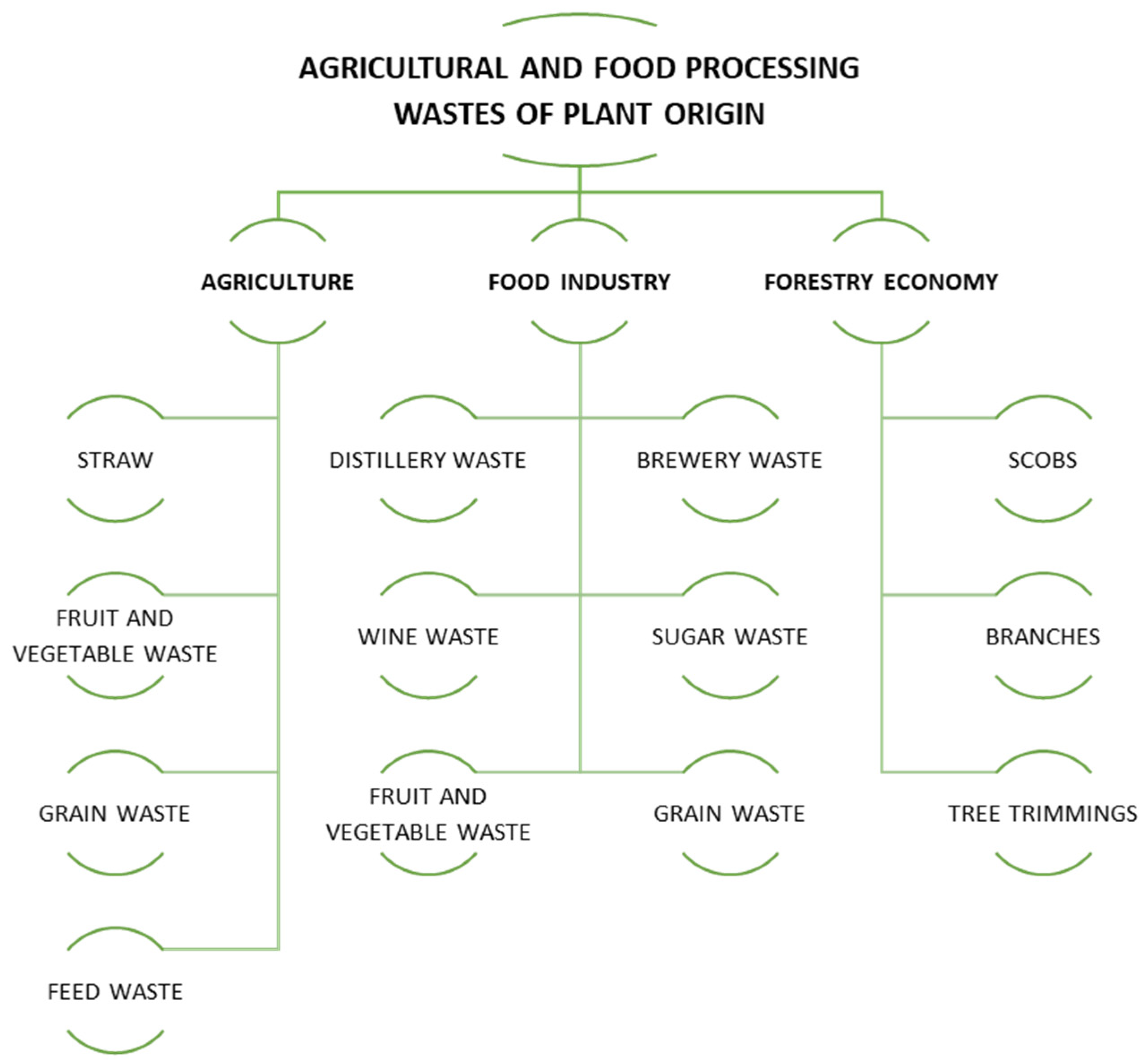
1. Introduction
1.1. Trends in the Literature Search Queries
1.2. General Characteristics of Lignocellulosic, Pectin, and Starch Wastes
1.3. Characterization of Selected Lignocellulosic, Pectin, and Starch Wastes
| Type | Component | Dry Mass Content Range/% | Reference |
|---|---|---|---|
| Brewer’s spent grain | Hemicellulose | 19.2–41.9 | [46] |
| Cellulose | 0.3–33 | ||
| Proteins | 14.2–31.0 | ||
| Lignin | 11.5–27.8 | ||
| Starch | 1.0–12.0 | ||
| Lipids | 3.0–10.6 | ||
| Ash | 1.1–4.6 | ||
| Brewer’s spent hops | Nitrogen free extract | 40.0 | [47] |
| Crude fiber | 23.0–26.0 | ||
| Proteins | 22.0–23.0 | ||
| Ash | 6.0–6.5 | ||
| Lipids | 4.5 | ||
| Sugar beet pulp | Hemicellulose | 25–36 | [55,56] |
| Cellulose | 20–25 | ||
| Pectin | 20–25 | ||
| Proteins | 10–15 | ||
| Ash | 3.7 | ||
| Lignin | 1–2 | ||
| Lipids | 1.4 | ||
| Sugarcane | Cellulose | 41.3 | [57] |
| Hemicellulose | 33.2 | ||
| Lignin | 17.3 | ||
| Ash | 2.8 | ||
| Wheat | Cellulose | 31.2 | [58] |
| Hemicellulose | 21.8 | ||
| Lignin | 22.8 | ||
| Ash | 8.7 | ||
| Rice | Cellulose | 36.3 | [59] |
| Hemicellulose | 20.7 | ||
| Lignin | 9.4 | ||
| Quinoa | Cellulose | 31 | [25] |
| Hemicellulose | 20.8 | ||
| Lignin | 20 | ||
| Rapeseed | Glucan | 31.5 | [60] |
| Hemicellulose | 17.4 | ||
| Lignin | 17.8 | ||
| Ash | 6.7 | ||
| Hemicellulose | 26.0 | ||
| Lignin | 12.2 |
2. Utilization of Selected Lignocellulosic, Pectin, and Starch Wastes in the Biotechnology of Food Additives and Other Important Chemicals
2.1. Microbial Proteins
2.2. Enzymes
2.3. Polyhydroxy Alcohols
2.4. Oligosaccharides
2.5. Biopolymers
2.6. Carboxylic Acids
2.7. Terpenes
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos, S.; Etxebarria, S.; Cidad, M.; Gutierrez, M.; San Martin, D.; Iñarra, B.; Olabarrieta, I.; Melado-Herreros, Á.; Zufia, J. Cleaner production strategies for the food industry. In The Interaction of Food Industry and Environment; Galanakis, C., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–34. [Google Scholar] [CrossRef]
- Prandecki, K.; Wrzaszcz, W.; Zieliński, M. Environmental and climate challenges to agriculture in Poland in the context of objectives adopted in the European Green Deal strategy. Sustainability 2021, 13, 10318. [Google Scholar] [CrossRef]
- Adedeji, A.A. Agri-food waste reduction and utilization—A sustainability perspective. J. ASABE 2022, 65, 471–479. [Google Scholar] [CrossRef]
- European Commission 2019. Communication from the Commission to the European Parliament, The European Council, The Council, The European Economic and Social Committee and The Committee of the Regions; The European Green Deal: Brussels, Belgium, 2019. [Google Scholar]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Moving towards the second generation of lignocellulosic biorefineries in the EU: Drivers, challenges, and opportunities. Renew. Sustain. Energy Rev. 2019, 101, 590–599. [Google Scholar] [CrossRef]
- Millati, R.; Cahyono, R.B.; Ariyanto, T.; Azzahrani, I.N.; Putri, R.U.; Taherzadeh, M.J. Agricultural, industrial, municipal, and forest wastes: An overview. In Sustainable Resource Recovery and Zero Waste Approaches; Taherzadeh, M.J., Bolton, K., Wong, J., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–22. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, S.W.; Bilal, M. Optimization of lignocellulolytic enzyme production by Pleurotus eryngii WC 888 utilizing agro-industrial residues and bio-ethanol production. Rom. Biotechnol. Lett. 2016, 21, 11133. [Google Scholar]
- Bilal, M.; Nawaz, M.Z.; Iqbal, H.; Hou, J.; Mahboob, S.; Al-Ghanim, K.A.; Cheng, H. Engineering ligninolytic consortium for bioconversion of lignocelluloses to ethanol and chemicals. Protein Pept. Lett. 2018, 25, 108–119. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Ligninolytic enzymes mediated ligninolysis: An untapped biocatalytic potential to deconstruct lignocellulosic molecules in a sustainable manner. Catal. Lett. 2020, 150, 524–543. [Google Scholar] [CrossRef]
- Monteiro de Oliveira, P.; Aborneva, D.; Bonturi, N.; Lahtvee, P.J. Screening and growth characterization of non-conventional yeasts in a hemicellulosic hydrolysate. Front. Bioeng. Biotechnol. 2021, 9, 659472. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.K.; Sharma, A.; Soni, R. Cellulases: Role in lignocellulosic biomass utilization. Cellulases. Methods in Molecular Biology; Lübeck, M., Ed.; Humana Press: Totowa, NJ, USA, 2018; Volume 1796, pp. 3–23. [Google Scholar] [CrossRef]
- Girma, E.; Worku, T. Extraction and characterization of pectin from selected fruit peel waste. Int. J. Sci. Res. 2016, 6, 447–454. [Google Scholar]
- Shivamathi, C.S.; Gunaseelan, S.; Soosai, M.R.; Vignesh, N.S.; Varalakshmi, P.; Kumar, R.S.; Karthikumar, S.; Kumar, R.V.; Baskar, R.; Rigby, S.P.; et al. Process optimization and characterization of pectin derived from underexploited pineapple peel biowaste as a value-added product. Food Hydrocoll. 2022, 123, 107141. [Google Scholar] [CrossRef]
- Freshfel Consumption Monitor 2021. Available online: https://freshfel.org/what-we-do/consumption-monitor/ (accessed on 22 January 2024).
- Ienczak, J.L.; Poletto, P.; Robl, D.; Rabelo, S.C. Transforming the lignocellulosic biomass into high value-added bioproducts. In Bio-Valorization of Waste: Trends and Perspectives; Shah, S., Venkatramanan, V., Prasad, R., Eds.; Springer: Singapore, 2021; pp. 21–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, J.; Han, B. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem. Rev. 2017, 117, 6834–6880. [Google Scholar] [CrossRef]
- Fatma, S.; Hameed, A.; Noman, M.; Ahmed, T.; Shahid, M.; Tariq, M.; Sohail, I.; Tabassum, R. Lignocellulosic biomass: A sustainable bioenergy source for the future. Protein Pept. Lett. 2018, 25, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Rampazzo, R.; Alkan, D.; Gazzotti, S.; Ortenzi, M.A.; Piva, G.; Piergiovanni, L. Cellulose nanocrystals from lignocellulosic raw materials, for oxygen barrier coatings on food packaging films. Packag. Technol. Sci. 2017, 30, 645–661. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Liu, K.; Yang, H.; Lin, Q.; Xu, T.; Song, X.; Du, H.; Bai, L.; Yao, S.; et al. Sustainable preparation of lignocellulosic nanofibrils and cellulose nanopaper from poplar sawdust. J. Clean. Prod. 2023, 384, 135582. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Hasunuma, T.; Ogino, C.; Kondo, A. Bioprocessing of bio-based chemicals produced from lignocellulosic feedstocks. Curr. Opin. Biotechnol. 2016, 42, 30–39. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Nilvebrant, N.O.; Reimann, A.; Larsson, S.; Jönsson, L.J. Detoxification of lignocellulose hydrolysates with ion-exchange resins. Appl. Biochem. Biotechnol. 2001, 91, 35–49. [Google Scholar] [CrossRef]
- Gupta, R.; Gautam, S.; Shukla, R.; Kuhad, R.C. Study of charcoal detoxification of acid hydrolysate from corncob and its fermentation to xylitol. J. Environ. Chem. Eng. 2017, 5, 4573–4582. [Google Scholar] [CrossRef]
- Jin, T.; Xing, X.; Xie, Y.; Sun, Y.; Bian, S.; Liu, L.; Chen, G.; Wang, X.; Yu, X.; Su, Y. Evaluation of preparation and detoxification of hemicellulose hydrolysate for improved xylitol production from quinoa straw. Int. J. Mol. Sci. 2023, 24, 516. [Google Scholar] [CrossRef]
- Peña-Castro, J.M.; Muñoz-Páez, K.M.; Robledo-Narvaez, P.N.; Vázquez-Núñez, E. Engineering the metabolic landscape of microorganisms for lignocellulosic conversion. Microorganisms 2023, 11, 2197. [Google Scholar] [CrossRef]
- Ciriminna, R.; Chavarría-Hernández, N.; Inés Rodríguez Hernández, A.; Pagliaro, M. Pectin: A new perspective from the biorefinery standpoint. Biofuels Bioprod. Biorefin. 2015, 9, 368–377. [Google Scholar] [CrossRef]
- Marras-Marquez, T.; Peña, J.; Veiga-Ochoa, M.D. Robust and versatile pectin-based drug delivery systems. Int. J. Pharm. 2015, 479, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Alsakhawy, M.A.; Abdelmonsif, D.A.; Haroun, M.; Sabra, S.A. Naringin-loaded Arabic gum/pectin hydrogel as a potential wound healing material. Int. J. Biol. Macromol. 2022, 222, 701–714. [Google Scholar] [CrossRef]
- Valle, K.Z.M.; Saucedo Acuña, R.A.; Ríos Arana, J.V.; Lobo, N.; Rodriguez, C.; Cuevas-Gonzalez, J.C.; Tovar-Carrillo, K.L. Natural film based on pectin and allantoin for wound healing: Obtaining, characterization, and rat model. BioMed Res. Int. 2020, 2020, 6897497. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Sandhya, R.; Nisha, P. Advances and prospects in the food applications of pectin hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 4393–4417. [Google Scholar] [CrossRef]
- Yang, J.S.; Mu, T.H.; Ma, M.M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, X.; Zhu, C. Physicochemical properties and antioxidant activity of pectin from hawthorn wine pomace: A comparison of different extraction methods. Int. J. Biol. Macromol. 2020, 158, 1239–1247. [Google Scholar] [CrossRef]
- Lupi, F.R.; Gabriele, D.; Seta, L.; Baldino, N.; de Cindio, B.; Marino, R. Rheological investigation of pectin-based emulsion gels for pharmaceutical and cosmetic uses. Rheol. Acta 2015, 54, 41–52. [Google Scholar] [CrossRef]
- Wahab, I.F.; Abd Razak, S.I. Polysaccharides as composite biomaterials. In Composites from Renewable and Sustainable Materials; Poletto, M., Ed.; IntechOpen: Rijeka, Croatia, 2016; pp. 65–84. [Google Scholar] [CrossRef]
- Kibar, E.A.A.; Aslan, Ö.; Bakan, A.; Özer, H. Determination of physicochemical and functional properties of cross-linked, hydroxypropylated, and dual modified corn starch and investigation of its use as gelling agent in soft confectionery. Starch-Stärke 2023, 76, 2300036. [Google Scholar] [CrossRef]
- He, J.; Han, Y.; Liu, M.; Wang, Y.; Yang, Y.; Yang, X. Effect of 2 types of resistant starches on the quality of yogurt. J. Dairy. Sci. 2019, 102, 3956–3964. [Google Scholar] [CrossRef]
- Engel, J.B.; Ambrosi, A.; Tessaro, I.C. Development of biodegradable starch-based foams incorporated with grape stalks for food packaging. Carbohydr. Polym. 2019, 225, 115234. [Google Scholar] [CrossRef]
- Mueller, E.; Hoffmann, T.G.; Schmitz, F.R.W.; Helm, C.V.; Roy, S.; Bertoli, S.L.; de Souza, C.K. Development of ternary polymeric films based on cassava starch, pea flour and green banana flour for food packaging. Int. J. Biol. Macromol. 2024, 256, 128436. [Google Scholar] [CrossRef] [PubMed]
- Maurer, H.W.; Kearney, R.L. Opportunities and challenges for starch in the paper industry. Starch-Stärke 1998, 50, 396–402. [Google Scholar] [CrossRef]
- Sun, Y.; Gu, J.; Tan, H.; Zhang, Y.; Huo, P. Physicochemical properties of starch adhesives enhanced by esterification modification with dodecenyl succinic anhydride. Int. J. Biol. Macromol. 2018, 112, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, K.; Ameen, H.; Morsy, M.; El-Ebiassy, A.; El-Sanabary, A.; Adel, M.; Salah, A. Production of high-performance textiles via pioneering strengthening approach using starch nanoparticles. J. Ind. Text. 2020, 50, 278–292. [Google Scholar] [CrossRef]
- Thanyapanich, N.; Jimtaisong, A.; Rawdkuen, S. Functional Properties of Banana Starch (Musa spp.) and Its Utilization in Cosmetics. Molecules 2021, 26, 3637. [Google Scholar] [CrossRef]
- Aruwajoye, G.S.; Faloye, F.D.; Kana, E.G. Soaking assisted thermal pretreatment of cassava peels wastes for fermentable sugar production: Process modelling and optimization. Energy Convers. Manag. 2017, 150, 558–566. [Google Scholar] [CrossRef]
- E Silva, J.O.V.; Almeida, M.F.; da Conceição Alvim-Ferraz, M.; Dias, J.M. Integrated production of biodiesel and bioethanol from sweet potato. Renew. Energy 2018, 124, 114–120. [Google Scholar] [CrossRef]
- Bianco, A.; Budroni, M.; Zara, S.; Mannazzu, I.; Fancello, F.; Zara, G. The role of microorganisms on biotransformation of brewers’ spent grain. Appl. Microbiol. Biotechnol. 2020, 104, 8661–8678. [Google Scholar] [CrossRef]
- Karlović, A.; Jurić, A.; Ćorić, N.; Habschied, K.; Krstanović, V.; Mastanjević, K. By-products in the malting and brewing industries—Re-usage possibilities. Fermentation 2020, 6, 82. [Google Scholar] [CrossRef]
- Habeeb, A.A.M.; Gad, A.E.; El-Tarabany, A.A.; Mustafa, M.M.; Atta, M.A.A. Using of sugar beet pulp by-product in farm animals feeding. Int. J. Sci. Res. Sci. Technol. 2017, 3, 107–120. [Google Scholar]
- Cambridge Dictionary 2023. Available online: https://dictionary.cambridge.org/dictionary/english/straw (accessed on 7 June 2023).
- Mubarak, M.; Salem, E.M.; Kenawey, M.K.; Saudy, H.S. Changes in calcareous soil activity, nutrient availability, and corn productivity due to the integrated effect of straw mulch and irrigation regimes. J. Soil Sci. Plant Nutr. 2021, 21, 2020–2031. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, C. Effect of straw and plastic film mulching on warming and insulation of tea plantation in winter. Chin. J. Eco-Agric. 2010, 18, 327–333. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Alkali delignification and Bacillus sp. BMP01 hydrolysis of rice straw for enhancing biofuel yields. Bull. Natl. Res. Cent. 2019, 43, 136. [Google Scholar] [CrossRef]
- Buchspies, B.; Kaltschmitt, M.; Junginger, M. Straw utilization for biofuel production: A consequential assessment of greenhouse gas emissions from bioethanol and biomethane provision with a focus on the time dependency of emissions. Glob. Chang. Biol. Bioenergy 2020, 12, 789–805. [Google Scholar] [CrossRef]
- Agrawal, S.; Sharma, D.; Nagpal, R.; Kaur, A.; Bhardwaj, N.; Mahajan, R. Valorisation of wheat straw into paper with improved quality characteristics using ultrafiltered xylano-pectinolytic pulping approach. 3 Biotech 2023, 13, 106. [Google Scholar] [CrossRef]
- Michel, F.; Thibault, J.F.; Barry, J.L.; de Baynast, R. Preparation and characterisation of dietary fibre from sugar beet pulp. J. Sci. Food Agric. 1988, 42, 77–85. [Google Scholar] [CrossRef]
- Zheng, Y.; Lee, C.; Yu, C.; Cheng, Y.S.; Zhang, R.; Jenkins, B.M.; VanderGheynst, J.S. Dilute acid pretreatment and fermentation of sugar beet pulp to ethanol. Appl. Energy 2013, 105, 1–7. [Google Scholar] [CrossRef]
- Abdeshahian, P.; Ascencio, J.J.; Philippini, R.R.; Antunes, F.A.F.; Dos Santos, J.C.; da Silva, S.S. Utilization of sugarcane straw for production of β-glucan biopolymer by Lasiodiplodia theobromae CCT 3966 in batch fermentation process. Bioresour. Technol. 2020, 314, 123716. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Xiong, L.; Chen, X.; Wang, C.; Huang, C.; Chen, X. Isolation of cellulose from wheat straw and its utilization for the preparation of carboxymethyl cellulose. Fiber. Polym. 2019, 20, 975–981. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Bhaduri, D.; Adak, T.; Munda, S.; Satapathy, B.S.; Dash, P.K.; Padhy, S.R.; Pattanayak, A.; Routray, S.; Chakraborti, M.; et al. Characterization of rice straw from major cultivars for best alternative industrial uses to cutoff the menace of straw burning. Ind. Crop. Prod. 2020, 143, 111919. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Romero, I.; Cara, C.; Castro, E.; Mussatto, S.I. Xylitol production by Debaryomyces hansenii and Candida guilliermondii from rapeseed straw hemicellulosic hydrolysate. Bioresour. Technol. 2018, 247, 736–743. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Paniagua-García, A.I.; Díez-Antolínez, R. Industrial potato peel as a feedstock for biobutanol production. New Biotechnol. 2018, 46, 54–60. [Google Scholar] [CrossRef]
- Toma, R.B.; Orr, P.H.; D’appolonia, B.; Dlntzis, F.R.; Tabekhia, M.M. Physical and chemical properties of potato peel as a source of dietary fiber in bread. J. Food Sci. 1979, 44, 1403–1407. [Google Scholar] [CrossRef]
- Parchami, M.; Mahboubi, A.; Agnihotri, S.; Taherzadeh, M.J. Biovalorization of brewer’s spent grain as single-cell protein through coupling organosolv pretreatment and fungal cultivation. Waste Manag. 2023, 169, 382–391. [Google Scholar] [CrossRef]
- Faria, N.T.; Marques, S.; Ferreira, F.C.; Fonseca, C. Production of xylanolytic enzymes by Moesziomyces spp. using xylose, xylan and brewery’s spent grain as substrates. New Biotechnol. 2019, 49, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Tiwari, B.K.; Williams, G.A.; Jaiswal, A.K. Bioprocessing of brewers’ spent grain for production of xylanopectinolytic enzymes by Mucor sp. Bioresour. Technol. Rep. 2020, 9, 100371. [Google Scholar] [CrossRef]
- Leite, P.; Silva, C.; Salgado, J.M.; Belo, I. Simultaneous production of lignocellulolytic enzymes and extraction of antioxidant compounds by solid-state fermentation of agro-industrial wastes. Ind. Crops Prod. 2019, 137, 315–322. [Google Scholar] [CrossRef]
- Liguori, R.; Pennacchio, A.; Vandenberghe, L.P.D.S.; De Chiaro, A.; Birolo, L.; Soccol, C.R.; Faraco, V. Screening of fungal strains for cellulolytic and xylanolytic activities production and evaluation of brewers’ spent grain as substrate for enzyme production by selected fungi. Energies 2021, 14, 4443. [Google Scholar] [CrossRef]
- Llimós, J.; Martínez-Avila, O.; Marti, E.; Corchado-Lopo, C.; Llenas, L.; Gea, T.; Ponsá, S. Brewer’s spent grain biotransformation to produce lignocellulolytic enzymes and polyhydroxyalkanoates in a two-stage valorization scheme. Biomass Convers. Biorefinery 2020, 12, 3921–3932. [Google Scholar] [CrossRef]
- Outeirino, D.; Costa-Trigo, I.; de Souza Oliveira, R.P.; Guerra, N.P.; Domínguez, J.M. A novel approach to the biorefinery of brewery spent grain. Process Biochem. 2019, 85, 135–142. [Google Scholar] [CrossRef]
- Terrasan, C.R.F.; Carmona, E.C. Solid-state fermentation of brewer’s spent grain for xylanolytic enzymes production by Penicillium janczewskii and analyses of the fermented substrate. Biosci. J. 2015, 31, 1826–1836. [Google Scholar] [CrossRef]
- Almowallad, S.A.; Aljobair, M.O.; Alkuraieef, A.N.; Aljahani, A.H.; Alsuhaibani, A.M.; Alsayadi, M.M. Utilization of agro-industrial orange peel and sugar beet pulp wastes for fungal endo-polygalacturonase production. Saudi J. Biol. Sci. 2022, 29, 963–969. [Google Scholar] [CrossRef]
- Campioni, T.S.; de Azevedo Carvalho, A.F.; de Figueiredo, F.C.; da Silva, D.F.; de Oliva Neto, P. Xylanases and cellulases biosynthesis by selected fungi in a simple and economic bio system using sugarcane straw. IJEAB 2020, 5, 217–230. [Google Scholar] [CrossRef]
- Gautam, A.; Kumar, A.; Bharti, A.K.; Dutt, D. Rice straw fermentation by Schizophyllum commune ARC-11 to produce high level of xylanase for its application in pre-bleaching. J. Genet. Eng. Biotechnol. 2018, 16, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Williams, G.A.; Jaiswal, A.K. Evaluation of brewer’s spent grain hydrolysate as a substrate for production of thermostable α-amylase by Bacillus stearothermophilus. Bioresour. Technol. Rep. 2019, 5, 141–149. [Google Scholar] [CrossRef]
- Araújo, D.; Costa, T.; Freitas, F. Biovalorization of lignocellulosic materials for xylitol production by the yeast Komagataella pastoris. Appl. Sci. 2021, 11, 5516. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.C.; Rodrigues, L.R. One-step process for producing prebiotic arabino-xylooligosaccharides from brewer’s spent grain employing Trichoderma species. Food Chem. 2019, 270, 86–94. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.C.; Silva, S.P.; Coelho, E.; Coimbra, M.A.; Prather, K.L.; Rodrigues, L.R. Single-step production of arabino-xylooligosaccharides by recombinant Bacillus subtilis 3610 cultivated in brewers’ spent grain. Carbohydr. Polym. 2018, 199, 546–554. [Google Scholar] [CrossRef]
- Zhang, W.; Roy, S.; Assadpour, E.; Cong, X.; Jafari, S.M. Cross-linked biopolymeric films by citric acid for food packaging and preservation. Adv. Colloid Interface Sci. 2023, 314, 102886. [Google Scholar] [CrossRef]
- Díaz, A.B.; González, C.; Marzo, C.; Caro, I.; Blandino, A. Feasibility of exhausted sugar beet pulp as raw material for lactic acid production. J. Sci. Food Agric. 2020, 100, 3036–3045. [Google Scholar] [CrossRef] [PubMed]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Effect of several pretreatments on the lactic acid production from exhausted sugar beet pulp. Foods 2021, 10, 2414. [Google Scholar] [CrossRef] [PubMed]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Valorisation of fungal hydrolysates of exhausted sugar beet pulp for lactic acid production. J. Sci. Food Agric. 2021, 101, 4108–4117. [Google Scholar] [CrossRef]
- Ascencio, J.J.; Philippini, R.R.; Gomes, F.M.; Pereira, F.M.; da Silva, S.S.; Kumar, V.; Chandel, A.K. Comparative highly efficient production of β-glucan by Lasiodiplodia theobromae CCT 3966 and its multiscale characterization. Fermentation 2021, 7, 108. [Google Scholar] [CrossRef]
- Matrawy, A.A.; Khalil, A.I.; Marey, H.S.; Embaby, A.M. Use of wheat straw for value-added product xylanase by Penicillium chrysogenum strain A3 DSM105774. J. Fungi 2021, 7, 696. [Google Scholar] [CrossRef]
- Shahryari, Z.; Fazaelipoor, M.H.; Setoodeh, P.; Nair, R.B.; Taherzadeh, M.J.; Ghasemi, Y. Utilization of wheat straw for fungal phytase production. Int. J. Recycl. Org. Waste Agric. 2018, 7, 345–355. [Google Scholar] [CrossRef]
- Ketsakhon, P.; Thammasittirong, A.; Thammasittirong, S.N.R. Adding value to rice straw waste for high-level xylanase production using a new isolate of Bacillus altitudinis RS3025. Folia Microbiol. 2022, 68, 87–99. [Google Scholar] [CrossRef]
- Ismail, S.A.; Hassan, A.A. Optimizing the production of rice straw hydrolytic cellulase under solid-state fermentation using Aspergillus terreus RS2. Egypt. Pharm. J. 2020, 19, 7. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, D.; Yadav, S.K.; Krishania, M. Process scale-up of an efficient acid-catalyzed steam pretreatment of rice straw for xylitol production by C. tropicalis MTCC 6192. Bioresour. Technol. 2021, 320, 124422. [Google Scholar] [CrossRef]
- Drzymała, K.; Mirończuk, A.M.; Pietrzak, W.; Dobrowolski, A. Rye and oat agricultural wastes as substrate candidates for biomass production of the non-conventional yeast Yarrowia lipolytica. Sustainability 2020, 12, 7704. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Bilal, M.; Chandra, R. Sustainable production of thermostable laccase from agro-residues waste by Bacillus aquimaris AKRC02. Catal. Lett. 2022, 152, 1784–1800. [Google Scholar] [CrossRef]
- Elegbede, J.A.; Lateef, A. Valorization of corn-cob by fungal isolates for production of xylanase in submerged and solid state fermentation media and potential biotechnological applications. Waste Biomass Valorization 2018, 9, 1273–1287. [Google Scholar] [CrossRef]
- Yao, F.; Liu, S.C.; Wang, D.N.; Liu, Z.J.; Hua, Q.; Wei, L.J. Engineering oleaginous yeast Yarrowia lipolytica for enhanced limonene production from xylose and lignocellulosic hydrolysate. FEMS Yeast Res. 2020, 20, foaa046. [Google Scholar] [CrossRef]
- Bi, H.; Xv, C.; Su, C.; Feng, P.; Zhang, C.; Wang, M.; Fang, Y.; Tan, T. β-Farnesene production from low-cost glucose in lignocellulosic hydrolysate by engineered Yarrowia lipolytica. Fermentation 2022, 8, 532. [Google Scholar] [CrossRef]
- Kirby, J.; Geiselman, G.M.; Yaegashi, J.; Kim, J.; Zhuang, X.; Tran-Gyamfi, M.B.; Prahl, J.-P.; Sundstrom, E.R.; Gao, Y.; Munoz, N.; et al. Further engineering of R. toruloides for the production of terpenes from lignocellulosic biomass. Biotechnol. Biofuels 2021, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Yang, R.; Liu, X.; Liu, X.; Chen, H. Green synthesis of bacterial cellulose via acetic acid pre-hydrolysis liquor of agricultural corn stalk used as carbon source. Bioresour. Technol. 2017, 234, 8–14. [Google Scholar] [CrossRef]
- Mardawati, E.; Hartono, A.T.; Nurhadi, B.; Fitriana, H.N.; Hermiati, E.; Ermawar, R.A. Xylitol production from pineapple cores (Ananas comosus (L.) Merr) by enzymatic and acid hydrolysis using microorganisms Debaryomyces hansenii and Candida tropicalis. Fermentation 2022, 8, 694. [Google Scholar] [CrossRef]
- Patelski, P.; Berłowska, J.; Balcerek, M.; Dziekońska-Kubczak, U.; Pielech-Przybylska, K.; Dygas, D.; Jędrasik, J. Conversion of potato industry waste into fodder yeast biomass. Processes 2020, 8, 453. [Google Scholar] [CrossRef]
- Mahmood, S.; Shahid, M.G.; Irfan, M.; Nadeem, M.; Syed, Q. Partial characterization of α-amylase produced from Aspergillus niger using potato peel as substrate. Punjab Univ. J. Zool. 2018, 33, 22–27. [Google Scholar] [CrossRef]
- Mukherjee, R.; Paul, T.; Soren, J.P.; Halder, S.K.; Mondal, K.C.; Pati, B.R.; Das Mohapatra, P.K. Acidophilic α-amylase production from Aspergillus niger RBP7 using potato peel as substrate: A waste to value added approach. Waste Biomass Valorization 2019, 10, 851–863. [Google Scholar] [CrossRef]
- Olakusehin, V.O.; Oyedeji, O. Production of α-amylase from Aspergillus flavus S2-OY using solid substrate fermentation of potato (Solanum tuberosum L.) peel. Int. J. Biol. Chem. Sci. 2021, 15, 1950–1967. [Google Scholar] [CrossRef]
- Niyomukiza, S.; Owino, W.; Maina, J.M.; Maina, N.; Issifu, M. Concomitant production of α-amylase and protease by Bacillus aerius strain FPWSHA isolated from food wastes. Biointerface Res. Appl. Chem. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Tuysuz, E.; Gonul-Baltaci, N.; Omeroglu, M.A.; Adiguzel, A.; Taskin, M.; Ozkan, H. Co-production of amylase and protease by locally isolated thermophilic bacterium Anoxybacillus rupiensis T2 in sterile and non-sterile media using waste potato peels as substrate. Waste Biomass Valorization 2020, 11, 6793–6802. [Google Scholar] [CrossRef]
- Ahmed, N.E.; Salem, S.S.; Hashem, A.H. Statistical optimization, partial purification, and characterization of phytase produced from Talaromyces purpureogenus NSA20 using potato peel waste and its application in dyes de-colorization. Biointerface Res. Appl. Chem. 2021, 12, 4417–4431. [Google Scholar] [CrossRef]
- Abdelraof, M.; Hasanin, M.S.; El-Saied, H. Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef]
- Tian, M.; Yuan, Q. Optimization of phytase production from potato waste using Aspergillus ficuum. 3 Biotech 2016, 6, 256. [Google Scholar] [CrossRef]
- Roukas, T.; Kotzekidou, P. Pomegranate peel waste: A new substrate for citric acid production by Aspergillus niger in solid-state fermentation under non-aseptic conditions. Environ. Sci. Pollut. Res. 2020, 27, 13105–13113. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Kapilan, R.; Merah, O.; Madhujith, T. Production of single-cell protein from fruit peel wastes using palmyrah toddy yeast. Fermentation 2022, 8, 355. [Google Scholar] [CrossRef]
- Atalla, S.M.; El Gamal, N.G. Production and characterization of xylanase from pomegranate peel by Chaetomium globosum and its application on bean under greenhouse condition. Bull. Natl. Res. Cent. 2020, 44, 104. [Google Scholar] [CrossRef]
- Pathania, S.; Sharma, S.; Kumari, K. Solid state fermentation of BSG for citric acid production. Indian J. Nat. Prod. Resour. 2018, 9, 70–74. [Google Scholar]
- Barathikannan, K.; Khusro, A.; Paul, A. Simultaneous production of xylitol and ethanol from different hemicellulose waste substrates by Candida tropicalis strain LY15. J. Bioprocess. Biotech. 2016, 6, 2. [Google Scholar] [CrossRef]
- Gooruee, R.; Hojjati, M.; Behbahani, B.A.; Shahbazi, S.; Askari, H. Extracellular enzyme production by different species of Trichoderma fungus for lemon peel waste bioconversion. Biomass Convers. Biorefinery 2022, 14, 2777–2786. [Google Scholar] [CrossRef]
- Yang, G.; Tan, H.; Li, S.; Zhang, M.; Che, J.; Li, K.; Chen, W.; Yin, H. Application of engineered yeast strain fermentation for oligogalacturonides production from pectin-rich waste biomass. Bioresour. Technol. 2020, 300, 122645. [Google Scholar] [CrossRef] [PubMed]
- Nasoha, N.Z.; Luthfi, A.A.I.; Roslan, M.F.; Hariz, H.B.; Bukhari, N.A.; Manaf, S.F.A. Exploring pineapple peel hydrolysate as a sustainable carbon source for xylitol production. Sci. Rep. 2023, 13, 19284. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Preparation and characterization of bacterial cellulose produced from fruit and vegetable peels by Komagataeibacter hansenii GA2016. Int. J. Biol. Macromol. 2020, 162, 1597–1604. [Google Scholar] [CrossRef]
- Kotarska, K.; Świerczyńska, A.; Dziemianowicz, W. Study on the decomposition of lignocellulosic biomass and subjecting it to alcoholic fermentation: Study on the decomposition of lignocellulosic biomass. Renew Energy 2015, 75, 389–394. [Google Scholar] [CrossRef]
- Ochoa-Chacón, A.; Martinez, A.; Poggi-Varaldo, H.M.; Villa-Tanaca, L.; Ramos-Valdivia, A.C.; Ponce-Noyola, T. Xylose metabolism in bioethanol production: Saccharomyces cerevisiae vs non-Saccharomyces yeasts. BioEnergy Res. 2021, 15, 905–923. [Google Scholar] [CrossRef]
- Persson, M.; Galbe, M.; Wallberg, O. Mitigation of pretreatment-derived inhibitors during lignocellulosic ethanol fermentation using spent grain as a nitrogen source. Biomass Convers. Biorefinery 2023, 13, 3349–3360. [Google Scholar] [CrossRef]
- Łaba, W.; Piegza, M.; Kawa-Rygielska, J. Evaluation of brewer’s spent grain as a substrate for production of hydrolytic enzymes by keratinolytic bacteria. J. Chem. Technol. Biotechnol. 2017, 92, 1389–1396. [Google Scholar] [CrossRef]
- Tišma, M.; Jurić, A.; Bucić-Kojić, A.; Panjičko, M.; Planinić, M. Biovalorization of brewers’ spent grain for the production of laccase and polyphenols. J. Inst. Brew. 2018, 124, 182–186. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Echeta, K.C. Current developments in sugar alcohols: Chemistry, nutrition, and health concerns of sorbitol, xylitol, glycerol, arabitol, inositol, maltitol, and lactitol. Int. J. Adv. Acad. Res. 2019, 5, 1–33. [Google Scholar]
- Samanta, A.K.; Jayapal, N.; Jayaram, C.; Roy, S.; Kolte, A.P.; Senani, S.; Sridhar, M. Xylooligosaccharides as prebiotics from agricultural by-products: Production and applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 62–71. [Google Scholar] [CrossRef]
- Medina-Córdova, N.; Reyes-Becerril, M.; Ascencio, F.; Castellanos, T.; Campa-Córdova, A.I.; Angulo, C. Immunostimulant effects and potential application of β-glucans derived from marine yeast Debaryomyces hansenii in goat peripheral blood leucocytes. Int. J. Biol. Macromol. 2018, 116, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Omara, I.I.; Pender, C.M.; White, M.B.; Dalloul, R.A. The modulating effect of dietary beta-glucan supplementation on expression of immune response genes of broilers during a coccidiosis challenge. Animals 2021, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Harriett, A.J.; Esher Righi, S.; Lilly, E.A.; Fidel, P., Jr.; Noverr, M.C. Efficacy of Candida dubliniensis and fungal β-glucans in inducing trained innate immune protection against inducers of sepsis. Front. Cell. Infect. Microbiol. 2022, 12, 898030. [Google Scholar] [CrossRef]
- Chaikliang, C.; Wichienchot, S.; Youravoug, W.; Graidist, P. Evaluation on prebiotic properties of β-glucan and oligo-β-glucan from mushrooms by human fecal microbiota in fecal batch culture. Funct. Foods Health Dis. 2015, 5, 395–405. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Bakry, A.M.; Xiong, S.; Yin, T.; Zhang, B.; Huang, J.; Liu, Z.; Huang, Q. Effect of yeast β-glucan on gel properties, spatial structure and sensory characteristics of silver carp surimi. Food Hydrocoll. 2019, 88, 256–264. [Google Scholar] [CrossRef]
- Aljewicz, M.; Mulet-Cabero, A.I.; Wilde, P.J.A. comparative study of the influence of the content and source of β-glucan on the rheological, microstructural properties and stability of milk gel during acidification. Food Hydrocoll. 2021, 113, 106486. [Google Scholar] [CrossRef]
- Khan, A.A.; Gani, A.; Khanday, F.A.; Masoodi, F.A. Biological and pharmaceutical activities of mushroom β-glucan discussed as a potential functional food ingredient. Bioact. Carbohydr. Diet. Fibre 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Rajwade, J.M.; Paknikar, K.M.; Kumbhar, J.V. Applications of bacterial cellulose and its composites in biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef]
- Azeredo, H.M.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial cellulose as a raw material for food and food packaging applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Brodnjak, U.V.; Sáha, P. Bacterial cellulose and guar gum based modified PVP-CMC hydrogel films: Characterized for packaging fresh berries. Food Packag. Shelf Life 2019, 22, 100402. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Patanè, G.T.; Corrado, I.; Giosafatto, C.V.L.; Ginestra, G.; Nostro, A.; Foti, A.; Gucciardi, P.G.; Mandalari, G.; Barreca, D.; et al. Functionalization of polyhydroxyalkanoates (PHA)-based bioplastic with phloretin for active food packaging: Characterization of its mechanical, antioxidant, and antimicrobial activities. Int. J. Mol. Sci. 2023, 24, 11628. [Google Scholar] [CrossRef]
- Al-Rousan, W.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Ajo, R.Y.; Holley, R.A. Use of acetic and citric acids to inhibit Escherichia coli O157: H7, Salmonella Typhimurium and Staphylococcus aureus in tabbouleh salad. Food Microbiol. 2018, 73, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Heir, E.; Solberg, L.E.; Jensen, M.R.; Skaret, J.; Grøvlen, M.S.; Holck, A.L. Improved microbial and sensory quality of chicken meat by treatment with lactic acid, organic acid salts and modified atmosphere packaging. Int. J. Food Microbiol. 2022, 362, 109498. [Google Scholar] [CrossRef]
- Hossain, M.M.; Jayaraman, B.; Kim, S.C.; Lee, K.Y.; Kim, I.H.; Nyachoti, C.M. Effects of a matrix-coated organic acids and medium-chain fatty acids blend on performance, and in vitro fecal noxious gas emissions in growing pigs fed in-feed antibiotic-free diets. Can. J. Anim. Sci. 2018, 98, 433–442. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lee, K.Y.; Mohammadigheisar, M.; Kim, I.H. Evaluation of the blend of organic acids and medium-chain fatty acids in matrix coating as antibiotic growth promoter alternative on growth performance, nutrient digestibility, blood profiles, excreta microflora, and carcass quality in broilers. Poult. Sci. 2018, 97, 4351–4358. [Google Scholar] [CrossRef]
- Aboyeji, O.O.; Oloke, J.K.; Arinkoola, A.O.; Oke, M.A.; Ishola, M.M. Optimization of media components and fermentation conditions for citric acid production from sweet potato peel starch hydrolysate by Aspergillus niger. Sci. Afr. 2020, 10, e00554. [Google Scholar] [CrossRef]
- Taib, N.A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Muller, J.; González-Martínez, C.; Chiralt, A. Poly (lactic) acid (PLA) and starch bilayer films, containing cinnamaldehyde, obtained by compression moulding. Eur. Polym. J. 2017, 95, 56–70. [Google Scholar] [CrossRef]
- Stanojević-Nikolić, S.; Dimić, G.; Mojović, L.; Pejin, J.; Djukić-Vuković, A.; Kocić-Tanackov, S. Antimicrobial activity of lactic acid against pathogen and spoilage microorganisms. J. Food Process. Preserv. 2016, 40, 990–998. [Google Scholar] [CrossRef]
- Moldovan, A.; Cuc, S.; Prodan, D.; Rusu, M.; Popa, D.; Taut, A.C.; Petean, I.; Bomboş, D.; Doukeh, R.; Nemes, O. Development and characterization of polylactic acid (PLA)-based nanocomposites used for food packaging. Polymers 2023, 15, 2855. [Google Scholar] [CrossRef] [PubMed]
- Koziol, A.; Stryjewska, A.; Librowski, T.; Salat, K.; Gawel, M.; Moniczewski, A.; Lochynski, S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini-Rev. Med. Chem. 2014, 14, 1156–1168. [Google Scholar] [CrossRef]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef]
- Fan, M.; Yuan, S.; Li, L.; Zheng, J.; Zhao, D.; Wang, C.; Wang, H.; Liu, X.; Liu, J. Application of terpenoid compounds in food and pharmaceutical products. Fermentation 2023, 9, 119. [Google Scholar] [CrossRef]



| Literature Query | Number of Results | Literature Query | Number of Results | Literature Query | Number of Results |
|---|---|---|---|---|---|
| Lignocellulose | 15,032 | Lignocellulosic waste | 2776 | Biotechnological use of lignocellulose | 606 |
| Brewer’s spent hops | 5 | Brewer’s spent hops waste | 2 | Biotechnological use of brewer’s spent hops | No results |
| Brewer’s spent grain | 402 | Brewer’s spent grain wastes | 163 | Biotechnological use of brewer’s spent grain | 16 |
| Straw | 16,620 | Straw waste | 2410 | Biotechnological use of straw | 111 |
| Wheat straw | 4658 | Wheat straw waste | 849 | Biotechnological use of wheat straw | 64 |
| Rice straw | 4529 | Rice straw waste | 987 | Biotechnological use of rice straw | 28 |
| Quinoa straw | 18 | Quinoa straw waste | 3 | Biotechnological use of quinoa straw | No results |
| Rapeseed straw | 188 | Rapeseed straw waste | 36 | Biotechnological use of rapeseed straw | 1 |
| Sugarcane straw | 398 | Sugarcane straw waste | 126 | Biotechnological use of sugarcane straw | 12 |
| Sugarcane bagasse | 2404 | Sugarcane bagasse waste | 630 | Biotechnological use of sugarcane bagasse | 89 |
| Oat bran | 655 | Oat bran waste | 13 | Biotechnological use of oat bran | 2 |
| Rice bran | 3104 | Rice bran waste | 250 | Biotechnological use of rice bran | 17 |
| Corn cob | 535 | Corn cob waste | 158 | Biotechnological use of corn cob | 10 |
| Corn stover | 2011 | Corn stover waste | 301 | Biotechnological use of corn stover | 16 |
| Corn stalk | 1524 | Corn stalk waste | 202 | Biotechnological use of corn stalk | 8 |
| Pineapple cores | 8 | Pineapple core waste | 2 | Biotechnological use of pineapple cores | No results |
| Starch | 86,431 | Starch wastes | 1859 | Biotechnological use of starch | 423 |
| Potato peels | 321 | Potato peel wastes | 101 | Biotechnological use of potato peels | 4 |
| Potato pulp | 203 | Potato pulp waste | 36 | Biotechnological use of Potato pulp | 1 |
| Sweet potato peel | 52 | Sweet potato peel waste | 13 | Biotechnological use of sweet potato peel | No results |
| Pectin | 14,351 | Pectin waste | 576 | Biotechnological use of pectin | 141 |
| Sugar beet pulp | 691 | Sugar beet pulp waste | 110 | Biotechnological use of sugar beet pulp | 15 |
| Papaya peels | 49 | Papaya peel waste | 19 | Biotechnological use of papaya peels | No results |
| Pomegranate peel | 904 | Pomegranate peel waste | 155 | Biotechnological use of pomegranate peel | 2 |
| Lemon peel | 2345 | Lemon peel waste | 456 | Biotechnological use of lemon peel | 13 |
| Mandarin peel | 215 | Mandarin peel waste | 36 | Biotechnological use of mandarin peel | 1 |
| Orange peel | 1487 | Orange peel waste | 378 | Biotechnological use of orange peel | 8 |
| Pineapple peel | 183 | Pineapple peel waste | 83 | Biotechnological use of pineapple peel | No results |
| Kiwi peel | 27 | Kiwi peel waste | 11 | Biotechnological use of kiwi peel | 1 |
| Component | Dry Mass Content Range/% | ||
|---|---|---|---|
| Raw Potato Peel Solanum tuberosum Variety Norchip [62] | Dried Potato Peel [61] | ||
| Hand Peeled | Peeled by Grating | Peeled by Abrasion | |
| Cellulose | 8.0 | 21.0 | 8.3 |
| Hemicellulose | ― | ― | 7.41 |
| Lignin | 6.2 | 19.0 | 32.88 |
| Protein | 17.1 | 10.5 | 10.73 |
| Starch | 12.0 | 9.0 | 23.01 |
| Ash | 8.4 | 5.0 | 7.45 |
| Water | 4.9 | 2.5 | ― |
| Lipids | 0 | 0 | 2.45 |
| Waste | Group of Compounds | Products | Productivity | Composition of the Medium | Microorganism | Reference |
|---|---|---|---|---|---|---|
| Brewer’s spent grain (BSG) | Cell biomass/single-cell protein | Fungal biomass concentration | 5.1 g L−1 | Organosolv liquor with dry matter content 9.4 g L−1 and with composition (dry mass/%) of total lignin 5.06 ± 0.05; glucan 37 ± 2; xylan 5.7 ± 0.3; galactan 0.90 ± 0.08; arabinan 0.89 ± 0.07; protein 26.5 ± 0.7 | Aspergillus oryzae var. oryzae CBS 819.72 | [63] |
| Single-cell protein (SCP) | 44.8 ± 0.7% | |||||
| Enzymes | Xylanase | 518.2 U mL−1 | [g L−1]: carbohydrates 40 (including 4% [w/v] d-xylose and beechwood xylan; 11% [w/v] BSG); MgSO4 0.3; KH2PO4 0.3; yeast extract 1 | Moesziomyces aphidis PYCC 5535 | [64] | |
| 67 U g−1 | The basic medium [g L−1]: KH2PO4 2.0; (NH4)2SO4 1.4; urea 0.3; MgSO4⋅7H2O 0.3; CaCl2 0.3; [mg L−1]: FeSO4⋅7H2O 5.0; MnSO4⋅H2O 1.56; ZnSO4⋅7H2O 1.4; CoCl2 2.0; BSG 15 g/250 mL; pH 6 | Mucor sp. (AB1) | [65] | |||
| 300–313 U g−1 | 2 g of dry substrate; solid medium with moisture level adjusted to 75% (wet basis) with distilled water and ratio C/N fixed to 15 (value of BSG) | Aspergillus ibericus | [66] | |||
| 1315.15 ± 37.5 U g−1 | 25 g of dry BSG/250 mL solution moisture adjusted to 70% by adding enough volume of distilled water and a mineral salt solution containing [g L−1] KH2PO4 1.5; CuSO4 0.4; CoSO4 0.0012 | Aspergillus niger LPB-334 | [67] | |||
| 268 ± 24 U g−1 | Preparation of BSG for solid-state fermentation: adding 2 × 2 cm sponge pieces as a bulking agent (3% w/w) then distilled water to reach 78 ± 2% moisture content (MC) | Aspergillus niger ATCC 16888 | [68] | |||
| 241 ± 10 U g−1 | Thermoascus aurantiacus ATCC 26904 | |||||
| 3152.39 ± 20.88 U g−1 | 400 g BSG (dry weight) moistened with a mineral salts solution containing [g L−1] 1.3 (NH4)2SO4; 5.0 NaNO3; 4.5 KH2PO4; 3 yeast extract in the proportion 1:2.5 [w/v] | Aspergillus brasiliensis CECT 2700 | [69] | |||
| 370.0 ± 30.1 U g−1 | 5 g dried BSG; 50% initial moisture, provided by Vogel’s salt solution | Penicillium janczewskii CRM 1348 | [70] | |||
| Pectinase | 137 U g−1 | The basic medium [g L−1]: KH2PO4 2.0; (NH4)2SO4 1.4; urea 0.3; MgSO4⋅7H2O 0.3; CaCl2 0.3; [mg L−1]: FeSO4⋅7H2O 5.0; MnSO4⋅H2O 1.56; ZnSO4⋅7H2O 1.4; CoCl2 2.0; BSG 15 g/250 mL; pH 6 | Mucor sp. (AB1) | [65] | ||
| Cellulase | 51–62 U g−1 | 2 g of dry substrate; solid medium with moisture level adjusted to 75% (wet basis) with distilled water and ratio C/N fixed to 15 (value of BSG) | Aspergillus ibericus | [66] | ||
| 118.04 ± 8.4 U g−1 | 25 g of dry BSG/250 mL solution; moisture adjusted to 70% by adding enough volume of distilled water and a mineral salt solution containing [g L−1] KH2PO4 1.5; CuSO4 0.4; CoSO4 0.0012 | Aspergillus niger LPB-334 | [67] | |||
| 7.26 ± 0.13 U g−1 | 400 g BSG (dry weight) moistened with a mineral salt solution containing [g L−1] 1.3 (NH4)2SO4; 5.0 NaNO3; 4.5 KH2PO4; 3 yeast extract in the proportion 1:2.5 [w/v] | Aspergillus brasiliensis CECT 2700 | [71] | |||
| Laccase | 560 U L−1 | A 50 g aliquot of BSG, particle size 2–3 mm, mixed with 10 mL of distilled water | Trametes versicolor TV-6 | [72] | ||
| β-Glucosidase | 94 ± 4 U g−1 | 2 g of dry substrate; solid medium with moisture level adjusted to 75% (wet basis) with distilled water and ratio C/N fixed to 15 (value of BSG) | Aspergillus niger CECT 2088 | [73] | ||
| 19.02 ± 0.04 U g−1 | 400 g BSG (dry weight) moistened with a mineral salt solution containing [g L−1] 1.3 (NH4)2SO4; 5.0 NaNO3; 4.5 KH2PO4; 3 yeast extract in the proportion 1:2.5 [w/v] | Aspergillus brasiliensis CECT 2700 | [69] | |||
| β-Xylosidase | 246.5 ± 14.7 mU g−1 | 5 g dried BSG; 50% initial moisture provided by Vogel’s salt solution | Penicillium janczewskii CRM 1348 | [70] | ||
| α-Amylase | 198.09 U mL−1 | [% w/v] starch 0.2; peptone 0.2; KCl⋅4H2O 0.02; MgSO4·7H2O 0.01; BSG hydrolysate (0.22% v/v) | Bacillus stearothermophilus LZT020 | [74] | ||
| Ferulic acid esterase | 1.05 ± 0.06 U g−1 | 400 g BSG (dry weight) moistened with a mineral salt solution containing [g L−1] 1.3 (NH4)2SO4; 5.0 NaNO3; 4.5 KH2PO4; 3 yeast extract in the proportion 1:2.5 [w/v] | Aspergillus brasiliensis CECT 2700 | [69] | ||
| α-L-Arabinofuranosidase | 674.8 ± 30.9 mU g−1 | 5 g dried BSG; 50% initial moisture provided by Vogel’s salt solution | Penicillium janczewskii CRM 1348 | [70] | ||
| Polyhydroxy alcohols | Xylitol | 3.97 ± 0.10 g L−1 | Detoxified BSG hydrolysates ([g L−1]: glucose 9.57 ± 1.76; xylose 13.00 ± 4.74; arabinose 8.85 ± 2.55) were used as cultivation medium supplemented with ammonium sulfate (10 mL, 13.6 g L−1) | Komagataella pastoris DSM 70877 | [75] | |
| Arabitol | 0.82 ± 0.05 g L−1 | |||||
| Oligosaccharides | Arabino-xylooligosacharide (AXOS) | 38.3 ± 1.8 mg g−1 | 20 g L−1 BSG in 2% [w/v] Vogel media; pH 7.0 | Trichoderma reesei MUM 9753 | [76] | |
| 54.24 ± 1.10 mg g−1 | recombinant Bacillus subtilis 3610 | [77] | ||||
| Biopolymers | Polyhydroxyalkanoate (PHA) | 9.0 ± 0.44 mg g−1 | 20 mL hydrolysates BSG; [g L−1] 1 (NH4)2SO4; 1.5 KH2PO4; 9.02 Na2HPO4⋅12H2O; 0.1 CaCl2⋅2H2O; 0.2 MgSO4⋅7H2O; 1 mL L−1 of microelement solution [g L−1]: 0.1 ZnSO4⋅7H2O; 0.03 MnCl2⋅4H2O; 0.3 H3BO3; 0.2 CoCl2; 0.02 CuSO4⋅7H2O; 0.02 NiCl2⋅6H2O; 0.03 Na2MoO4⋅2H2O | Cupriavidus necator DSM428 | [68] | |
| Carboxylic acids | Citric acid | 0.12–0.23 g 100 g−1 | 150 mL of fermentation medium [g L−1]: peptone 2; yeast extract 1.5: potassium dihydrogen phosphate 2; magnesium sulfate 2; ammonium sulphate 2; brewer’s spent grain 30 g, containing water in 1:5 ratio; pH 5.5 | Aspergillus niger MTCC 281 | [78] | |
| Sugar beet pulp (SBP) | Enzymes | Endo-polygalacturonase | 54.44 ± 1.4% | [g L−1]: SBP powder 30; (NH4)2HPO4 2; NH4H2PO4 0.9; MgSO4 0.1; KCl 0.5; pH 5.6 and 7.0 | Aspergillus niger AUMC 4156 | [71] |
| 52.94 ± 2.0% | Penicillium oxalicum AUMC 4153 | |||||
| Exhausted sugar beet pulp | Carboxylic acids | Lactic acid | 26.88 ± 0.69 g L−1 | 50 mL phosphate buffer (50 mmol L−1, pH 6.5);10 mL MRS media; 6 g exhausted sugar beet pulp pellets (ESBPP) hydrolysates; 30 g L−1 CaCO3 | Lactobacillus casei 2246 | [79] |
| 50 g L−1 | 60 mL ESBP hydrolysates; [g L−1] 5 yeast extract; 18 CaCO3; pH 6.5 | Lactiplantibacillus plantarum CECT 748 | [80] | |||
| 30 g L−1 | 50 mL ESBPP hydrolysate; 5 g L−1 yeast extract; different concentrations of CaCO3 (9.0 or 18.0 or 27.0 g L−1) | Lactobacillus plantarum CECT 748 | [81] | |||
| Sugarcane bagasse (SCB) | Biopolymer | Lasiodiplodan ((1→6)-β-d-glucan) | 22.0 g L−1 | 50 mL of medium (40 g L−1 sugarcane bagasse cellulosic hydrolysate (SCBCH); 10 g L−1 rice bran extract (RBE)) | Lasiodiplodia theobromae CCT 3966 | [82] |
| 16.2 g L−1 | 50 mL of medium (40 g L−1 sugarcane bagasse cellulosic hydrolysate (SCBCH); 10 g L−1 soybean bran extract (SBE)) | |||||
| Birch (Betula pendula) | Cell biomass | Yeast biomass | 1.1 OD | Lignocellulosic hydrolysate-enriched C5-sugars. Dilutions made using minimal medium ([g L−1] monopotassium phosphate 3; magnesium sulfate, 0.5); C/N ratio adjusted by modifying concentration of added ammonium sulfate; pH 6.0 | Candida parapsilosis DSM 70125 | [10] |
| 1.0 OD | Kluyveromyces marxianus CBS 6556 | |||||
| Sugarcane straw | Enzymes | Xylanase | 90.2 U mL−1 | [m/v]: 3.0% pretreated sugarcane straw; 0.1% (NH4)2SO4; 0.0017% MgSO4·7H2O; 0.1% K2HPO4; 0.0028% ZnSO4; 0.1% NH4H2PO4; 0.06% KCl; 0.1% yeast extract; 0.1% sucrose; pH 4.5 | Trichoderma reesei QM9414 | [72] |
| Cellulase | 0.5 FPU mL−1 | |||||
| Biopolymer | β-Glucan | 4.7% | [g L−1]: SCS hydrolysate (40 glucose concentration); 2 KH2PO4; 2 MgSO4 · 7 H2O; 1 yeast extract; pH 7.0 | Lasiodiplodia theobromae CCT3966 | [57] | |
| Wheat straw | Enzymes | Xylanase | 53.7 U mL−1 | [g L−1]: (NH4)2SO4 1.3; KH2PO4 0.37; MgSO4⋅7H2O 0.25; CaCl2⋅2H2O 0.07; FeCl3 0.02; yeast extract 1.0; beechwood xylan 0.5% [w/v], 2% [w/v] agar | Penicillium chrysogenum A3 DSM105774 | [83] |
| Phytase | 16.46 ± 0.56 U g−1 of dry substrate | Wheat straw 5 g dry weight; [g g−1 of dry substrate]: glucose 0.17; (NH4)2SO4 0.068; [g kg−1] 655 moisture | Aspergillus ficuum PTCC 5288 | [84] | ||
| Rice straw | Enzymes | Xylanase | 6721.9 U g−1 of dry substrate | Rice straw waste 5 g; Mandel Weber medium (77.5% initial moisture content; [g L−1]: 1.4 (NH4)2SO4; 2.0 KH2PO4; 0.3 CaCl2; 0.3 MgSO4⋅7H2O; 0.02 Tween-80; 0.005 FeSO4⋅7H2O; 0.0016 MnSO4⋅7H2O; 0.0014 ZnSO4⋅7H2O; 0.002 CoCl2⋅6H2O) | Schizophyllum commune ARC-11 | [73] |
| Xylanase | 2518.51 U mL−1 | [g L−1]: 5 yeast extract; 1 peptone; 1 NaNO3; 1 KH2PO4; 0.02 MgSO4⋅7H2O; 10 rice straw | Bacillus altitudinis RS3025 | [85] | ||
| Cellulase | 124.94 U g−1 | 3.75 g (1.5% w/v) of rice straw moistened with 11.25 mL (1:3 biomass to moistening agent) of modified Mandel’s medium ([g L−1]: (NH4)2SO4 1.4; KH2PO4 2; urea 0.63; CaCl2 0.3; MgSO4⋅7H2O 0.3; peptone 0.75; 1 mL of trace element solution); pH 7 | Aspergillus terreus RS2 | [86] | ||
| Polyhydroxy alcohols | Xylitol | 25.8 g L−1 | Rice straw hydrolysate [g L−1]: xylose 45.0; yeast extract 0.5; peptone 0.5; antifoam agent 60–100 μL; pH 5 | Candida tropicalis MTCC 6192 | [87] | |
| Quinoa straw | Polyhydroxy alcohols | Xylitol | 26.05 ± 0.31 (g L−1) (0.5 g g−1) | Detoxified hydrolysate; yeast extract 5 g L−1; tryptone 4 g L−1; pH 5.5 | Candida tropicalis CICC 1779 | [25] |
| Rapeseed straw | Polyhydroxy alcohols | Xylitol | 0.55 g g−1 | Detoxified hydrolysate [g L−1]: glucose 11.68 ± 0.09; xylose 40.59 ± 0.21; galactose 8.67 ± 0.12; arabinose 6.79 ± 0.09; mannose 2.22 ± 0.06 | Candida guilliermondii FTI 20037 (ATCC 201 935) | [60] |
| 0.45 g g−1 | Debaryomyces hansenii (NRRL Y-7426) | |||||
| Oat bran | Cell biomass | Yeast biomass | 9.35 ± 0.55 g L−1 | 50 mL of hydrolysate; 6.7 g L−1 Yeast Nitrogen Base (Sigma-Aldrich). | Yarrowia lipolytica A101 | [88] |
| Rice bran | Enzymes | Laccase | 4.58 U mL−1 | 2 g rice bran; 100 mL mineral basal salt solution (MBSS) [g L−1]: dextrose 10.0; peptone 3.0; K2HPO4 0.4; ZnSO4 0.01; MnSO4 0.5; KH2PO4 0.6; FeSO4 0.0005; MnSO4 0.5 | Bacillus aquimaris AKRC02 | [89] |
| Corn cob | Enzymes | Xylanase | 50.55 U mL−1 | [g L−1]: corn cob 20.0; MgSO4 2.0; NaNO3 1.4; KH2PO4 1.8; NH4Cl 2.0; CaCO3 1.2 | Aspergillus fumigatus SD5A | [90] |
| 48.63 U g−1 | Aspergillus fumigatus L1 | |||||
| Corn stover | Terpenes | Limonene | 20.57 mg L−1 | YPBiomass medium [g L−1]: 10 yeast extract; 20 peptone; 50% detoxified lignocellulosic hydrolysate (v/v) | Engineered Yarrowia lipolytica | [91] |
| β-Farnesene | 7.38 ± 0.24 g L−1 | 500 mL of the initial 100% lignocellulosic hydrolysate media (40.3 ± 0.4 g L−1 glucose, 14.7 ± 0.3 g L−1 xylose, 5 mM magnesium sulfate, pH 6.0) | Engineered Yarrowia lipolytica ATCC MYA2613 | [92] | ||
| 1,8-Cineole | 1.4 g L−1 | Lignocellulosic hydrolysate derived from corn stover; 5 g L−1 ammonium sulfate; 100 µM iron sulfate; 100 mM potassium phosphate | Rhodosporidium toruloides | [93] | ||
| α-Bisabolene | 2.6 g L−1 | |||||
| Corn stalk | Biopolymer | Bacterial cellulose | 2.86 g L−1 | Detoxified hydrolysate; [%] 0.5 baco-peptone; 0.5 yeast extract; 1.5 D-mannitol; 0.2 magnesium sulfate; 0.5 anhydrous ethanol; pH 68 | Acetobacter xylinum ATCC 23767 | [94] |
| Pineapple cores | Polyhydroxy alcohols | Xylitol | 0.371 g g−1 glucose | 100 mL of hydrolysate; 10 mL of tenfold-concentrated nutritional medium containing [g L−1]: 9.44 (NH4)2SO4; 2.5 KH2PO4; 0.5 MgSO4⋅7H2O; 0.05 CaCl2⋅2 H2O; 0.5 citric acid; 0.1 Myo-inositol; 0.035 FeSO4·7 H2O; 0.02 calcium pantothenate; 0.011 ZnSO4·7H2O; 0.0092 MnSO4·7H2O; 0.005 pyridoxal hydrochloride; 0.005 nicotine acid; 0.005 thiamine hydrochloride; 0.0035 KI; 0.002 CoCl2⋅6H2O; 0.002 H3BO3; 0.0013 Na2CoO4⋅2H2O; 0.001 CuSO4⋅7H2O; 0.001 aminobenzoic acid; 0.0005 Al2(SO4)3; 0.0001 D-biotin | Candida tropicalis | [95] |
| Potato pulp waste | Cell biomass | Fodder yeast biomass | 39.3% | Potato pulp hydrolysate (200 g potato pulp; [mL]: 800 water; 0.05 Thermamyl; 0.1 San Extra; 0.1 Cellic CTec 2 (15 FPU)); (g L−1): 0.2 (NH4)2HPO4, 0.06 MgSO4⋅7H2O, pH 4.8–5.2 | Candida guilliermondii ATCC 6260 | [96] |
| Potato peel | Enzymes | α-Amylase | 3014.30 U g−1 | 20 g raw potato peel; 2 mL salt solution [g L−1]: MgSO4 2; KH2PO4 10; MnSO4 0.5; NaCl 2 | Aspergillus niger | [97] |
| 1112.25 U g−1 of dry substrate | 1 g potato peel; 1 mL liquid medium [%, w/v]: NaNO3 0.3; MgSO4 0.05; KCl 0.05; FeSO4 0.002; K2HPO4 0.1; pH 3.0 | Aspergillus niger RBP7 | [98] | |||
| 48.14 ± 0.43 U mL−1 | 5 g raw potato peel powder amended with 10 mL minimal salt solution [g L−1]: KH2PO4 2.0; MgSO4⋅7H2O 0.2; NaCl 0.1; CaCl2 0.1; MnSO4 0.5; peptone 0.2; pH 6.0 | Aspergillus flavus S2-OY | [99] | |||
| Amylase | 16.9 U mL−1 | [g L−1]: 20 potato peel powder; 2 yeast extract; 5 peptones; 0.5 MgSO4; 0.5 KH2PO4; 1.5 NaCl; 0.5 CaCl2 | Bacillus aerius FPWSHA | [100] | ||
| 64.9 U mL−1 | [g L−1]: 10 potato peel powder; 1.5 KH2PO4; 0.5 MgSO4; 0.01 CaCl2; 0.003 FeSO4; pH 8.0 | Anoxybacillus rupiensis T2 | [101] | |||
| Protease | 12.3 U mL−1 | [g L−1]: 20 potato peel powder; 2 yeast extract; 5 peptones; 0.5 MgSO4; 0.5 KH2PO4; 1.5 NaCl; 0.5 CaCl2 | Bacillus aerius FPWSHA | [100] | ||
| 26.2 U mL−1 | [g L−1]: 10 potato peel powder; 1.5 KH2PO4; 0.5 MgSO4; 0.01 CaCl2; 0.003 FeSO4; pH 8.0 | Anoxybacillus rupiensis T2 | [101] | |||
| Pytase | 138.4 U mL−1 | [g L−1]: glucose 10.0; (NH4)2SO4 3.0; KCl 0.5; MgSO4⋅7H2O 0.5; CaCl2 0.1; calcium phytate 0.5%; pH 5.5 | Talaromyces purpureogenus NSA20 | [102] | ||
| Biopolymer | Bacterial cellulose | 4.7 g L−1 | PPW acid hydrolysate; pH 6.0 | Gluconacetobacter xylinus | [103] | |
| Potato waste | Enzymes | Phytase | 12.93 ± 0.47 U g−1 | Potato waste (moisture content 79%); 4% (w/w) (NH4)2SO4 | Aspergillus ficuum ATCC 6687 | [104] |
| Sweet potato peel | Carboxylic acids | Citric acid | 4.36 ± 006 mg mL−1 | 90 mL sweet potato peel starch hydrolysate; 10 mL nutrient solution [g L−1]: 2.23 NH4NO3; 0.23 MgSO4⋅7H2O; 1.0 KH2PO4; pH 6.5 | Aspergillus niger | [105] |
| Papaya peels | Cell biomass/single-cell protein | Biomass | 11.73 ± 0.81 g L−1 | 1000 mL fruit peel medium (10%, v/v): 100 mL fruit juice; inorganic supplements [g]: 1.0 KH2PO4; 0.5 MgSO4⋅7H2O; 0.1 NaCl; 0.1 CaCl2; 900 mL distilled water | Palmyrah toddy sample as the source of natural mixed culture of yeast and bacteria | [106] |
| Single-cell protein (SCP) | 52.4 ± 0.4% | |||||
| Pomegranate peel | Enzymes | Xylanase | 1469.40 U mL−1 | [g L−1]: NaNO3 2.0; K2HPO4 0.5; KCl 0.5; MgSO4 0.7; H2O 0.5; pomegranate peel 20.0 | Chaetomium globosum | [107] |
| Carboxylic acids | Citric acid | 306.8 g kg−1 | 10 g crushed pomegranate peels (0.5–1.0 cm diameter) without dryin;, 75% moisture content; pH 8.0; 3% (w/w) methanol | Aspergillus niger B60 | [108] | |
| Polyhydroxy alcohols | Xylitol | 55.57 g L−1 | [g L−1]: detoxified hydrolysate about the content pomegranate peel 20; yeast extract 1.0; peptone 2.0; KH2PO4 2.0; MgSO4·7 H2O 0.3; pH 7 | Candida tropicalis LY15 (KJ734199) | [109] | |
| Lemon peel | Enzymes | Total cellulase | 10.96 ± 0.51 U mL−1 | [g L−1]: lemon peel powder 0.5% w/v; urea 0.3; KH2PO4 2.0; (NH4)2SO4 1.4; MgSO4⋅7H2O 0.3; CaCl2⋅6H2O 0.3; FeSO4⋅7H2O 0.005; MnSO4 0.002; ZnSO4 0.002; CoSO4⋅7H2O 0.002; 2 mL L−1 Tween 80 | Trichoderma afroharzianum NAS107 | [110] |
| Exoglucanase | 5.42 ± 0.12 U mL−1 | |||||
| Endoglucanase | 6.02 ± 0.19 U mL−1 | |||||
| β-Glucosidase | 3.97 ± 0.15 U mL−1 | |||||
| Pectinase | 1.62 ± 0.11 U mL−1 | |||||
| Xylanase | 4.11 ± 0.49 U mL−1 | |||||
| Mandarin peel | Oligosaccharides | Oligosaccharide | 4.49 ± 0.48 mg mL−1 | Citrus peel waste powder liquid medium (substrate concentration: 1–11% (w/v); 50 mM buffer (acetate buffer from 3.0 to 5.0; phosphate buffer from 6.0 to 8.0); 0.0004% biotin; 0.5% methanol); final culture volume 25 mL | Pichia pastoris X-33 | [111] |
| Orange peel | 1.99 ± 0.13 mg mL−1 | |||||
| Pineapple peel | Polyhydroxy alcohols | Xylitol | 0.31 g g−1 | [g L−1] hydrolysate supplemented with 10 yeast extract (control)/urea; 2.0 (NH4)2SO4; 0.1 CaCl2⋅2H2O | Candida tropicalis FTI 20037 | [112] |
| Kiwi peel | Biopolymer | Bacterial cellulose | 11.53% | Peel hydrolysates 500 mL; 500 mL HS (Hestrin–Schramm) medium ([g L −1]: 20 glucose; 5 yeast extract; 5 peptone; 2.7 Na2PO4; 1.15 citric acid) | Komagataeibacter hansenii GA2016 | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popielarz, D.; Farkaš, P.; Bzducha-Wróbel, A. Current Directions of Selected Plant-Origin Wastes’ Valorization in Biotechnology of Food Additives and Other Important Chemicals. Foods 2025, 14, 954. https://doi.org/10.3390/foods14060954
Popielarz D, Farkaš P, Bzducha-Wróbel A. Current Directions of Selected Plant-Origin Wastes’ Valorization in Biotechnology of Food Additives and Other Important Chemicals. Foods. 2025; 14(6):954. https://doi.org/10.3390/foods14060954
Chicago/Turabian StylePopielarz, Dominika, Pavol Farkaš, and Anna Bzducha-Wróbel. 2025. "Current Directions of Selected Plant-Origin Wastes’ Valorization in Biotechnology of Food Additives and Other Important Chemicals" Foods 14, no. 6: 954. https://doi.org/10.3390/foods14060954
APA StylePopielarz, D., Farkaš, P., & Bzducha-Wróbel, A. (2025). Current Directions of Selected Plant-Origin Wastes’ Valorization in Biotechnology of Food Additives and Other Important Chemicals. Foods, 14(6), 954. https://doi.org/10.3390/foods14060954





