Temporal and Workshop Heterogeneity of Microbial Communities with Physicochemical Properties and Flavor Substances During Stacked Fermentation of Sauce-Flavor Baijiu
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Amplicon Sequencing
2.3. Measurement of Physicochemical Indicators
2.4. Analysis of Volatiles
2.5. Statistical Analysis
3. Results
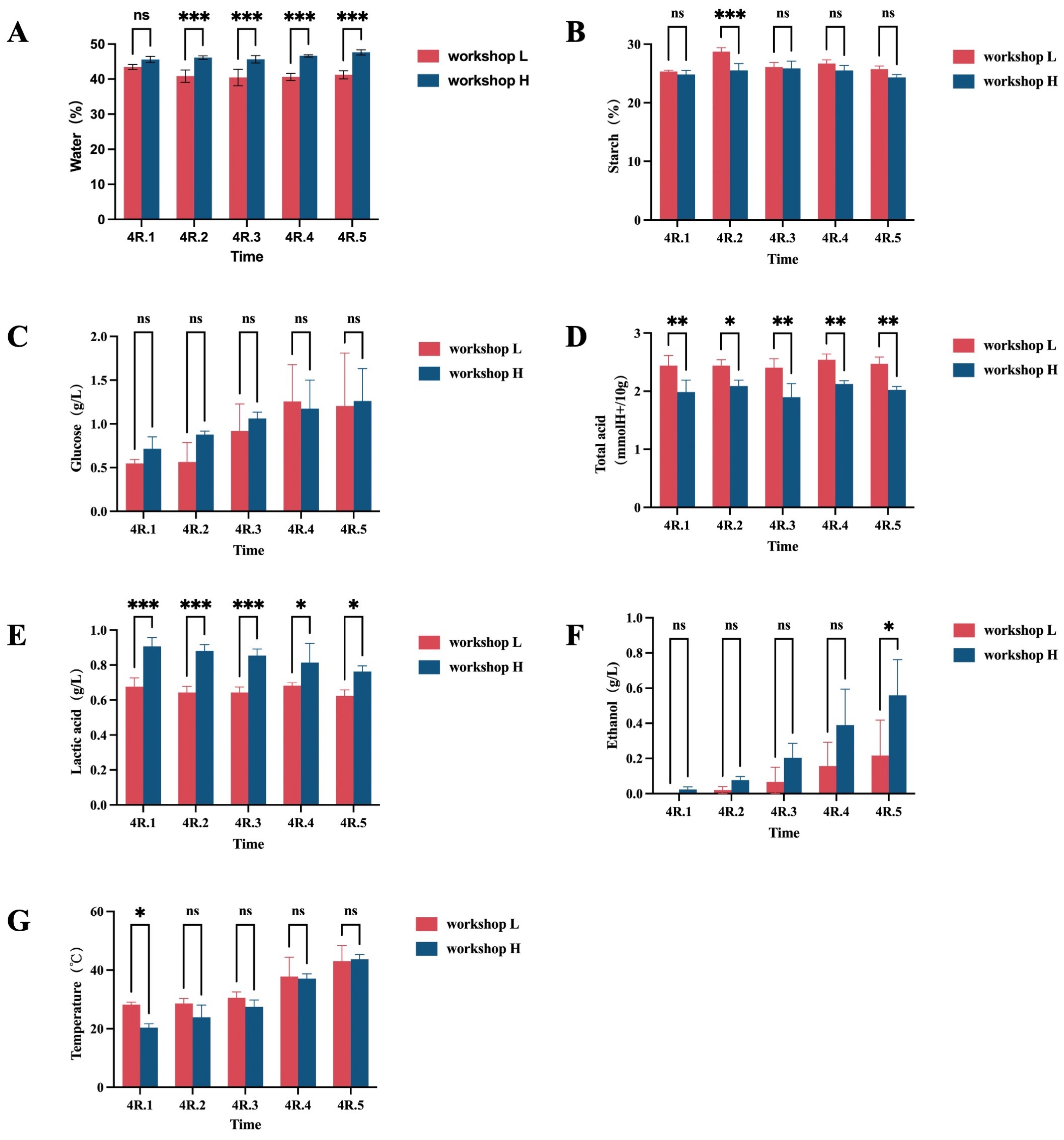
3.1. Dynamic Changes in Physicochemical Factors
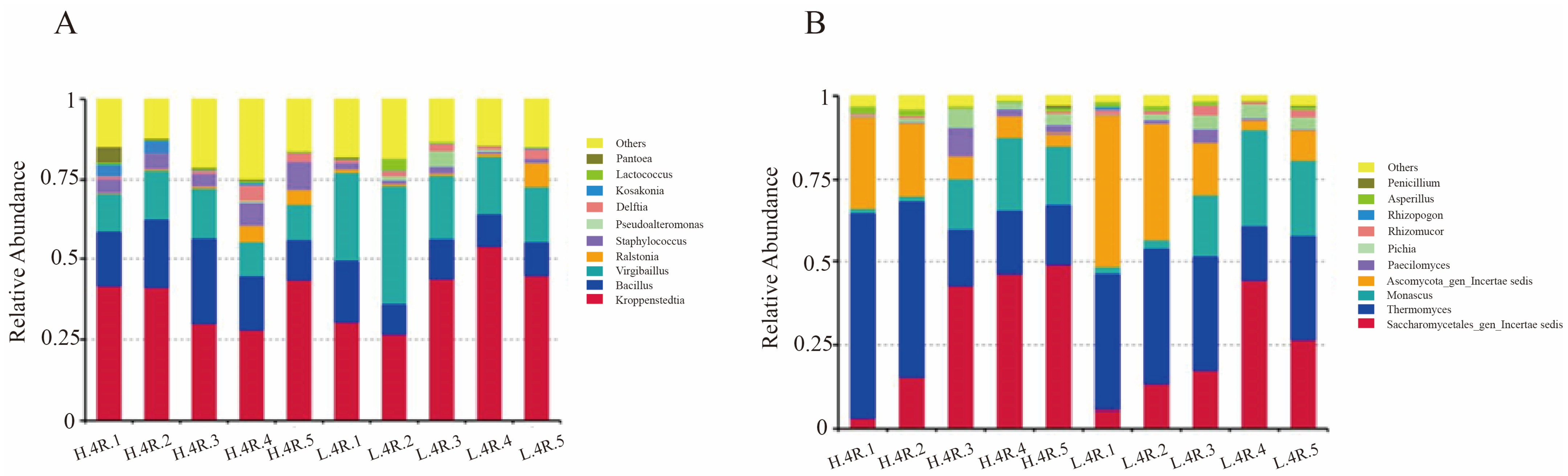
3.2. Microbial Diversity and Community Composition During Stacked Fermentation
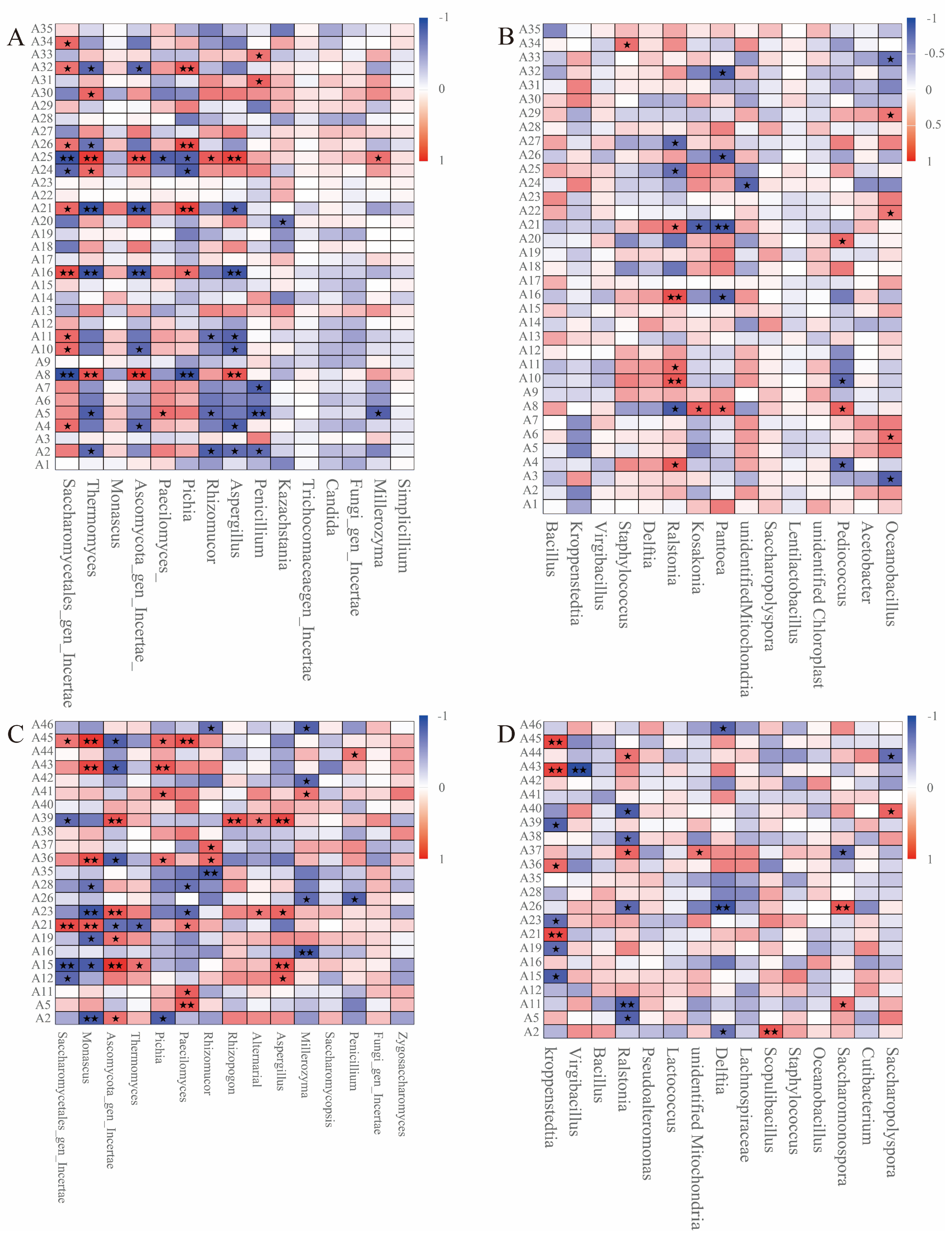
3.3. Bacterial Community Succession During Stacked Fermentation
3.4. Fungal Community Succession During Stacked Fermentation
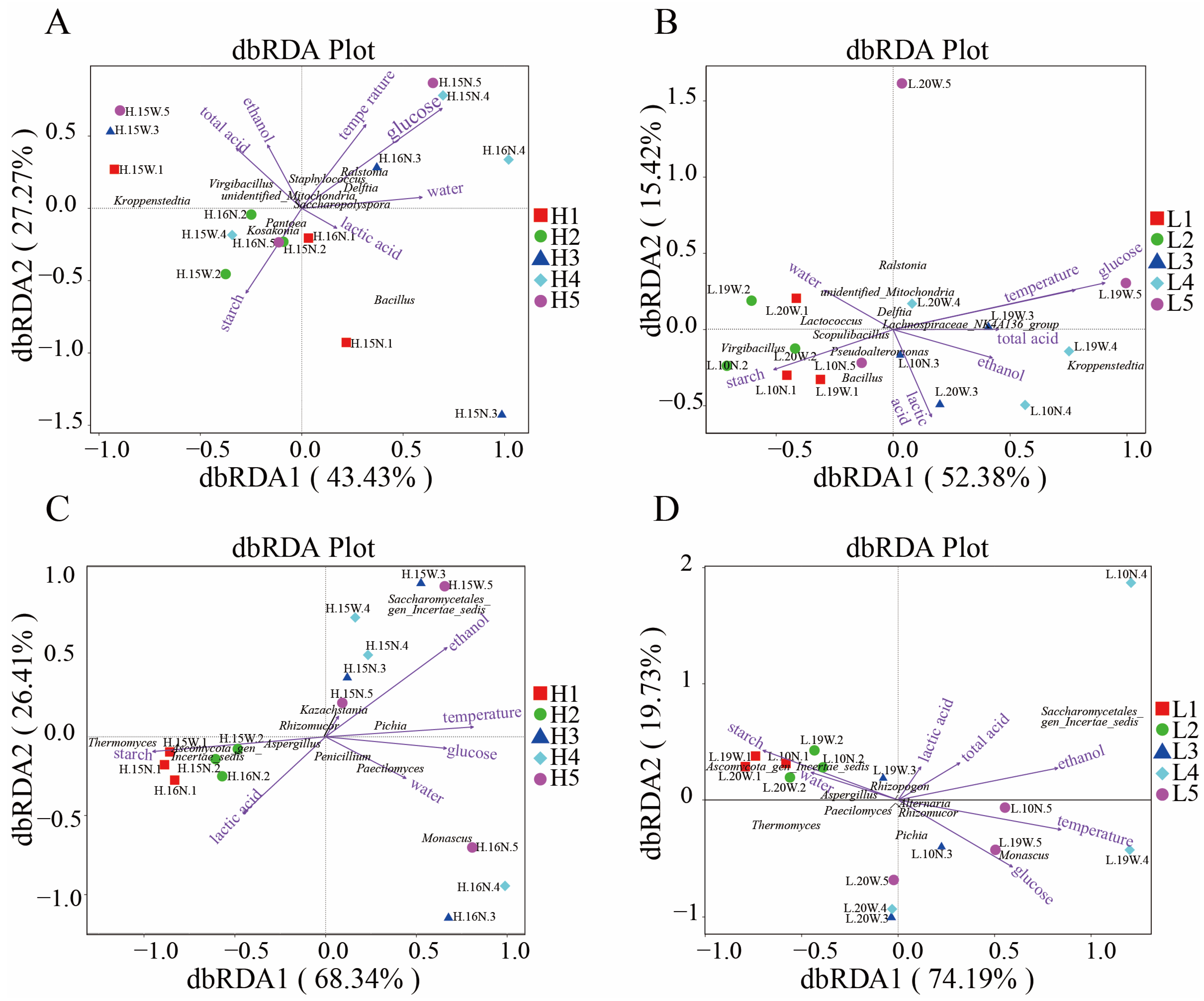
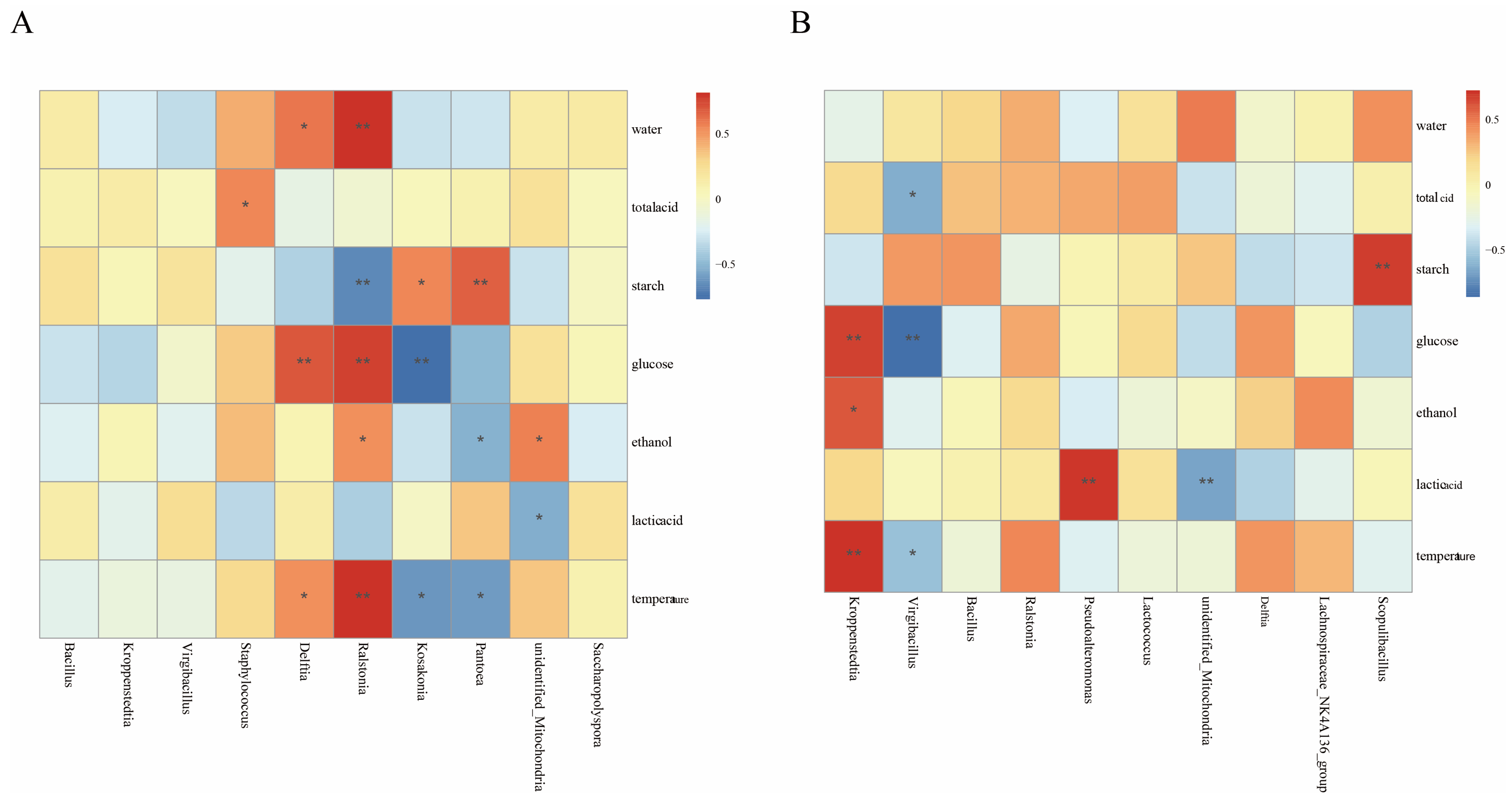
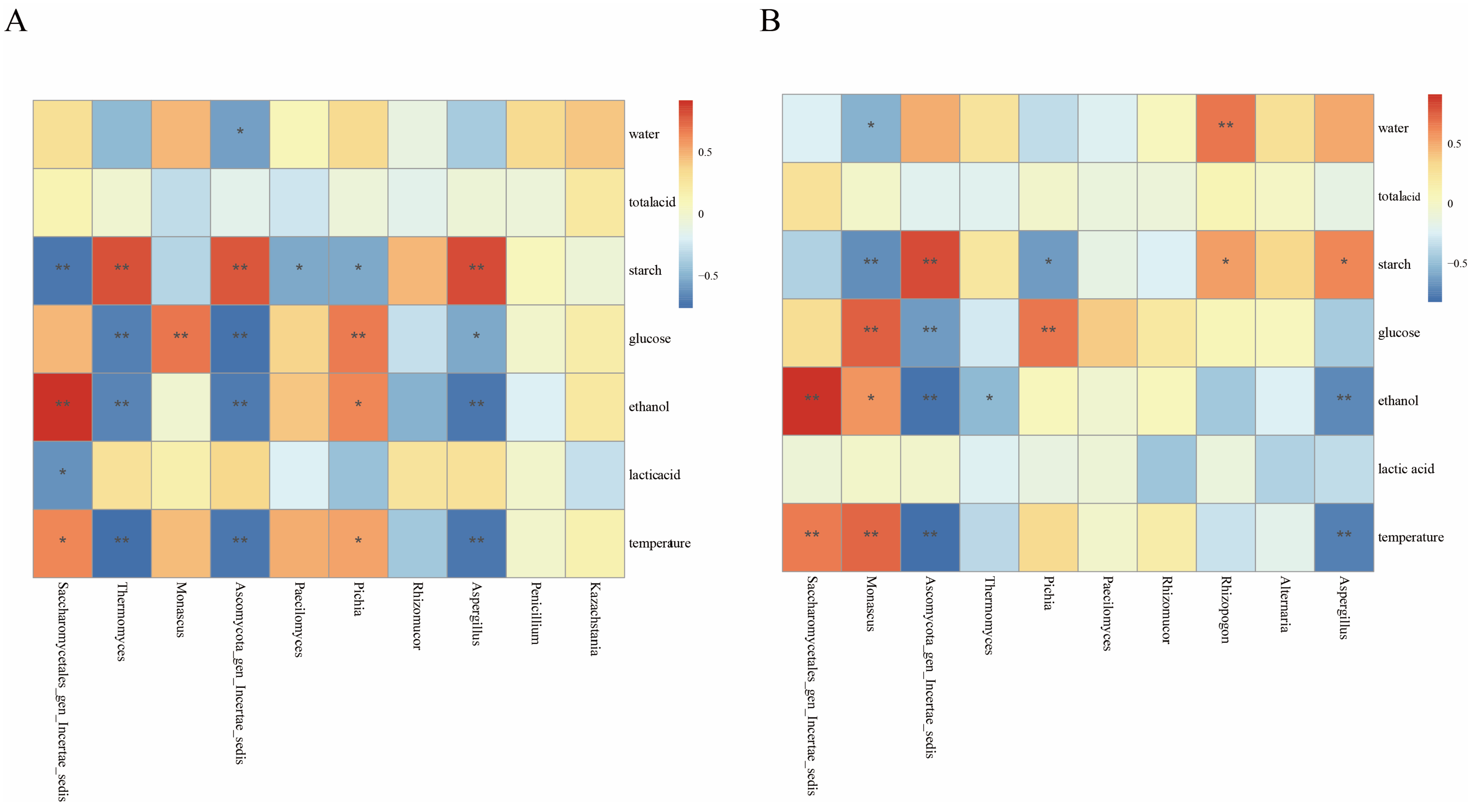
3.5. Relationships Between Physicochemical Factors and Microbial Communities
3.6. Relationships Between Flavor Substances and Microbial Communities
4. Discussion
4.1. Differences in Key Microorganisms Between Two Workshops
4.2. Influence Mechanism of Physicochemical Factors on Microorganisms
4.3. Microbial-Mediated Flavor Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duan, J.; Cheng, W.; Lv, S.; Deng, W.; Hu, X.; Li, H.; Sun, J.; Zheng, F.; Sun, B. Characterization of key aroma compounds in soy sauce flavor baijiu by molecular sensory science combined with aroma active compounds reverse verification method. Food Chem. 2024, 443, 138487. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Yang, S.; Shen, Y.; Cheng, W.; Li, H.; Sun, J.; Huang, M.; Sun, B. What Are the Main Factors That Affect the Flavor of Sauce-Aroma Baijiu. Foods 2022, 11, 3534. [Google Scholar] [CrossRef]
- Wu, X.J. Diversity and Dynamics of Yeasts and Bacteria During the Solid State Fermentative Process Contributing to Chinese Maotai-Flavor Liquor Making. Master’s Thesis, Jiangnan University, Wuxi, China, 2013. (In Chinese with English Abstract). [Google Scholar]
- Huang, Y.; Wang, Y.; Xu, Y. Purification and characterisation of an acid protease from the Aspergillus hennebergii HX08 and its potential in traditional fermentation. J. Inst. Brew. 2017, 123, 432–441. [Google Scholar] [CrossRef]
- Hu, X.X.; Huang, Y.G.; Tu, H.B.; Hu, F.; Cheng, P.Y.; You, X.L. Bacterial diversity structure during the first round of pile and pit fermentation of Moutai-flavor Baijiu. Food Sci. 2020, 41, 175–182, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Luo, L.-J.; Song, L.; Han, Y.; Zhen, P.; Han, D.-Y.; Zhao, X.; Zhou, X.; Wei, Y.-H.; Yu, H.-X.; Han, P.-J.; et al. Microbial communities and their correlation with flavor compound formation during the mechanized production of light-flavor Baijiu. Food Res. Int. 2023, 172, 113139. [Google Scholar] [CrossRef]
- Ren, T.; Su, W.; Mu, Y.; Qi, Q.; Zhang, D. Study on the correlation between microbial communities with physicochemical properties and flavor substances in the Xiasha round of cave-brewed sauce-flavor Baijiu. Front. Microbiol. 2023, 14, 1124817. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, X.; Hu, J.; Deng, H.; He, J.; Zhang, C. The spatiotemporal heterogeneity of microbial community assembly during pit fermentation of soy sauce flavor baijiu. Food Biosci. 2024, 61, 104438. [Google Scholar] [CrossRef]
- Wang, H. Microbiome-Based Study on Brewing Microorganisms and Flavor of Sauce-Flavor Baijiu. Master’s Thesis, Guizhou University, Guiyang, China, 2019. (In Chinese with English Abstract). [Google Scholar]
- Zhang, C.L.; Yang, L.; Li, Z.; Gan, G.D.; He, J.J. Correlation between microbial community and physicochemical indexes in fermented grains of stacking fermentation process of the second rounds sauce-flavor Baijiu. China Brew. 2021, 40, 31–36, (In Chinese with English Abstract). [Google Scholar]
- Yang, Y.Q. Structure Solid State Fermentation Simulation System and Microbial Community Structure Analysis of Maotai-Liquor Stacking Fermentation, Fermentation Cellar. Master’s Thesis, Fujian Normal University, Fuzhou, China, 2015. (In Chinese with English Abstract). [Google Scholar]
- Liu, X.; Mu, Y.; Lv, X.; Chen, N.; Chen, L.; Wen, T.; Su, W. Analysis of fermentation characteristics in fermented grains across seven rounds of sauce-flavored Baijiu: Microbial communities structure, physicochemical parameters, volatile and non-volatile flavor compounds. Food Chem. X 2025, 25, 102228. [Google Scholar] [CrossRef]
- Dai, Y.; Tian, Z.; Meng, W.; Li, C.; Li, Z. Changes in Microbial Diversity, Physicochemical Characteristics, and Flavor Substances During Maotai-Flavored Liquor Fermentation and Their Correlations. J. Biobased Mater. Bioenergy 2019, 13, 290–307. [Google Scholar] [CrossRef]
- Chen, X.; Du, B.; Liu, J.; Zhang, C.; Zhu, H.; Wang, K.; Sun, B.; Li, X. Exploring the significance of the 2nd and 4 h round fermentations in the brewing process of sauce-flavor baijiu. Food Biosci. 2024, 59, 104114. [Google Scholar] [CrossRef]
- Yang, L.; Xian, C.; Li, P.; Wang, X.; Song, D.; Zhao, L.; Zhang, C. The spatio-temporal diversity and succession of microbial community and its environment driving factors during stacking fermentation of Maotai-flavor baijiu. Food Res. Int. 2023, 169, 112892. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Wu, Y.-L.; Lo, D.; Wu, M.-C. Physicochemical property changes during the fermentation of longan (Dimocarpus longan) mead and its aroma composition using multiple yeast inoculations. J. Inst. Brew. 2013, 119, 303–308. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Hao, W.; Duan, C.; Wang, S.; Wei, J.; Liu, G. Metatranscriptomics Unravel Composition, Drivers, and Functions of the Active Microorganisms in Light-Flavor Liquor Fermentation. Microbiol. Spectr. 2022, 10, e02151-21. [Google Scholar] [CrossRef]
- Wang, P.; Wu, Q.; Jiang, X.; Wang, Z.; Tang, J.; Xu, Y. Bacillus licheniformis affects the microbial community and metabolic profile in the spontaneous fermentation of Daqu starter for Chinese liquor making. Int. J. Food Microbiol. 2017, 250, 59–67. [Google Scholar] [CrossRef]
- Jin, Y.; Li, D.; Ai, M.; Tang, Q.; Huang, J.; Ding, X.; Wu, C.; Zhou, R. Correlation between volatile profiles and microbial communities: A metabonomic approach to study Jiang-flavor liquor Daqu. Food Res. Int. 2019, 121, 422–432. [Google Scholar] [CrossRef]
- Qi, Z.; Zhou, X.; Tian, L.; Zhang, H.; Cai, L.; Tang, F. Distribution of mycotoxin-producing fungi across major rice production areas of China. Food Control 2022, 134, 108572. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y.; Huang, Y. Microbiome diversity and evolution in stacking fermentation during different rounds of Jiang-flavoured Baijiu brewing. LWT 2021, 143, 111119. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Hu, X.; Li, Y. The impact of environmental factors on the environmental bacterial diversity and composition in the Jiang-flavoured Baijiu production region. LWT 2021, 149, 111784. [Google Scholar] [CrossRef]
- Wang, H.; Sun, C.; Yang, S.; Ruan, Y.; Lyu, L.; Guo, X.; Wu, X.; Chen, Y. Exploring the impact of initial moisture content on microbial community and flavor generation in Xiaoqu baijiu fermentation. Food Chem. X 2023, 20, 100981. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yu, X.; Zhang, L.; Wu, Q.; Chen, F.; Guo, F.; Xu, Y. Acidity drives volatile metabolites in the spontaneous fermentation of sesame flavor-type baijiu. Int. J. Food Microbiol. 2023, 389, 110101. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, D.; Dhandapani, B.; Mahadevan, S. Recent Advances in Organic Acid Production from Microbial Sources by Utilizing Agricultural By-Products as Substrates for Industrial Applications. In The Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Meng, D.J.; Zhang, J.J.; Guo, B.H.; Yu, R.S.; Xue, X.X.; Hu, J.H.; Jiang, W.; Han, X.L. Correlation Analysis of Acid Flavor Substances and Microorganisms in Fermented Grains of Maotai Flavor Baijiu. Food Sci. Technol. 2022, 10, 77–83. [Google Scholar]
- Kang, J.; Hu, Y.; Jia, L.; Zhang, M.; Zhang, Z.; Huang, X.; Chen, X.; Han, B.-Z. Response of microbial community assembly and succession pattern to abiotic factors during the second round of light-flavor Baijiu fermentation. Food Res. Int. 2022, 162, 111915. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Wang, H.; Yang, F.; Xu, Y. Effects of initial temperature on microbial community succession rate and volatile flavors during Baijiu fermentation process. Food Res. Int. 2020, 141, 109887. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, J.; Wu, C.; Zhang, E.; Li, L. A preliminary study on temperature rising mechanism of fermented grains during heap fermentation process of sauce-flavor Baijiu in winter. China Brew. 2024, 43, 68–73. [Google Scholar]
- Wang, X.; Du, H.; Xu, Y. Source tracking of prokaryotic communities in fermented grain of Chinese strong-flavor liquor. Int. J. Food Microbiol. 2017, 244, 27–35. [Google Scholar] [CrossRef]
- Dong, W.; Yu, X.; Wang, L.; Zou, M.; Ma, J.; Liu, J.; Feng, Y.; Zhao, S.; Yang, Q.; Hu, Y.; et al. Unveiling the microbiota of sauce-flavor Daqu and its relationships with flavors and color during maturation. Front. Microbiol. 2024, 15, 1345772. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Wang, C.-T.; Shen, C.-H.; Wang, S.-T.; Mao, J.-Q.; Li, Z.; Gänzle, M.; Mao, J. Microbiota stratification and succession of amylase-producing Bacillus in traditional Chinese Jiuqu (fermentation starters). J. Sci. Food Agric. 2020, 100, 3544–3553. [Google Scholar] [CrossRef]
- Tu, W.; Cao, X.; Cheng, J.; Li, L.; Zhan, T.; Wu, Q.; Xiang, P.; Shen, C.; Li, Q. Chinese Baijiu: The Perfect Works of Microorganisms. Front. Microbiol. 2022, 13, 919044. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Toyokawa, Y.; Sugimoto, Y.; Azuma, H.; Tsukahara, K.; Nasuno, R.; Watanabe, D.; Tsukahara, M.; Takagi, H. Characterization of a New Saccharomyces cerevisiae Isolated from Hibiscus Flower and Its Mutant with L-Leucine Accumulation for Awamori Brewing. Front. Genet. 2019, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Tan, Y.; Lv, X.; Chen, L.; Yang, F.; Wang, H.; Du, H.; Wang, L.; Xu, Y. Microbial Community Succession and Its Environment Driving Factors During Initial Fermentation of Maotai-Flavor Baijiu. Front. Microbiol. 2021, 12, 669201. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, L.; Peng, Z.; Zhang, Q.; Huang, W.; Yang, F.; Du, G.; Zhang, J.; Wang, L. Analysis of environmental driving factors on core functional community during daqu fermentation. Food Res. Int. 2022, 157, 111286. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Niu, M.; Yu, H.; Shi, W.; Chai, L.; Lu, Z.; Liu, X.; Shen, C.; Xu, Z.; Wang, S.; et al. Exploring the contribution of temperature-adapted microbiota to enzyme profile of saccharification in Daqu using metagenomics and metaproteomics. LWT 2024, 197, 115916. [Google Scholar] [CrossRef]
- Yi, Z.; Jin, Y.; Xiao, Y.; Chen, L.; Tan, L.; Du, A.; He, K.; Liu, D.; Luo, H.; Fang, Y.; et al. Unraveling the Contribution of High Temperature Stage to Jiang-Flavor Daqu, a Liquor Starter for Production of Chinese Jiang-Flavor Baijiu, with Special Reference to Metatranscriptomics. Front. Microbiol. 2019, 10, 472. [Google Scholar] [CrossRef]
- Wu, S.; Chai, L.; Huang, T.; Shi, W.; Lu, Z.; Zhang, X.; Wang, S.; Shen, C.; Xu, Z. Isolation, screening, and metabolic characterization of Kroppenstedtia eburnea from the fermented grains of Jiang-flavor Baijiu. Acta Microbiol. Sin. 2024, 64, 2502–2521. [Google Scholar]
- Fan, W.; Xu, Y.; Qian, M. Current practice and future trends of aroma and flavor research in Chinese Baijiu. In Sex, Smoke, and Spirits: The Role of Chemistry; American Chemical Society: Washington, DC, USA, 2019; pp. 145–175. [Google Scholar]
- Zhang, H.; Du, H.; Xu, Y. Volatile organic compound-mediated antifungal activity of Pichia spp. and its effect on the metabolic profiles of fermentation communities. Appl. Environ. Microbiol. 2021, 87, e02992-20. [Google Scholar] [CrossRef]
- Kruis, A.; Bohnenkamp, A.; Patinios, C.; van Nuland, Y.; Levisson, M.; Mars, A.; Berg, C.v.D.; Kengen, S.; Weusthuis, R. Microbial production of short and medium chain esters: Enzymes, pathways, and applications. Biotechnol. Adv. 2019, 37, 107407. [Google Scholar] [CrossRef] [PubMed]
- Ru, H.; Xu, H.; Li, Q.; Yu, Y.; Zheng, Q. Equilibrium of esterification in Chinese distilled liquor (Baijiu) during ageing. LWT 2024, 192, 115735. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Wang, X.; Zhu, J.; Xu, J.; Zhang, R.; Cai, F.; Zhu, Z.; Cao, J.; Yu, Q. Optimizing Baijiu fermentation with high-yield ethyl caproate-producing Candida parapsilosis strain. LWT 2024, 203, 116347. [Google Scholar] [CrossRef]
- Chen, L.; Qin, X.; Wang, G.; Teng, M.; Zheng, Y.; Yang, F.; Du, H.; Wang, L.; Xu, Y. Oxygen influences spatial heterogeneity and microbial succession dynamics during Baijiu stacking process. Bioresour. Technol. 2024, 403, 130854. [Google Scholar] [CrossRef]

| Samples | Shannon | Simpson | Chao1 | Ace | Goods_Coverage | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | |
| L.4R.1 | 3.642 | 2 | 0.85 | 0.629 | 134.071 | 177.75 | 135.01 | 179.598 | 0.9999 | 0.9999 |
| L.4R.2 | 3.614 | 2.424 | 0.816 | 0.696 | 131.765 | 170.85 | 132.527 | 174.381 | 0.9999 | 0.9999 |
| L.4R.3 | 3.526 | 2.838 | 0.838 | 0.786 | 106.248 | 171.13 | 105.793 | 173.517 | 0.9998 | 0.9997 |
| L.4R.4 | 2.996 | 2.621 | 0.796 | 0.72 | 124.012 | 176.23 | 119.945 | 183.195 | 0.9999 | 0.9999 |
| L.4R.5 | 3.521 | 2.925 | 0.83 | 0.79 | 140.376 | 187.04 | 137.677 | 187.074 | 0.9999 | 0.9998 |
| H.4R.1 | 3.647 | 1.807 | 0.844 | 0.529 | 142.999 | 217.863 | 143.95 | 220.22 | 0.9999 | 0.9999 |
| H.4R.2 | 3.485 | 2.396 | 0.844 | 0.645 | 144.424 | 254.32 | 146.77 | 235.034 | 0.9997 | 0.9998 |
| H.4R.3 | 3.401 | 2.908 | 0.791 | 0.777 | 124.437 | 222.557 | 123.038 | 230.22 | 0.9998 | 0.9999 |
| H.4R.4 | 4.027 | 2.517 | 0.896 | 0.718 | 107.333 | 170.113 | 106.704 | 177.512 | 0.9998 | 0.9999 |
| H.4R.5 | 3.548 | 2.855 | 0.846 | 0.76 | 83.032 | 234.322 | 83.607 | 234.205 | 0.9999 | 0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, J.; Yan, Y.; Zhang, G.; Shen, Y.; Cheng, W.; Li, H.; Duan, Z.; Sun, J.; Wang, B.; Wu, J.; et al. Temporal and Workshop Heterogeneity of Microbial Communities with Physicochemical Properties and Flavor Substances During Stacked Fermentation of Sauce-Flavor Baijiu. Foods 2025, 14, 924. https://doi.org/10.3390/foods14060924
Niu J, Yan Y, Zhang G, Shen Y, Cheng W, Li H, Duan Z, Sun J, Wang B, Wu J, et al. Temporal and Workshop Heterogeneity of Microbial Communities with Physicochemical Properties and Flavor Substances During Stacked Fermentation of Sauce-Flavor Baijiu. Foods. 2025; 14(6):924. https://doi.org/10.3390/foods14060924
Chicago/Turabian StyleNiu, Jiao, Yahan Yan, Guihu Zhang, Yi Shen, Wei Cheng, Hehe Li, Zhongfu Duan, Jinyuan Sun, Bowen Wang, Jihong Wu, and et al. 2025. "Temporal and Workshop Heterogeneity of Microbial Communities with Physicochemical Properties and Flavor Substances During Stacked Fermentation of Sauce-Flavor Baijiu" Foods 14, no. 6: 924. https://doi.org/10.3390/foods14060924
APA StyleNiu, J., Yan, Y., Zhang, G., Shen, Y., Cheng, W., Li, H., Duan, Z., Sun, J., Wang, B., Wu, J., & Sun, B. (2025). Temporal and Workshop Heterogeneity of Microbial Communities with Physicochemical Properties and Flavor Substances During Stacked Fermentation of Sauce-Flavor Baijiu. Foods, 14(6), 924. https://doi.org/10.3390/foods14060924





