1. Introduction
Fenugreek (
Trigonella foenum graecum) is an annual plant with significant nutritional and pharmacological value. The leaves and seeds of the fenugreek plant are used in cooking where it confers a distinct smell along with the color and texture of food [
1]. Fenugreek has been used for centuries for its laxative, demulcent, galactagogue, expectorant, and carminative properties, and the herb/spice features in pharmacopeia worldwide, including in the Ayurvedic, Chinese, Arabic, Greek, and Latin systems of traditional medicine [
2]. These official monographs cite the characteristics and uses of the different parts of the plant, mainly the seeds. The fenugreek seed is widely known for its diverse phytochemical profile due to the presence of metabolites that impart unique medicinal, food, and nutritional properties to the seeds [
3]. The phytochemical classes such as phenolics and flavonoids are widely known for their protective and therapeutic role against various types of human cancers due to the presence of functional groups with an ability to bind the signaling proteins in order to inhibit their prospective actions. The phytochemicals recognized for the aforementioned activities consist of saponins (diosgenin), flavonoids (quercetin, kaempferol, luteolin, and rutin), alkaloids (trigonelline), coumarins, vitamins, soluble fibers, and carbohydrates like galactomannan [
4]. A plethora of literature reviews are available for fenugreek exploring the widely covering range, highlighting the huge potential the plant possesses for the development of new drugs. The prevalent scope may include its antioxidant, hypocholesterolemic, antineoplastic, anti-inflammatory, hepatoprotective, antiulcerogenic, antipyretic, immunomodulatory, and antitumor properties. Fenugreek seeds may also have a potential role in diabetes management, as they have been found to contain polyphenolic flavonoids, evident for its hypoglycemic, hypocholesterolemic, hypotriglyceridemics, and antiperoxidative effects [
4]. Likewise, the presence of galactomannan, trigonelline, and diosgenin have been noticed with significant effects on diabetes through a number of different physiological pathways, e.g., the reduction in carbohydrate absorption, inhibition of alpha-amylase and sucrase activities, and restoration of pancreatic β-cell function [
2]. A recent study revealed a lower fasting and post-prandial blood glucose levels for diabetic patients using the powder from fenugreek supplement for three months [
5]. Fenugreek seeds have been reported to arrest the cancer cell cycle, inhibit cell proliferation in tumors, and reduce cell migration rates [
2]. The selective cytotoxic effects of fenugreek seeds have been documented in various cancer cell lines including the colonic, pancreatic, prostate, breast, and T-cell lymphoma [
1]. In addition, all parts of the fenugreek plant have demonstrated antimicrobial properties against bacteria such as
Pseudomonas aeruginosa,
Escherichia coli,
Staphylococcus aureus,
Helicobacter pylori, and
Rhizoctonia solani [
4]. Studies suggest the antibacterial role of fenugreek seed extracts is principally due to the naturally occurring combination of alkaloids, flavonoids, tannins, saponins, terpenoids, and steroids [
5].
It is noteworthy to mention that a plethora of the literature has been reported with regard to the characterization and extraction of different phytochemical classes present in fenugreek seeds such as flavonoids, phenolics, alkaloids, saponins, and flavonoids glycosides [
6,
7,
8,
9,
10,
11]. In addition, a number of studies have reported the various biological and pharmacological potentials of fenugreek seeds in the prevention and treatment of inflammation, diabetes, cardiovascular diseases, hypercholesteremia, bacterial and fungal infections, cancer, and to boost a mother’s milk [
12,
13,
14,
15,
16]. However, a research gap in the shape of a comprehensive correlation for the biological and pharmacological potential vs. the phytochemical classes of fenugreek seeds responsible for the reported activities still exists. There is an intense need to correlate the different phytochemical classes of the fenugreek seeds with the respective biological activities. This may further explore the phytochemical class and chemical components thereof, playing a vital role in the health-related activities of the fenugreek seeds.
The concept presented in the current study deal with the phytochemical analysis of the fenugreek seeds vs. the correlation with the biological activities of cytotoxicity, antibacterial, antifungal, and α-amylase conducted in-house. The detailed studies for the phytochemical classes of flavonoids and alkaloid were performed previously as reported [
6,
17], followed by the biological activities as mentioned. The statistical models were implicated to extract and highlight the significant correlations among the phytochemical classes and biological activities performed for fenugreek. The study recruited forty-five different origins of fenugreek seed samples in order to broaden the data pool for an effective statistical application.
There is a significant correlation between the phytochemical composition (saponins, phenolics, flavonoids, and alkaloids) of fenugreek seeds and their biological activities, including cytotoxicity, antibacterial, antifungal, and α-amylase inhibition. This study aims to investigate the phytochemical composition of fenugreek seeds from diverse origins and analyze their correlation with biological activities (cytotoxicity, antibacterial, antifungal, and α-amylase inhibition) using statistical models. The findings will contribute to understanding the functional potential of fenugreek phytochemicals in relation to their bioactivities.
2. Materials and Methods
2.1. Strains, Cells, Cell Culture Media, and Standard Drugs/Chemicals Used
The strains used in this study consisted of Streptococcus faecalis (ATCC*29212), Staphylococcus aureus (ATCC*29213), Acinetobacter baumannii (ATCC*1605), Pseudomonas aeruginosa (ATCC* 15442), and Candida albicancs (ATCC* 14053). Muller Hinton agar (Oxoid, Hampshire, United Kingdom, CM0337), nutrient agar, blood agar, Sabouraud dextrose agar, Sabouraud dextrose broth, and Muller Hinton broth (Oxoid, CM0405) were used for culture and susceptibility testing (diffusion method and broth dilution method) to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentrations (MBCs). The human breast adenocarcinoma (MCF-7; ATCC-HTB22), human colon adenocarcinoma (HT-29; ATCC- HTB-38™), hepatocellular carcinoma (HepG2; ATCC HB-8065), and normal human fetal lung fibroblast (MRC5; ATCC-CCL171) were tested in this study. The cell lines of MCF7, HT-29, and MRC5 were maintained in Roswell Park Memorial Institute Medium (RPMI-1640), whereas the HepG2 cell line was cultured in Dulbecco’s Modified Eagle Medium (DMEM) obtained from Gibco, Life Technologies, Carlsbad, CA, USA. The heat-inactivated fetal bovine serum (FBS) and antibiotics (1% penicillin-streptomycin) were also purchased from Gibco, Life Technologies, Carlsbad, CA, USA. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Invitrogen, MA, USA) alpha-amylase (Sigma-Aldrich, MA, USA), Acarbose (Sigma-Aldrich), Paclitaxel (MedChemExpress, NJ, USA), Oxaliplatin (MedChemExpress), and Olaparib (MedChemExpress) were used.
2.2. Collection, Extraction, and Analysis for Fenugreek Samples
The fenugreek seed samples from five different origins were collected and extracted in three different solvents. The extracts were dried and the yield were calculated as reported in the previous studies [
6]. The samples were subjected to flavonoids [
6] and trigonelline [
17] quantification using the in-house developed UHPLC-DAD analytical methods.
2.3. Cell Culture Studies
For cell culture studies, the three cell lines of MCF7, HT29, and MRC5 were maintained in the RPMI1640 medium whereas the HepG2 cell line was cultured in DMEM. The media were supplemented with 10%FBS and 1% penicillin-streptomycin antibiotic (10,000 units of penicillin +10,000 µg of streptomycin) and maintained at 37 °C in 5% CO2 with 100% relative humidity.
Determination of Cytotoxicity and Selectivity
Previously reported MTT assay was used to determine the cytotoxicity of the extracts in MCF7, HT29, HepG2, and MRC5 cell lines [
18]. Briefly, the cells were cultured in 96-well plates and incubated (37 °C) overnight in order to screen the cytotoxic potential for the extracts at a concentration of 100 μg/mL (DMSO 0.4%;
n = 3). The plates were kept for 48 h (37 °C in 5% CO
2) followed by the addition of MTT and further incubation for 3 h. The supernatant was discarded whereas the MTT crystals were solubilized via the addition of DMSO to each well. The absorbance was recorded using a multi-plate reader (BIORAD, PR 4100, Hercules, CA, USA). The inhibition percentage compared to the control cells was calculated and the extracts with more %inhibition were selected for cytotoxicity determination (IC
50 determination) in fibroblasts cell lines. The extract concentration studied ranged from 1 to 500 μg/mL (500, 250, 100, 50, 10, 1) whereas Paclitaxel, Oxaliplatin, and Olaparib were used as positive controls in order to determine the IC
50 values using GraphPad Prism (San Diego, CA, USA).
2.4. Antimicrobial Studies
2.4.1. Preparation of Standard Inoculum
The tested bacterial strains were grown on Muller Hinton (MH) agar medium whereas Candida albicans was grown on Sabouraud dextrose (SD) agar (37 °C; 24 h). To prepare inoculum, the selected colonies were inoculated in the MH and SB broth to form a homogenous suspension for the tested strains, standardized to 0.5 McFarland turbidity using a calibrated Vitek Densichek Biomerieux Analyzer (bioMérieux, Marcy-l’Étoile, France).
2.4.2. Diffusion Assay on Agar Plates
For the agar well diffusion assay, tested strains (100 μL) with 0.5 McFarland turbidity were swabbed on the agar plate surfaces as per the reported protocol [
19]. The plates were dried (10 min), and the wells were penetrated (6 mm) and filled with 100 µL of the tested extract (50 mg/mL) as well as positive (Vancomycin and Teicoplanin; 30 µg disks) and negative controls (Amikacin 30 µg and Imipenem 10 µg disks) for the bacterial strains. The solvent of dimethyl sulfoxide (DMSO) was used as a negative control. The process was performed in triplicate and the agar plates were incubated (24 h; 37 °C), examined, and zones of inhibition (mm) were measured.
2.4.3. MIC and MBC Determination
The extracts with a significant zone of inhibitions were further assessed for MIC and MBC where a 96-well microtiter plate was used to prepare two-fold dilutions for the extracts at 50, 25, 12.5, 6.2, and 3.1 mg/mL. The 0.5 McFarland turbidity standards (10 μL) for
S. faecalis,
S. aureus,
A. baumannii,
P. aeruginosa, and
C. albicans were poured into the wells of the selected samples and positive controls. The plates were incubated overnight (37 °C) followed by calculation of the MIC and MBC as per the guidelines of the clinical and laboratory standards institute [
20,
21].
2.5. Alpha-Amylase Inhibition Activity
A previously reported method was adopted for α-amylase inhibition [
22]. Initially, the extracts were screened at a concentration of 500 μg/mL where the samples with significant α-amylase enzyme inhibition activity were further investigated in the concentration range of 1000, 500, 250, 100, 50, 25, and 5 μg/mL. The positive standard used was Acarbose. An aliquot of
Aspergillus. oryzae-based α-amylase (1 mg) in phosphate buffer was prepared. To each well, 20 μL was added along with the extract samples (20 μL), mixed properly, and incubated for 10 min (37 °C). Following the incubation, 30 μL of the starch (0.05% in deionized water) solution was added to individual wells and incubated further for 8 min at 37 °C. The reaction was halted by the addition of 20 μL of hydrochloric acid (1 M) followed by 100 μL of the iodine reagent (0.25 mM) into each well. The control wells were prepared by replacing the enzyme with buffer solution and addition of Acarbose. The data were recorded by measuring the absorbance at 550 nm using a multi-plate reader (BIORAD, PR 4100, Hercules, CA, USA). The %inhibition was calculated for the wells using the formula
* absorbance of the reaction mixture in the presence of the extract, ** absorbance of the mixture without the enzyme, and *** absorbance of the reaction mixture in the absence of any extract.
2.6. Statistical Analysis
The results for the dataset were entered into an SPSS (statistical package for social science students V27.0) software and statistical models for the correlation and paired differences were applied. The descriptive data, i.e., mean ± standard deviation (SD), were generated from triplicate readings. The GraphPad Prism 9.2.0 (GraphPad, San Diego, CA, USA) software was used to determine the significant differences for the control vs. standard groups at p < 0.05.
4. Discussion
Fenugreek seeds are acknowledged for their diverse biological activities associated with the presence of a number of phytochemical classes. This research study aims to establish a potential correlation for the key phytochemical classes of flavonoids and alkaloids reported in the fenugreek seeds with the cytotoxicity, antimicrobial, and α-amylase inhibitory activity tested herein. The extracts for the fenugreek seeds put into use for the biological activities were extracted in our previous study [
6]. To assess the cytotoxicity potential for the fenugreek seed extracts, HT29, MCF7, MRC5, and HepG2 cell lines were utilized where the cell lines of HT29 and MCF7 were tested for the preliminary cytotoxicity potential of the extracts. The idea was to extricate the fenugreek samples with a potential to inhibit the cell viability with an initial checkup value of >50%. The extracts of E1M, E2M, E3M, Y1M, IR2M, IR3M, and Q1M were seen with an inhibition of 50% or above for both the cell lines of HT29 and MCF7. Hence, these extracts were selected for further testing in order to determine the IC
50 values for these sample. To investigate the selectivity and observe the cell lines coverage for these extracts, HepG2 and MRC5 cell lines along with six further MTT concentrations were included for IC
50 determination. The extracts of IR2M (HepG2) and IR3M (HT29, MCF7, and MRC5) showed significantly lower IC
50 values compared to the other extracts. The samples with cytotoxic potential observed herein were the methanol extracted seeds of fenugreek. Previous studies revealed a dose-dependent cytotoxic effect and apoptosis induction for the methanolic extract of the fenugreek seeds against MCF-7 breast and HepG2 hepatocellular carcinoma cell lines [
23,
24]. These results corroborate the findings for cytotoxicity observed in our study.
The antimicrobial potential for the fenugreek seed extracts was studied against the bacterial (SF, SA, AB, and PA) and fungal strains (CA). The extracts exhibited potential antibacterial activity against SF (E2M, E3C, and E2C), SA (E1M, E2C, and E2H), AB (Y2M), and PA (Q3H and Y2M); however, a lack of antifungal activity was recorded for all the extracts against CA. The results from the previous studies presented a potential effect for fenugreek extract against Gram-positive (
S. aureus) and Gram-negative bacteria (
P. aeruginosa) [
25]. The literature herein supports the prospective role for the antibacterial uses of fenugreek seeds. The selected samples were tested for MIC and MBD determination. The data showed the lowest MIC against SF (E2C, E2M, E3C, I3H), SA (E1M, Y3C, IR2H, IR3H, IR3C) as well as the MBC against SF (E2C, E2M, E3C, I3H) and SA (E1M, Y3C, IR2H, IR3H, IR3C).
The α-amylase inhibition for the extracts was studied where an initial screening at a dose of 500 μg/mL for each fenugreek extract was employed to acquire the extracts with an α-amylase inhibitory potential of >50%. The result yielded the Y3C, Q1H, Y2C, I3C, Q3C, E3H, I2C, I1C, E1C, and E3C extracts with an inhibition of >50% for α-amylase. The mentioned extracts were selected and studied further at six different low-dose concentrations to calculate the IC
50 values. The extracts of E3H, I3C, Q1H, YC, and Y3C resulted in the lower IC
50 values comparable to the standard drugs tested in the study. The ability of the fenugreek seeds to modify and inhibit the carbohydrate-metabolizing enzymes (α-amylase) in a dose-dependent manner have been reported previously [
26,
27]. The reported studies demarcate the antidiabetic role for fenugreek seeds as observed in current study.
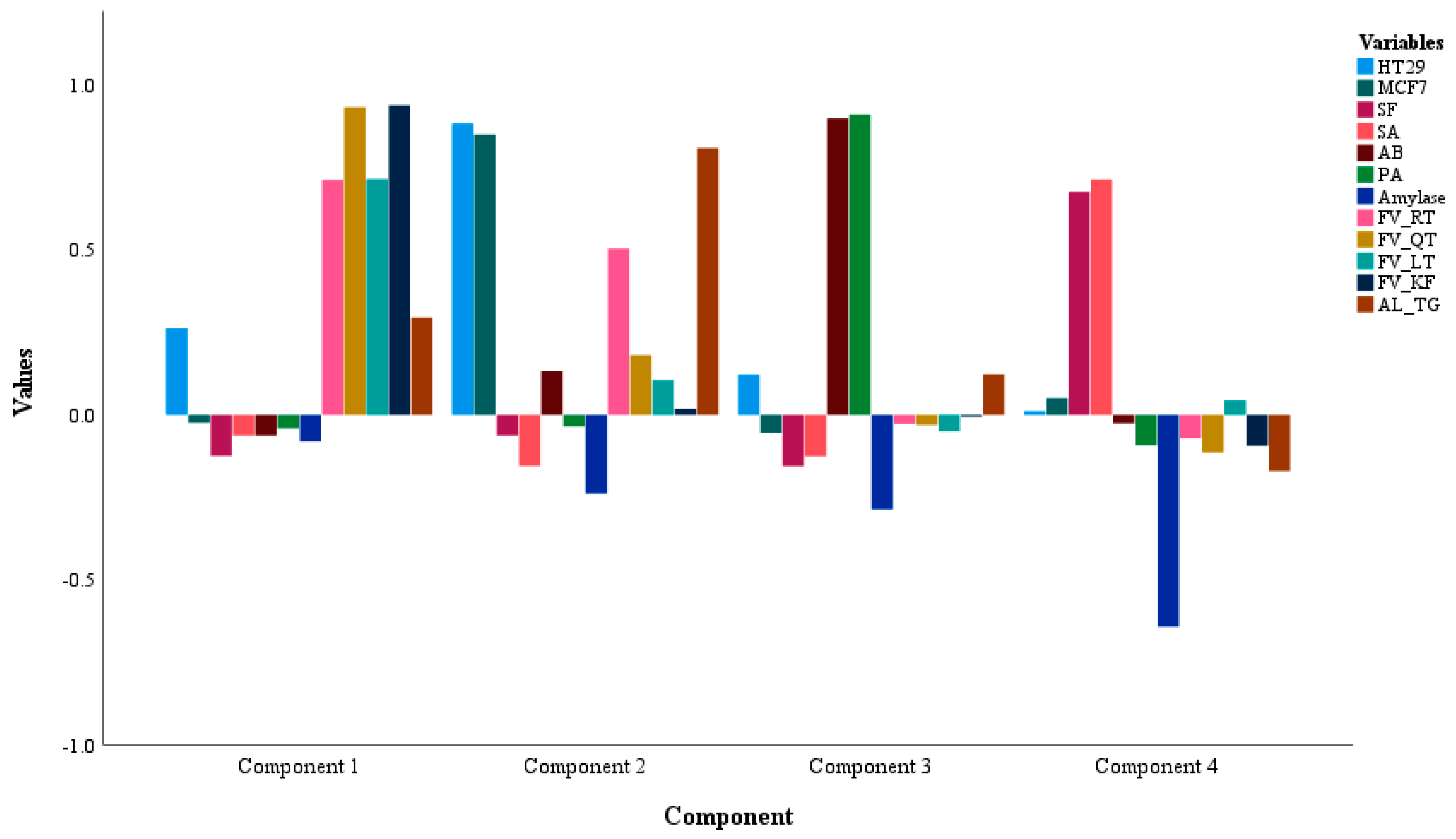
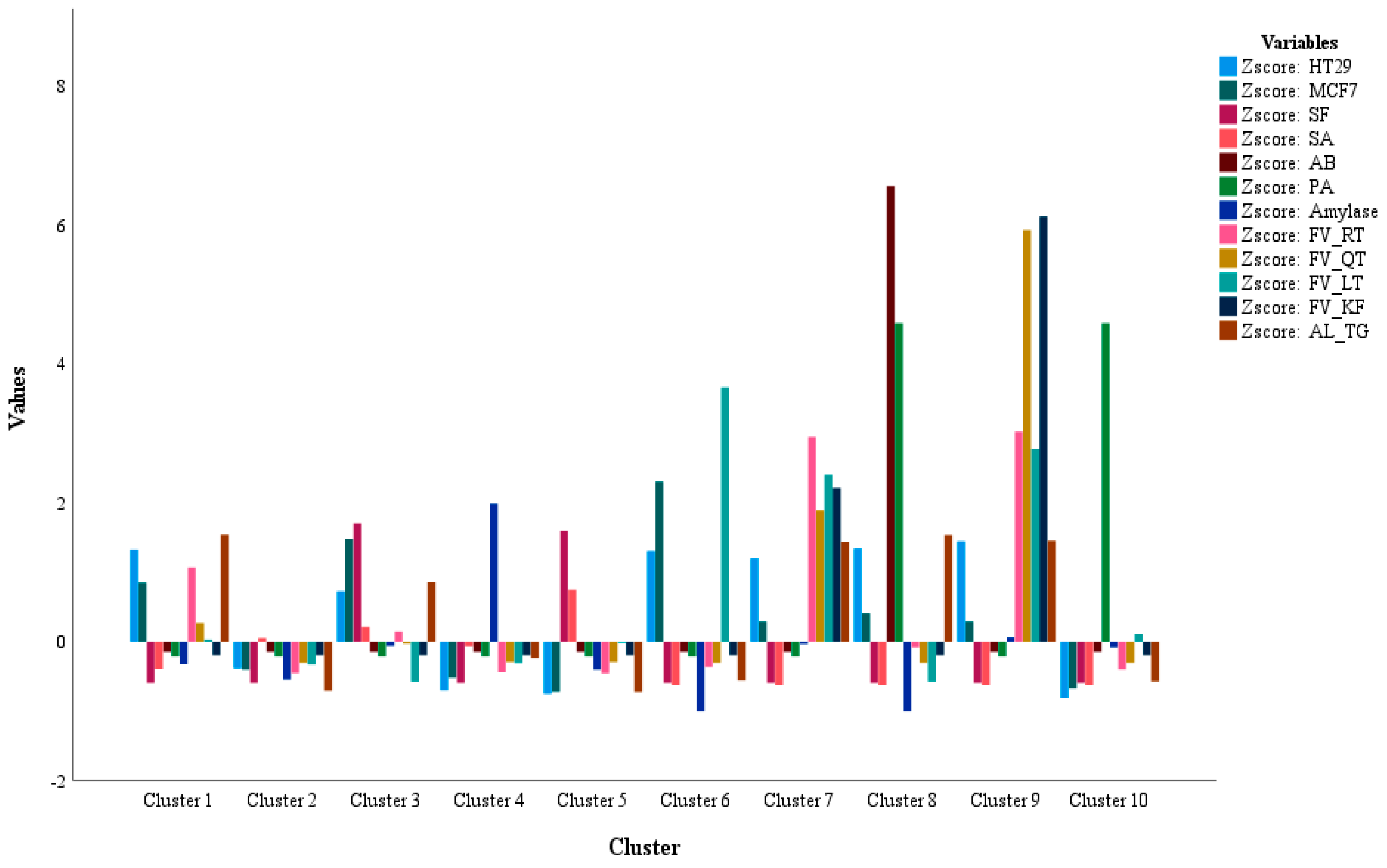
The statistical models were applied to link the significant correlations and paired differences among the biological activities vs. the phytochemicals analyzed for these extracts. The Pearson’s analysis revealed a high positive correlation for the inhibitory effect upon the HT29 and MCF7 cell lines with the extracts possessing the phytochemical classes of flavonoids (RT, QT, LT, and KF) and the alkaloid TG. As well as cytotoxicity, the α-amylase inhibitory activity was noticed in the extracts containing a significant amount of the flavonoids and alkaloid. Likewise, the antimicrobial activity against SF and SA was recognized in the fenugreek extracts containing more amounts of TG and LT. To comprehend the correlation link between the phytochemical classes and biological activities, component analysis was performed to bring out the variables with less inter-variability, i.e., more correlation, loaded in the same components. As evident from the PCA data, the cytotoxicity and α-amylase activities were loaded alongside the flavonoids and alkaloid whereas the variables for antimicrobial activity were loaded in one cluster, suggesting a prominent role for the analyzed phytochemical classes in the biological activities of fenugreek seeds. However, the sample pool was larger with 45 different fenugreek seed extracts, hence K-mean analysis was utilized to highlight the seed extracts with more phytochemical amounts and higher activity. Interestingly, K-mean analysis clustered the extracts with the most potent biological activities containing a significant amount of either flavonoids and alkaloid, or altogether. For instance, the designated extracts (phytochemical class) were: IR2M [Iranian origin] with more cytotoxicity against MCF7 and HT29; I2M [Indian origin] with moderate HT29 and MCF7; Y2M [Yemen origin] with the highest antimicrobial activity against AB and PA alongwith a moderate activity for HT29 and MCF7; I3M [Indian origin] with a significant activity against HT29 and MCF7 activity as well as a minor activity for amylase inhibition; Q3H [Saudi Origin] with the highest antimicrobial activity against PA; E3H [Egyptian origin], I2C & I3C [Indian origin], Y2C & Y3C [Yemen origin], and Q1H [Saudi origin] with the highest inhibition for amylase activity.
The K-mean cluster activity was further confirmed by the paired sample t-test. The paired differences for the phytochemical classes of flavonoids (QT, LT, KF, and AG) and alkaloid (TG) are shown in the table where the differences observed confirms an alike pattern for the correlation pattern seen in the statistical models of Pearson’s correlation, PCA, and K-mean cluster analysis.
The variation in the phytochemical composition of the fenugreek seeds and its biological activities thereof are prone to a number of factors including the geographical variation where the factors of humidity, salinity, altitude, soil composition, temperature, water irrigation, soil water retention capacity, annual rainfall level, and pH of the soil play a substantial role. In addition, the harvesting time, seeds processing, shipment, and its storage are the key factors to be considered for preserving the optimal quality of the seeds or plants [
28,
29].
These phytochemical classes owe the potential to inhibit the initiation of carcinogenesis via modulation of the phase I and II carcinogen-metabolizing enzymes as well as inhibition of oxygen radical-forming enzymes that contribute to DNA synthesis and inhibition of protein kinases playing a role in the proliferative signal transduction and cell cycle. The flavonoids suppress tumor development through the induction of cellular apoptosis mediated via suppression of DNA topoisomerase and p53, induction of autophagy, inhibition of telomerase, and triggering of mitochondrial toxicity [
30,
31].
Likewise, the phytochemical class of alkaloids is well known for its antioxidant, anticancer, and antidiabetic medicinal uses. Alkaloids are accepted as one of the richest and most productive sources of drug discovery, particularly with regard to cancer treatments. The in vitro and in vivo experiments have revealed the potential anticancer properties of alkaloids, mediated through interactions with various aspects of tumor progression. The alkaloids may induce caspase-dependent and caspase-independent apoptosis as well as modulate both the pro-apoptotic BAX/BAK and anti-apoptotic BCL-2 protein expression. They also regulate autophagy through the modulation of phosphatidylinositol 3-kinase, mitogen-activated protein kinases, and reactive oxygen species. In addition, they arrest the cell cycle at different stages through the regulation of a cyclin-dependent kinase family of proteins. They suppress angiogenesis, invasion and metastasis in a variety of cancer cell lines through inhibition of focal adhesion kinase, vascular endothelial growth factor, and matrix metalloproteinase [
32,
33]. The research work herein, crosslinking the statistical data for the biological activities vs. the phytochemical classes of flavonoids and alkaloid analyzed in our studies, elucidate a substantial role for these phytochemical classes in the health care applications of fenugreek seeds in terms of antioxidant, anticancer, antimicrobial, and as an antidiabetic. The support from the previous literature data favor the presence of flavonoids and alkaloids being the key components behind the traditional as well as the biological role of fenugreek seeds.
5. Conclusions
This research work provides a comprehensive correlation for the multiple classes of fenugreek seed extracts (flavonoids and alkaloid) in establishing its role in cytotoxicity, antimicrobial, and antidiabetic activity (α-amylase inhibition). The in-depth statistical analysis for the multiple classes of the fenugreek seed extracts substantiates a significant role for the flavonoids (QT, LT, KF, AG) and alkaloid (TG) to inhibit the cell growth for MCF7 and HT29, microorganisms (SF, and SA), and α-amylase activity. Herein, the study establishes the pharmacological role for the fenugreek seeds to be properly correlated with the traditional uses of fenugreek. The samples of IR2M, IR2 and 3H, IR2 and 3H, IR3C [Iran origin samples], E2 and 3C, E1 and 2M [Egypt origin samples], I3H [Indian origin], and Q1H [Saudi origin] were more effective for the biological activities. This study may be helpful for the quality control and standardization of fenugreek to evaluate and authenticate the fenugreek seeds as well as the pharmaceutical products and dietary supplements for the presence of the essential key elements of flavonoids and alkaloid. The major insight that the current study provides is the identification and correlation of the fenugreek phytochemical class and its respective chemical moiety responsible for the claimed medicinal uses; hence, this paves a way for researchers to isolate and utilize the same chemical moiety for the established medicinal use. The identified compounds may be utilized as biomarkers for plant-based drug discovery and bioengineering to open avenues for genetic modification in order to enhance the yield for the phytochemical compounds. The future directives may include in-depth study for the geographical variation affecting the metabolites’ number and concentration. In addition, the identified metabolites may be enhanced for their yield within the same source, utilizing the genetic biodiversity and gene manipulations technique.