Advancing Stable Isotope Analysis for Alcoholic Beverages’ Authenticity: Novel Approaches in Fraud Detection and Traceability
Abstract
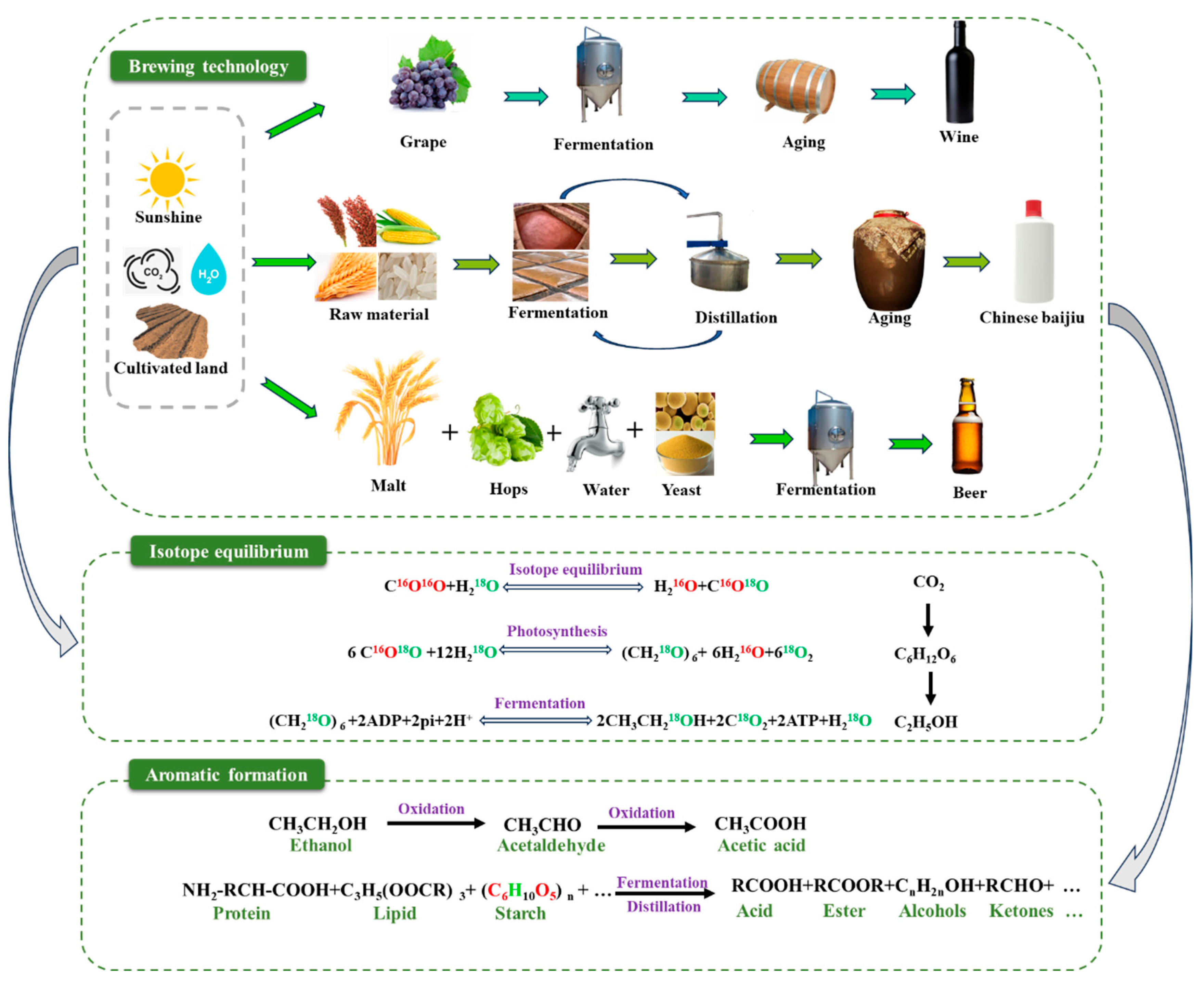
1. Introduction
2. Origin Traceability
2.1. Wine
2.2. Chinese Baijiu
2.3. Beer
2.4. Other Alcoholic Beverages
2.5. General Principles for the Authenticity Assessment of Alcoholic Beverages
- (1)
- Ensure sample authenticity: Representative and authentic samples are essential for training a robust authenticity model.
- (2)
- Select appropriate stable isotope indicators: Carbon isotopes of ethanol are commonly used to detect ethanol adulteration and ingredient composition. Oxygen isotopes of water can identify origin and water adulteration. Carbon isotopes of ethanol and glycine can differentiate vintage.
- (3)
- Establish a comprehensive database: The database should include information on geographic location, climatic conditions, production date, raw ingredient sources, and brewing processes. Integration with heavy isotopes (e.g., Sr, Pb) and elemental fingerprinting enhance traceability accuracy.
- (4)
- Develop discriminant models: Combine chemometric methods with mass spectrometry data to identify authenticity markers and statistically evaluate traceability models.
3. Adulteration Identification
3.1. Adulteration with Exogenous Alcohol
3.1.1. Wine
3.1.2. Chinese Baijiu
3.1.3. Other Alcoholic Beverages
3.2. Exogenous Water
3.2.1. Wine
3.2.2. Chinese Baijiu and Beer
3.2.3. Other Alcoholic Beverages
3.3. Chaptalization
3.3.1. Wine
3.3.2. Beer
3.3.3. Other Alcoholic Beverages
3.4. Trace Compounds
3.4.1. Wine
3.4.2. Chinese Baijiu
3.4.3. Other Alcoholic Beverages
4. Vintage
4.1. Vintage Wine
4.2. Aged Chinese Baijiu
4.3. Other Alcoholic Beverages
5. Conclusions and Perspective
- (1)
- Constructing a comprehensive database: Establish a worldwide stable isotope database of a wide range of alcoholic beverages and regions to facilitate rapid comparative analyses.
- (2)
- Developing novel markers: Identify new indicator molecules that integrate stable isotope data with other chemical and sensory analyses for more accurate authentication.
- (3)
- Advancing analytical technologies: Incorporate emerging technologies such as LC-IRMS, AI-driven isotopic analysis, and IoT-enabled real-time authentication tools.
- (4)
- Promoting interdisciplinary collaboration: Leverage expertise from fields such as ecology, food science, and information technology to drive innovation and application of stable isotope technology.
- (5)
- Addressing practical challenges: Explore ways to reduce the costs of isotopic analysis and improve accessibility for small-scale producers.
- (6)
- Considering climate change impacts: Climate change is emerging as a significant factor that could influence the authenticity and traceability of alcoholic beverages, particularly wine. Rising temperatures, altered precipitation patterns, and increased frequency of extreme weather events are already affecting grape-growing regions worldwide [28]. These changes can lead to shifts in the isotopic signatures of grapes and wines, as stable isotopes are influenced by climatic conditions during plant growth [28]. As climate change progresses, it will be essential to develop new research lines that account for these shifts. This includes updating isotopic databases to reflect changing climatic conditions, exploring novel biomarkers that are less sensitive to climate variability, and integrating isotopic analysis with other tools such as geographic information systems (GIS) and machine learning to enhance traceability and authenticity verification [101].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coelho, I.; Matos, A.S.; Epova, E.N.; Barre, J.; Cellier, R.; Ogrinc, N.; Castanheira, I.; Bordado, J.; Donard, O.F.X. Multi-element and multi-isotopic profiles of Port and Douro wines as tracers for authenticity. J. Food Compos. Anal. 2023, 115, 104988. [Google Scholar] [CrossRef]
- Horacek, M.; Ogrinc, N.; Magdas, D.A.; Wunderlin, D.; Sucur, S.; Maras, V.; Misurovic, A.; Eder, R.; Čuš, F.; Wyhlidal, S.; et al. Isotope Analysis (13C, 18O) of Wine From Central and Eastern Europe and Argentina, 2008 and 2009 Vintages: Differentiation of Origin, Environmental Indications, and Variations Within Countries. Front. Sustain. Food Syst. 2021, 5, 638941. [Google Scholar] [CrossRef]
- McCool, W.C.; Brenner Coltrain, J. A Potential Oxygen Isotope Signature of Maize Beer Consumption: An Experimental Pilot Study. Ethnoarchaeology 2018, 10, 56–67. [Google Scholar] [CrossRef]
- Bong, Y.S.; Ryu, J.S.; Choi, S.H.; La, M.R.; Lee, K.S. Investigation of the geographical provenance of the beer available in South Korea using multielements and isotopes. Food Control. 2016, 60, 378–381. [Google Scholar] [CrossRef]
- Gajek, M.; Pawlaczyk, A.; Maćkiewicz, E.; Albińska, J.; Wysocki, P.; Jóźwik, K.; Szynkowska-Jóźwik, M.I. Assessment of the Authenticity of Whisky Samples Based on the Multi-Elemental and Multivariate Analysis. Foods 2022, 11, 2810. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Aguiñaga, R.; Navarro-Arteaga, U.E.; Muñoz-Sánchez, M.; Gómez-Ruiz, H.; Warren-Vega, W.M.; Romero-Cano, L.A. Design of a Novel Auxiliary Diagnostic Test for the Determination of Authenticity of Tequila 100% Agave Silver Class Based on Chemometrics Analysis of the Isotopic Fingerprint of the Beverage. Foods 2023, 12, 2605. [Google Scholar] [CrossRef]
- Ciepielowski, G.; Pacholczyk-Sienicka, B.; Frczek, T.; Klajman, K.; Paneth, P.; Albrecht, U. Comparison of quantitative NMR and IRMS spectrometry for the authentication of “Polish Vodka”. J. Sci. Food Agric. 2018, 99, 263–268. [Google Scholar] [CrossRef]
- Pepi, S.; Vaccaro, C. Geochemical fingerprints of “Prosecco” wine based on major and trace elements. Environ. Geochem. Health 2017, 40, 833–847. [Google Scholar] [CrossRef]
- Song, X.B.; Hou, M.; Li, Z.; Zhu, L.; Zheng, F.P.; Huang, M.Q.; Sun, X.T.; Li, H.H.; Chen, F.; Sun, B.G. Multi-element analysis of Baijiu (Chinese liquors) by ICP-MS and their classification according to geographical origin. Food Qual. Saf. 2018, 2, 43–49. [Google Scholar] [CrossRef]
- Yin, X.L.; Peng, Z.X.; Pan, Y.; Lv, Y.; Long, W.J.; Gu, H.W.; Fu, H.Y.; She, Y.B. UHPLC-QTOF-MS-based untargeted metabolomic authentication of Chinese red wines according to their grape varieties. Food Res. Int. 2024, 178, 113923. [Google Scholar] [CrossRef]
- Rapa, M.; Ferrante, M.; Rodushkin, I.; Paulukat, C.; Conti, M.E. Venetian Protected Designation of origin wines traceability: Multi-elemental, isotopes and chemometric analysis. Food Chem. 2023, 404, 134771. [Google Scholar] [CrossRef] [PubMed]
- Rossmann, A. Determination of stable isotope ratios in food analysis. Food Res. Int. 2001, 17, 347–381. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef]
- Kelly, S.; Heaton, K.; Hoogewerff, J. Tracing the geographical origin of food: The application of multi-element and multi-isotope analysis. Trends Food Sci. Technol. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Primrose, S.; Woolfe, M.; Rollinson, S. Food forensics: Methods for determining the authenticity of foodstuffs. Trends Food Sci. Technol. 2010, 21, 582–590. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Miranda, C.; Barbarin, I.; Royo, J.B. Application of the measurement of the natural abundance of stable isotopes in viticulture: A review. Aust. J. Grape Wine Res. 2015, 21, 157–167. [Google Scholar] [CrossRef]
- Christoph, N.; Hermann, A.; Wachter, H. 25 Years authentication of wine with stable isotope analysis in the European Union—Review and outlook. BIO Web Conf. 2015, 5, 02020. [Google Scholar] [CrossRef]
- Yue, T.; Wang, D.B.; Li, A.J.; Li, G.H.; Yue, H.W.; Zhang, L.Q.; Zhong, Q.D. Stable carbon isotopic variation in ethanol during baijiu processing. Food Ferment. Ind. 2023, 49, 63–67, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Bowen, G.J.; Cai, Z.Y.; Fiorella, R.P.; Putman, A.L. Isotopes in the Water Cycle: Regional- to Global-Scale Patterns and Applications. Annu. Rev. Earth Planet. Sci. 2019, 47, 453–479. [Google Scholar] [CrossRef]
- Meng, Y.C.; Jin, B.H.; Rogers, K.M.; Zhou, H.C.; Song, X.; Zhang, Y.H.; Lin, G.H.; Wu, H. Hydrogen and Oxygen Isotope Fractionation Effects in Different Organ Tissues of Grapes under Drought Conditions. J. Agric. Food Chem. 2023, 71, 13662–13671. [Google Scholar] [CrossRef]
- Jin, G.Y.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Liu, H.L.; Sun, B.G. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- Zava, A.; Sebastião, P.J.; Catarino, S. Wine traceability and authenticity: Approaches for geographical origin, variety and vintage assessment. Ciência Técnica Vitivinícola 2020, 35, 133–147. [Google Scholar] [CrossRef]
- Raco, B.; Dotsika, E.; Poutoukis, D.; Battaglini, R.; Chantzi, P. O–H–C isotope ratio determination in wine in order to be used as a fingerprint of its regional origin. Food Chem. 2015, 168, 588–594. [Google Scholar] [CrossRef]
- Gherghely, I.; Rácz-Fazakas, T.; Gór, C.; Kapiller-Dezsőfi, R.; Romhányi, A.R. Effect of the production site on stable isotopes of ethanol in fruit spirits. Talanta Open 2022, 5, 100107. [Google Scholar] [CrossRef]
- Laghi, L.; Versari, A.; Ricci, A.; Laurie, V.F.; Parpinello, G.P. Progress in authentication, typification and traceability of grapes and wines by chemometric approaches. Food Res. Int. 2014, 60, 2–18. [Google Scholar] [CrossRef]
- Gao, F.; Zeng, G.; Hao, X.; Wang, H.; Li, H. Variation and fractionation of δ2H, δ18O, and δ17O stable isotopes from irrigation water to soil, grapes, and wine for the traceability of geographical origins. Food Chem. 2025, 462, 141012. [Google Scholar] [CrossRef]
- Janovsk, M.P.; Ferenczi, L.; Truba, J.; Klír, T. Stable isotope analysis in soil prospection reveals the type of historic land-use under contemporary temperate forests in Europe. Sci. Rep. 2024, 14, 14746. [Google Scholar] [CrossRef]
- Dinca, O.R.; Ionete, R.E.; Costinel, D.; Geana, I.E.; Popescu, R.; Stefanescu, I.; Radu, G.L. Regional and Vintage Discrimination of Romanian Wines Based on Elemental and Isotopic Fingerprinting. Food Anal. Methods 2016, 9, 2406–2417. [Google Scholar] [CrossRef]
- Akamatsu, F.; Shimizu, H.; Igi, Y.; Kamada, A.; Koyama, K.; Yamada, O.; Goto-Yamamoto, N. Prediction method for determining the carbon stable isotopic composition of berry sugars in the original must of Chardonnay wines. Food Chem. 2022, 369, 130854. [Google Scholar] [CrossRef]
- Spangenberg, J.E.; Zufferey, V. Carbon isotope compositions of whole wine, wine solid residue, and wine ethanol, determined by EA/IRMS and GC/C/IRMS, can record the vine water status-a comparative reappraisal. Anal. Bioanal. Chem. 2019, 411, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lin, G.H.; Tian, L.; Yan, Z.; Yi, B.Q.; Bian, X.H.; Jin, B.H.; Xie, L.Q.; Zhou, H.C.; Rogers, K.M. Origin verification of French red wines using isotope and elemental analyses coupled with chemometrics. Food Chem. 2021, 339, 127760. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Y.; Gao, J.; Zhao, Y.F.; Wen, H.S.; Zhang, J.J.; Zhang, A.; Yuan, C.L. Geographical Origin Classification of Chinese Wines Based on Carbon and Oxygen Stable Isotopes and Elemental Profiles. J. Food Prot. 2020, 83, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Leder, R.; Petric, I.V.; Jusup, J.; Banović, M. Geographical Discrimination of Croatian Wines by Stable Isotope Ratios and Multielemental Composition Analysis. Front. Nutr. 2021, 8, 625613. [Google Scholar] [CrossRef]
- Kokkinofta, R.; Fotakis, C.; Zervou, M.; Zoumpoulakis, P.; Savvidou, C.; Poulli, K.; Louka, C.; Economidou, N.; Tzioni, E.; Damianou, K.; et al. Isotopic and Elemental Authenticity Markers: A Case Study on Cypriot Wines. Food Anal. Methods 2017, 10, 3902–3913. [Google Scholar] [CrossRef]
- Akamatsu, F.; Shimizu, H.; Hayashi, S.; Kamada, A.; Igi, Y.; Koyama, K.; Yamada, O.; Goto-Yamamoto, N. Chemometric approaches for determining the geographical origin of Japanese Chardonnay wines using oxygen stable isotope and multi-element analyses. Food Chem. 2022, 371, 131113. [Google Scholar] [CrossRef]
- Camin, F.; Dordevic, N.; Wehrens, R.; Neteler, M.; Delucchi, L.; Postma, G.; Buydens, L. Climatic and geographical dependence of the H, C and O stable isotope ratios of Italian wine. Anal. Chim. Acta 2015, 853, 384–390. [Google Scholar] [CrossRef]
- Su, Y.Y.; Zhao, Y.; Cui, K.X.; Wang, F.; Zhang, J.J.; Zhang, A. Wine characterisation according to geographical origin using analysis of mineral elements and rainfall correlation of oxygen isotope values. Int. J. Food Sci. Technol. 2022, 57, 552–565. [Google Scholar] [CrossRef]
- Epova, E.N.; Bérail, S.; Séby, F.; Vacchina, V.; Bareille, G.; Médina, B.; Sarthou, L.; Donard, O.F.X. Strontium elemental and isotopic signatures of Bordeaux wines for authenticity and geographical origin assessment. Food Chem. 2019, 294, 35–45. [Google Scholar] [CrossRef]
- Durante, C.; Bertacchini, L.; Cocchi, M.; Manzini, D.; Marchetti, A.; Rossi, M.C.; Sighinolfi, S.; Tassi, L. Development of 87 Sr/86 Sr maps as targeted strategy to support wine quality. Food Chem. 2018, 255, 139–146. [Google Scholar] [CrossRef]
- Braschi, E.; Marchionni, S.; Priori, S.; Casalini, M.; Tommasini, S.; Natarelli, L.; Buccianti, A.; Bucelli, P.A.C.; Costantini, E.; Conticelli, S. Tracing the 87Sr/86Sr from rocks and soils to vine and wine: An experimental study on geologic and pedologic characterisation of vineyards using radiogenic isotope of heavy elements. Sci. Total Environ. 2018, 628–629, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, L.; Sighinolfi, S.; Ulrici, A.; Maletti, L.; Durante, C.; Marchetti, A.; Tassi, L. Tracing geographical origin of Lambrusco PDO wines using isotope ratios of oxygen, boron, strontium, lead and their elemental concentration. Curr. Res. Food Sci. 2021, 4, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Geană, E.I.; Sandru, C.; Stanciu, V.; Ionete, R.E. Elemental Profile and 87Sr/86Sr Isotope Ratio as Fingerprints for Geographical Traceability of Wines: An Approach on Romanian Wines. Food Anal. Methods 2017, 10, 63–73. [Google Scholar] [CrossRef]
- Su, Y.Y.; Li, Y.F.; Zhang, J.C.; Wang, L.S.; Rengasamy, K.R.; Ma, W.; Zhang, A. Analysis of soils, grapes, and wines for Sr isotope characterisation in Diqing Tibetan Autonomous Prefecture (China) and combining multiple elements for wine geographical traceability purposes. J. Food Compos. Anal. 2023, 122, 105470. [Google Scholar] [CrossRef]
- Tescione, I.; Marchionni, S.; Casalini, M.; Vignozzi, N.; Mattei, M.; Conticelli, S. 87Sr/86Sr isotopes in grapes of different cultivars: A geochemical tool for geographic traceability of agriculture products. Food Chem. 2018, 258, 374–380. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, J.; Wang, L.; Jin, G.; Zhang, A. Signature of Sr isotope ratios and the contents of elements as a tool to distinguish wine regions in China. Food Chem. 2024, 446, 138812. [Google Scholar] [CrossRef]
- Wu, H.; Tian, L.; Chen, B.; Jin, B.H.; Tian, B.; Xie, L.Q.; Rogers, K.M.; Lin, G.H. Verification of imported red wine origin into China using multi isotope and elemental analyses. Food Chem. 2019, 301, 125137. [Google Scholar] [CrossRef]
- Dutra, S.V.; Adami, L.; Marcon, A.R.; Carnieli, G.J.; Roani, C.A.; Spinelli, F.R.; Leonardelli, S.; Vanderlinde, R. Characterization of wines according the geographical origin by analysis of isotopes and minerals and the influence of harvest on the isotope values. Food Chem. 2013, 141, 2148–2153. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, J.; Wang, L.; Araujo, L.D.; Tan, D.; Yuan, C.; Zhang, A. Assessing geographical origin of Diqing wines based on their elemental and isotopic profiles. J. Food Compos. Anal. 2024, 125, 105671. [Google Scholar] [CrossRef]
- Griboff, J.; Horacek, M.; Wunderlin, D.A.; Monferrán, M.V. Differentiation Between Argentine and Austrian Red and White Wines Based on Isotopic and Multi-Elemental Composition. Front. Sustain. Food Syst. 2021, 5, 657412. [Google Scholar] [CrossRef]
- Fan, S.X.; Zhong, Q.D.; Wang, D.B.; Li, G.H.; Huang, Z.B. Elemental profile and oxygen isotope ratio (δ18O) for verifying the geographical origin of Chinese wines. J. Food Drug Anal. 2018, 26, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Q.C.; Yao, C.X.; Song, W.G. Geographical origin traceability discrimination of chongming rice wine. Food Mach. 2020, 36, 77–82, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Xi, H.; Huang, Y.M.; Gorska-Horczyczak, E.; Wierzbicka, A.; Jeleń, H.H. Rapid analysis of Baijiu volatile compounds fingerprint for their aroma and regional origin authenticity assessment. Food Chem. 2021, 337, 128002. [Google Scholar] [CrossRef]
- Niu, Y.W.; Chen, X.M.; Xiao, Z.B.; Ma, N.; Zhu, J.C. Characterization of aroma-active compounds in three Chinese Moutai liquors by gas chromatography-olfactometry, gas chromatography-mass spectrometry and sensory evaluation. Nat. Prod. Res. 2017, 31, 938–944. [Google Scholar] [CrossRef]
- He, Y.X.; Liu, Z.P.; Qian, M.; Yu, X.W.; Xu, Y.; Chen, S. Unraveling the chemosensory characteristics of strong-aroma type Baijiu from different regions using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry and descriptive sensory analysis. Food Chem. 2020, 331, 127335. [Google Scholar] [CrossRef]
- Li, H.H.; Hu, X.M.; Sun, B.G.; Zhang, F.G.; Sun, J.Y.; Huang, M.Q.; Sun, X.T. Application of Carbon Stable Isotope in the Authenticity of Baijiu. J. Chin. Inst. Food Sci. Technol. 2019, 19, 183–189, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- FigeiraI, R.; Venturini Filho, W.G.; Ducatti, C.; Nogueira, A.M.P. Isotope analysis (δ13C) of pulpy whole apple juice. Ciência Tecnol. Aliment. 2011, 31, 660–665. [Google Scholar] [CrossRef]
- Renee Brooks, J.; Buchmann, N.; Phillips, S.; Ehleringer, B.; David Evans, R.; Lott, M. Heavy and Light Beer: A Carbon Isotope Approach To Detect C4 Carbon in Beers of Different Origins, Styles, and Prices. J. Agric. Food Chem. 2002, 50, 6413–6418. [Google Scholar] [CrossRef]
- Chesson, L.A.; Valenzuela, L.O.; O’Grady, S.P.; Cerling, T.E.; Ehleringer, J.R. Links between Purchase Location and Stable Isotope Ratios of Bottled Water, Soda, and Beer in the United States. J. Agric. Food Chem. 2010, 58, 7311–7316. [Google Scholar] [CrossRef]
- Carter, J.F.; Yates, H.S.A.; Tinggi, U. A global survey of the stable isotope and chemical compositions of bottled and canned beers as a guide to authenticity. Sci. Justice 2015, 55, 18–26. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, Z.Q.; Ma, Y.Q.; Shao, F.L.; Huang, J.L.; Wu, H.; Tian, L. Origin Identification of the Sauce-Flavor Chinese Baijiu by Organic Acids, Trace Elements, and the Stable Carbon Isotope Ratio. J. Food Qual. 2019, 2019, 7525201. [Google Scholar] [CrossRef]
- Dehelean, A.; Cristea, G.; Feher, I.; Hategan, A.R.; Magdas, D.A. Differentiation of Transylvanian fruit distillates using supervised statistical tools based on isotopic and elemental fingerprint. J. Sci. Food Agric. 2022, 103, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Tibola, C.S.; Bonnet, M.; Dossa, A.A.; Kus-Yamashita, M.M.M.; Marciano, M.A.M.; Cano, C.B. Strategies to Mitigate Economically Motivated Food Frauds. Encycl. Food Saf. 2024, 1, 309–320. [Google Scholar] [CrossRef]
- Geana, E.I.; Popescu, R.; Costinel, D.; Dinca, O.R.; Stefanescu, I.; Ionete, R.E.; Bala, C. Verifying the red wines adulteration through isotopic and chromatographic investigations coupled with multivariate statistic interpretation of the data. Food Control. 2016, 62, 1–9. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Commission Implementing Regulation (EU) 2025/340. Available online: https://eur-lex.europa.eu/eli/reg_impl/2025/340/oj (accessed on 19 February 2025).
- Perini, M.; Camin, F. δ18O of Ethanol in Wine and Spirits for Authentication Purposes. J. Food Sci. 2013, 78, C839–C844. [Google Scholar] [CrossRef]
- Zhang, P.M.; Zhong, Q.D.; Wang, D.B.; Yue, H.W.; Li, G.H. The application of tartaric acid as the internal standardization of stable carbon isotope of food. Food Ferment. Ind. 2018, 44, 258–264, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Chen, H.; Shen, B.H.; Zhang, S.J.; Xiang, P.; Zhou, X.Y.; Shen, M. Analysis of stable carbon isotope ratio of ethanol in Jiangxiang baijiu by GC-IRMS. Liquor. Mak. Sci. Technol. 2018, 7, 116–123, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wang, D.B. Research on Stable Isotopic Characters of Traditional Chinese Spirits and the Technology System for Authenticity Assessment; China University of Mining & Technology: Beijing, China, 2015. [Google Scholar]
- Rhodes, C.N.; Heaton, K.; Goodall, I.; Brereton, P.A. Gas chromatography carbon isotope ratio mass spectrometry applied to the detection of neutral alcohol in Scotch whisky: An internal reference approach. Food Chem. 2009, 114, 697–701. [Google Scholar] [CrossRef]
- Fonseca-Aguiñaga, R.; Gómez-Ruiz, H.; Miguel-Cruz, F.; Romero-Cano, L.A. Analytical characterization of tequila (silver class) using stable isotope analyses of C, O and atomic absorption as additional criteria to determine authenticity of beverage. Food Control. 2020, 112, 107161. [Google Scholar] [CrossRef]
- Neo, P. China Revises Wine Labelling Regulations to Protect Domestic Manufactures. Available online: https://www.foodnavigator-asia.com/Article/2023/03/08/china-revises-wine-labelling-regulations-to-protect-domestic-manufacturers (accessed on 8 March 2023).
- Magdas, D.A.; Moldovan, Z.; Cristea, G. The use of stable isotopes in quantitative determinations of exogenous water and added ethanol in wines. AIP Conf. Proc. 2012, 1425, 175–177. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.F.; Liu, Y.X.; Kang, T.H.; Tan, D.; Liu, D.H.; Zhang, A. Identification of watered-down wine based on stable oxygen isotope. Liquor. Mak. Sci. Technol. 2023, 2, 102–106, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Meier-Augenstein, W.; Kemp, H.F.; Hardie, S.M.L. Detection of counterfeit scotch whisky by 2H and 18O stable isotope analysis. Food Chem. 2012, 133, 1070–1074. [Google Scholar] [CrossRef]
- Smajlovic, I.; Wang, D.B.; Túri, M.; Zhong, Q.D.; Futó, I.; Veres, M.; Sparks, K.L.; Sparks, J.P.; Jakšić, D.; Vuković, A.; et al. Quantitative analysis and detection of chaptalization and watering down of wine using isotope ratio mass spectrometry. In Proceedings of the BIO Web of Conferences, Geneva, Switzerland, 15–19 July 2019; pp. 1–9. [Google Scholar] [CrossRef]
- Jiang, W.; Xue, J.; Fu, F.Y.; Wang, L.; Liu, X. The application of SNIF-NMR in chaptalizing of grape wine. Liquor. Mak. Sci. Technol. 2015, 1, 68–72, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Guyon, F.; Gaillard, L.; Salagoity, M.H.; Medina, B. Intrinsic ratios of glucose, fructose, glycerol and ethanol 13C/12C isotopic ratio determined by HPLC-co-IRMS: Toward determining constants for wine authentication. Anal. Bioanal. Chem. 2011, 401, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Mardegan, S.F.; Andrade, T.M.B.; Neto, E.R.d.S.; Vasconcellos, E.B.d.C. Stable carbon isotopic composition of Brazilian beers—A comparison between large- and small-scale breweries. J. Food Compos. Anal. 2013, 29, 52–57. [Google Scholar] [CrossRef]
- Sleiman, M.; Filho, W.G.V.; Ducatti, C.; Nojimoto, T. Carbon and nitrogen stable isotopes used to determine the percentage of malt in Pilsen beer. Braz. J. Food Technol. 2008, 11, 95–102. Available online: www.researchgate.net/publication/289118046 (accessed on 18 November 2024).
- Voica, C.; Magdas, D.A.; Feher, I. Metal Content and Stable Isotope Determination in Some Commercial Beers from Romanian Markets. J. Chem. 2015, 2015, 192032. [Google Scholar] [CrossRef]
- Kitagaki, H.; Kitamoto, K. Breeding Research on Sake Yeasts in Japan: History, Recent Technological Advances, and Future Perspectives. Annu. Rev. Food Sci. Technol. 2013, 4, 215–235. [Google Scholar] [CrossRef]
- Toshihiko, I.; Mahito, K.; Yoichiro, S.; Seiei, W.; Hitoshi, T. Formation of Guaiacol by Spoilage Bacteria from Vanillic Acid, a Product of Rice Koji Cultivation, in Japanese Sake Brewing. J. Agric. Food Chem. 2016, 64, 4599–4605. [Google Scholar] [CrossRef]
- Kanai, M.; Kawata, T.; Yoshida, Y.; Kita, Y.; Ogawa, T.; Mizunuma, M.; Watanabe, D.; Shimoi, H.; Mizuno, A.; Yamada, O. Sake yeast YHR032W/ERC1 haplotype contributes to high S-adenosylmethionine accumulation in sake yeast strains. J. Biosci. Bioeng. 2017, 123, 141–147. [Google Scholar] [CrossRef]
- Akamatsu, F.; Igi, Y.; Fujita, A. Separation and Purification of Glucose in Sake for Carbon Stable Isotope Analysis. Food Anal. Methods 2020, 13, 885–891. [Google Scholar] [CrossRef]
- Akamatsu, F.; Oe, T.; Hashiguchi, T.; Hisatsune, Y.; Kawao, T.; Fujii, T. Application of carbon and hydrogen stable isotope analyses to detect exogenous citric acid in Japanese apricot liqueur. Food Chem. 2017, 228, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the European Union. Commission Regulation (EU) 2024/1451. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32024R1451 (accessed on 24 May 2024).
- Cui, W.; Wang, X.; Han, S.; Guo, W.; Meng, N.; Li, J.; Sun, B.; Zhang, X. Research progress of tartaric acid stabilization on wine characteristics. Food Chemistry: X 2024, 23, 101728. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.X.; Gao, J.H.; Mi, J.B.; Liu, Y.; Ge, B.K.; Zheng, W.J. Determination of carbon isotope ratio for six flavour characters in wine by GC-C-IRMS. Food Res. Dev. 2019, 40, 137–141, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Jin, X.Y.; Zhang, L.M.; Wu, S.M.; Huang, M.Q.; Yu, W.J.; Zhang, S.S. Developing an authentication approach using SPME-GC-IRMS based on compound-specific δ 13C analysis of six typical volatiles in wine. Food Quality and Safety 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Yin, X.J.; Xia, Y.L.; Su, J. Study on the identification of ethyl caproate doped in Wuliangye strong aromatic liquor produced by solid-state fermentation process. Packag. Food Mach. 2021, 39, 46–51, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zhong, Q.D.; Wang, D.B.; Xiong, Z.H. Application of Stable Isotope Technique on Distinguish Between Chinese Spirit by Traditional Fermentation and Chinese Spirit Made from Traditional and Liquid Fermentation. J. Chin. Mass Spectrom. Soc. 2014, 35, 66–71, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, Z.M.; Wei, J.P.; Huang, J.; Fan, T.; Ye, H.X.; An, M.Z. Detection of stable oxygen isotope of flavor compounds in nongxiang baijiu. Liquor. Mak. Sci. Technol. 2022, 7, 136–144, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Yu, H.X.; Chu, Y.H.; Bian, X.H.; Chen, S.L.; Jin, B.H.; Rogers, K.M.; Xu, D.M.; Chen, X.Z.; Wu, H. Identifying the vintage of French wine using stable isotopes, elemental fingerprints, and a data-driven but explainable approach. Food Chem. 2025, 464, 141907. [Google Scholar] [CrossRef]
- Liu, Q.R.; Zhang, X.J.; Zheng, L.; Meng, L.J.; Liu, G.Q.; Yang, T.; Lu, Z.M.; Chai, L.J.; Wang, S.T.; Shi, J.S.; et al. Machine learning based age-authentication assisted by chemo-kinetics: Case study of strong-flavor Chinese Baijiu. Food Res. Int. 2023, 167, 112594. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, X.L.; Song, X.B.; Zheng, F.P.; Li, H.H.; Chen, F.; Zhang, Y.H.; Zhang, F.Y. Evolution of the key odorants and aroma profiles in traditional Laowuzeng baijiu during its one-year ageing. Food Chem. 2020, 310, 125898. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lu, J.L.; Qian, M.; He, H.K.; Li, A.; Zhang, J.; Shen, X.M.; Gao, J.J.; Xu, Y. Untargeted Headspace-Gas Chromatography-Ion Mobility Spectrometry in Combination with Chemometrics for Detecting the Age of Chinese Liquor (Baijiu). Foods 2021, 10, 2888. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Zhou, W.; Burr, G.S.; Fu, Y.; Fan, Y.; Wu, S. Authentication of Chinese vintage liquors using bomb-pulse 14C. Sci. Rep. 2016, 6, 38381. [Google Scholar] [CrossRef] [PubMed]
- Christoph, N.; Rossmann, A.; Schlicht, C.; Voerkelius, S. Wine authentication using stable isotope ratio analysis: Significance of geographic origin, climate, and viticultural parameters. In Authentication of Food and Wine; Oxford University Press Inc.: Oxford, UK, 2007; Volume 7, pp. 166–179. [Google Scholar] [CrossRef]
- Laursen, K.; Bontempo, L.; Camin, F.; Roßmann, A. Advances in Isotopic Analysis for Food Authenticity Testing. In Advances in Food Authenticity Testing; Woodhead Publishing: Sawston, UK, 2016; pp. 227–252. [Google Scholar] [CrossRef]
- Stavins, R.N.; Catena, L.; Forrestel, E.; Rivail, J.-B.; Sahn, M.; Sumner, D.A. Global Climate Change and Wine Production: Industry and Academic Perspectives. Harv. Data Sci. Rev. 2025, 7, 1–7. [Google Scholar] [CrossRef]

| Model of Origin Traceability | Geographical Origins | Amount | Stable Isotope and Methods | Machine Learning | Accuracy Rate (%) | References |
|---|---|---|---|---|---|---|
| Country | France, Spain, Italy, Australia, the United States, South Africa, Chile, and China | 600 | δ13C of glycerol and ethanol: GC-C-IRMS, δ18O of wine water: Gasbench-IRMS | DA | 83.9 | [47] |
| RF | 55.8 | |||||
| ANN | 93.1 | |||||
| Argentina and Austria | 62 | δ18O of wine water: Gasbench-IRMS, δ13C of ethanol: EA-IRMS | LDA | 98.4 | [50] | |
| America, Asia, Europe, and Oceania | 80 | δ18O of beer water: Gasbench-IRMS, δ13C of beer: EA-IRMS, 87Sr/86Sr of beer: MC-ICP-MS | DA | 99 | [4] | |
| State/Province | Changji, Mile, and Changli regions in China | 188 | δ18O of wine water: GC-P-IRMS | PLS-DA | 95 | [51] |
| SVM | 97 | |||||
| Hebei Province, Helanshan, Diqing, and Yantai in China | 62 | δ13C of glycerol and ethanol: GC-C-IRMS, δ18O of wine water: Gasbench-IRMS | DA | 100 | [47] | |
| Helan Mountain, Xinjiang, Yunchuanzang, Yanhuai Valley, and Hexi Corridor in China | 142 | δ13C of glycerol and ethanol: LC-IRMS, δ18O of wine water: Gasbench-IRMS | LDA | 90.8 | [33] | |
| Helan Mountain, Xinjiang, Southwest Mountain, and Loess Plateau regions in China | 104 | δ18O of wine water: Gasbench-IRMS | ANN | 90.9 | [38] | |
| LDA | 88.5 | |||||
| Zone B (Croatian Uplands), Zone CI (Slavonia and Croatian Danube), and Zone CII (Croatian Istria and Kvarner) in Croatia | 190 | δ18O of wine water: Gasbench-IRMS, δ13C of wine: EA-IRMS | GDA | 86.3 | [34] | |
| Bordeaux, Burgundy, Languedoc-Roussillon and Rhone regions in French | 240 | δ13C of glycerol and ethanol: GC-C-IRMS, δ18O of wine water: Gasbench-IRMS | ANN | 98.2 | [32] | |
| PLS-DA | 92.5 | |||||
| Shanghai, Shaoxing, Xiaogan Beijing in China and Korea | 44 | δ2H and δ18O: Gasbench-IRMS | PLS-DA | 97.73 | [52] | |
| Village/Town | Three towns (Foshan, Yunling, and Benzilan) Deqin County, plateau of Diqing, Yunnan, China | 36 | δ13C of wine: EA-IRMS, δ18O of wine: EQ-IRMS | DA | 80.6 | [49] |
| ANN | 87.7 | |||||
| Six counties (YL, BZL, YM, DW, YuL, FS) in Diqing, Yunnan, China | / | δ13C of wine: EA-IRMS, δ18O of wine water: Gasbench-IRMS, 87Sr/86Sr: MC-ICP-MS | DA | 100 | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Li, Y.; Shao, F.; Lu, Y.; Meng, W.; Rogers, K.M.; Sun, D.; Wu, H.; Peng, X. Advancing Stable Isotope Analysis for Alcoholic Beverages’ Authenticity: Novel Approaches in Fraud Detection and Traceability. Foods 2025, 14, 943. https://doi.org/10.3390/foods14060943
Ma Y, Li Y, Shao F, Lu Y, Meng W, Rogers KM, Sun D, Wu H, Peng X. Advancing Stable Isotope Analysis for Alcoholic Beverages’ Authenticity: Novel Approaches in Fraud Detection and Traceability. Foods. 2025; 14(6):943. https://doi.org/10.3390/foods14060943
Chicago/Turabian StyleMa, Yiqian, Yalan Li, Feilong Shao, Yuanyu Lu, Wangni Meng, Karyne M. Rogers, Di Sun, Hao Wu, and Xiaodong Peng. 2025. "Advancing Stable Isotope Analysis for Alcoholic Beverages’ Authenticity: Novel Approaches in Fraud Detection and Traceability" Foods 14, no. 6: 943. https://doi.org/10.3390/foods14060943
APA StyleMa, Y., Li, Y., Shao, F., Lu, Y., Meng, W., Rogers, K. M., Sun, D., Wu, H., & Peng, X. (2025). Advancing Stable Isotope Analysis for Alcoholic Beverages’ Authenticity: Novel Approaches in Fraud Detection and Traceability. Foods, 14(6), 943. https://doi.org/10.3390/foods14060943






