Advances in Detection Technologies for Pesticide Residues and Heavy Metals in Rice: A Comprehensive Review of Spectroscopy, Chromatography, and Biosensors
Abstract
:1. Introduction
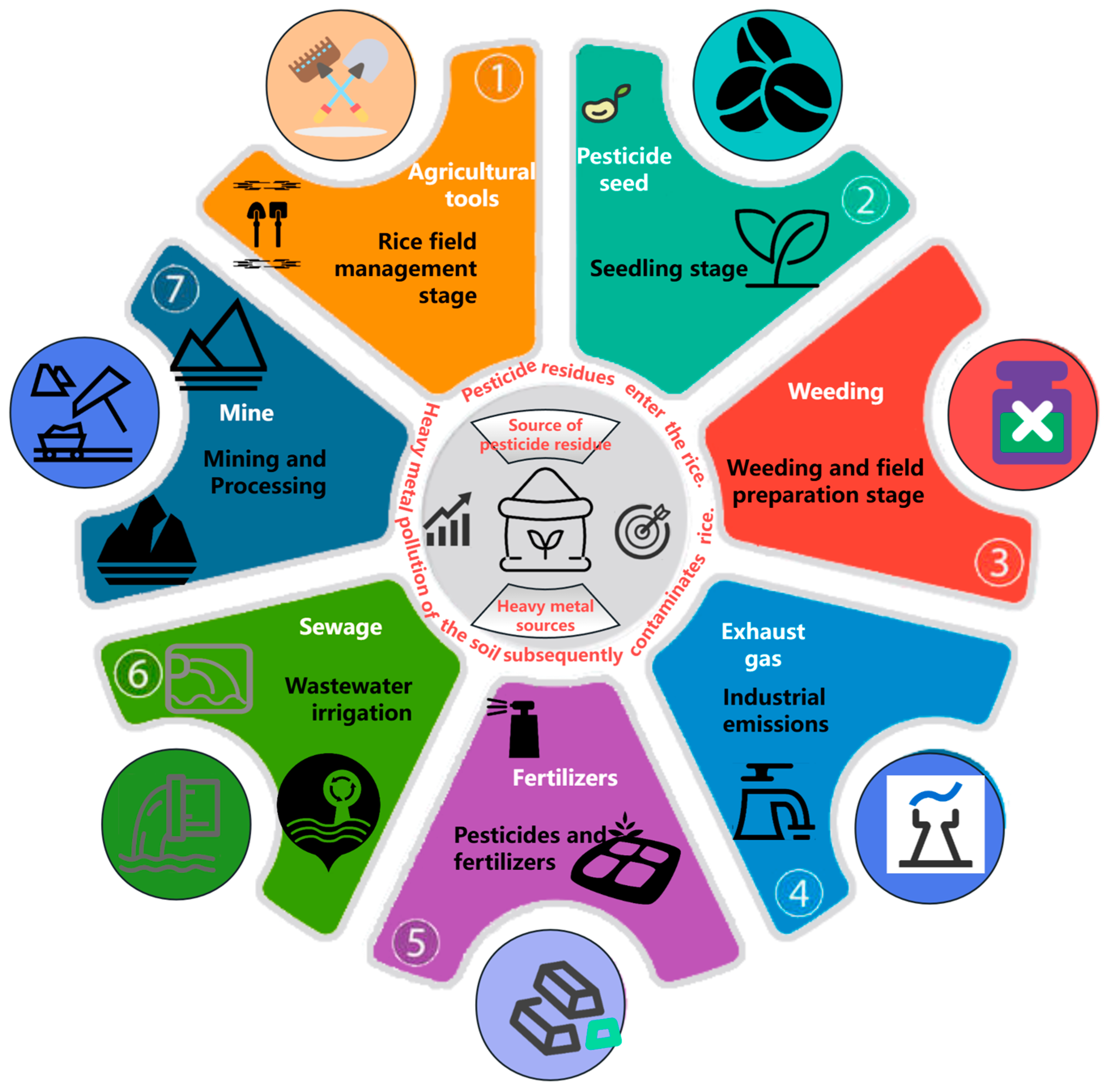
2. Types and Sources of Pesticide Residues and Heavy Metals in Rice
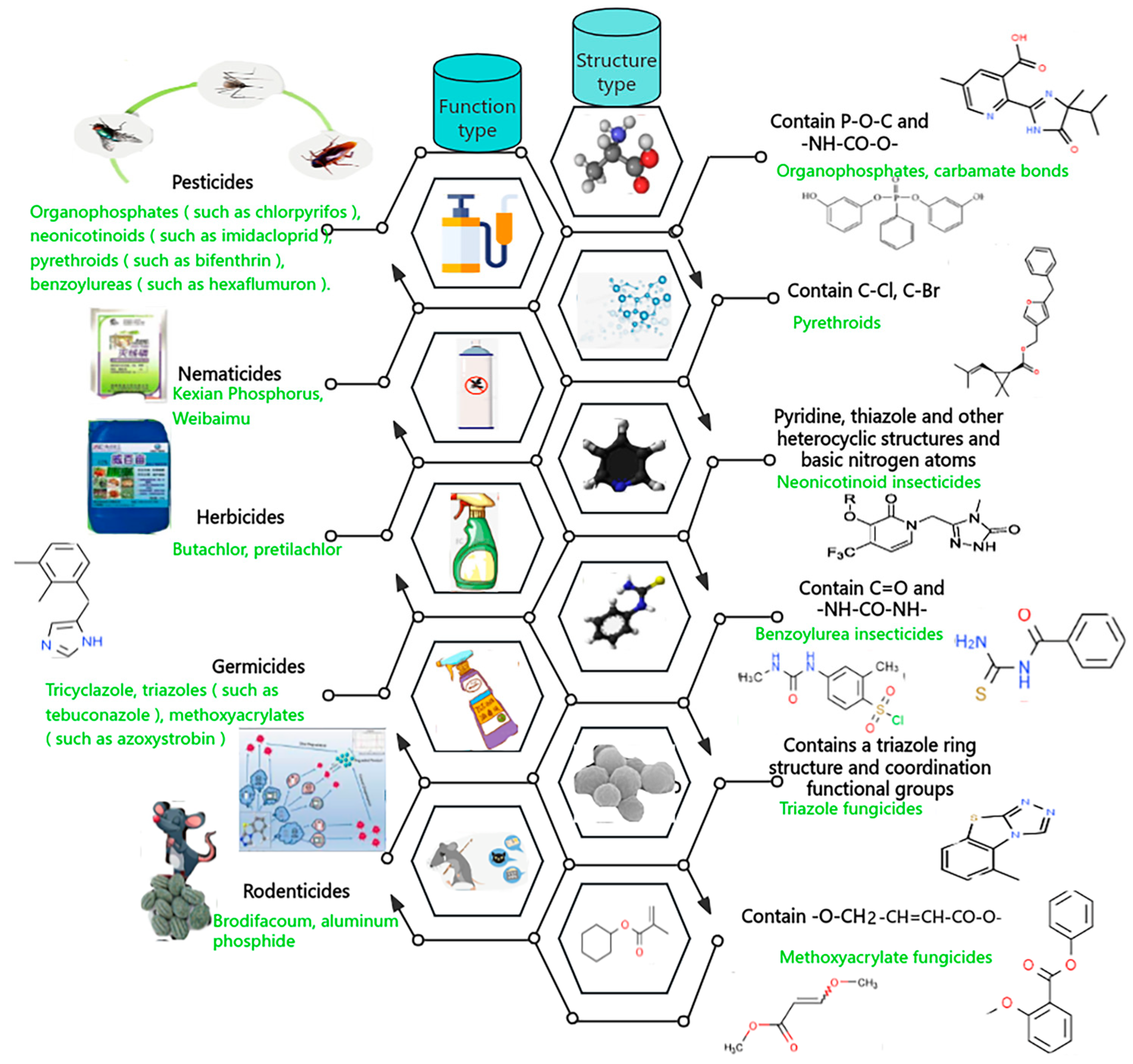
2.1. Types of Pesticide Residues
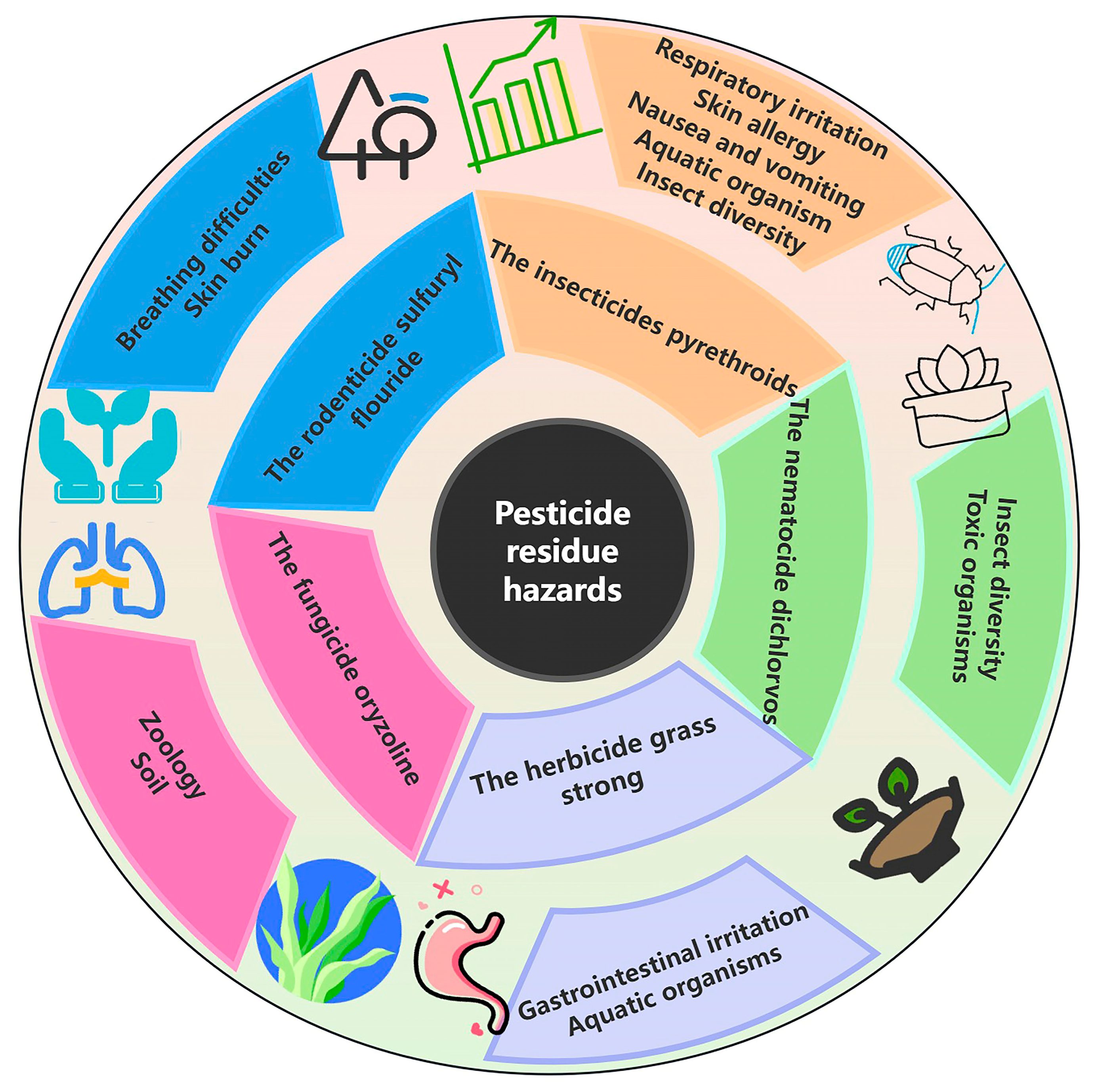
2.2. Health and Ecological Risks of Pesticide Residues
2.3. Types of Heavy Metals
2.3.1. Classification Based on Bioavailability
2.3.2. Classification Based on the Toxic Mechanisms of Action
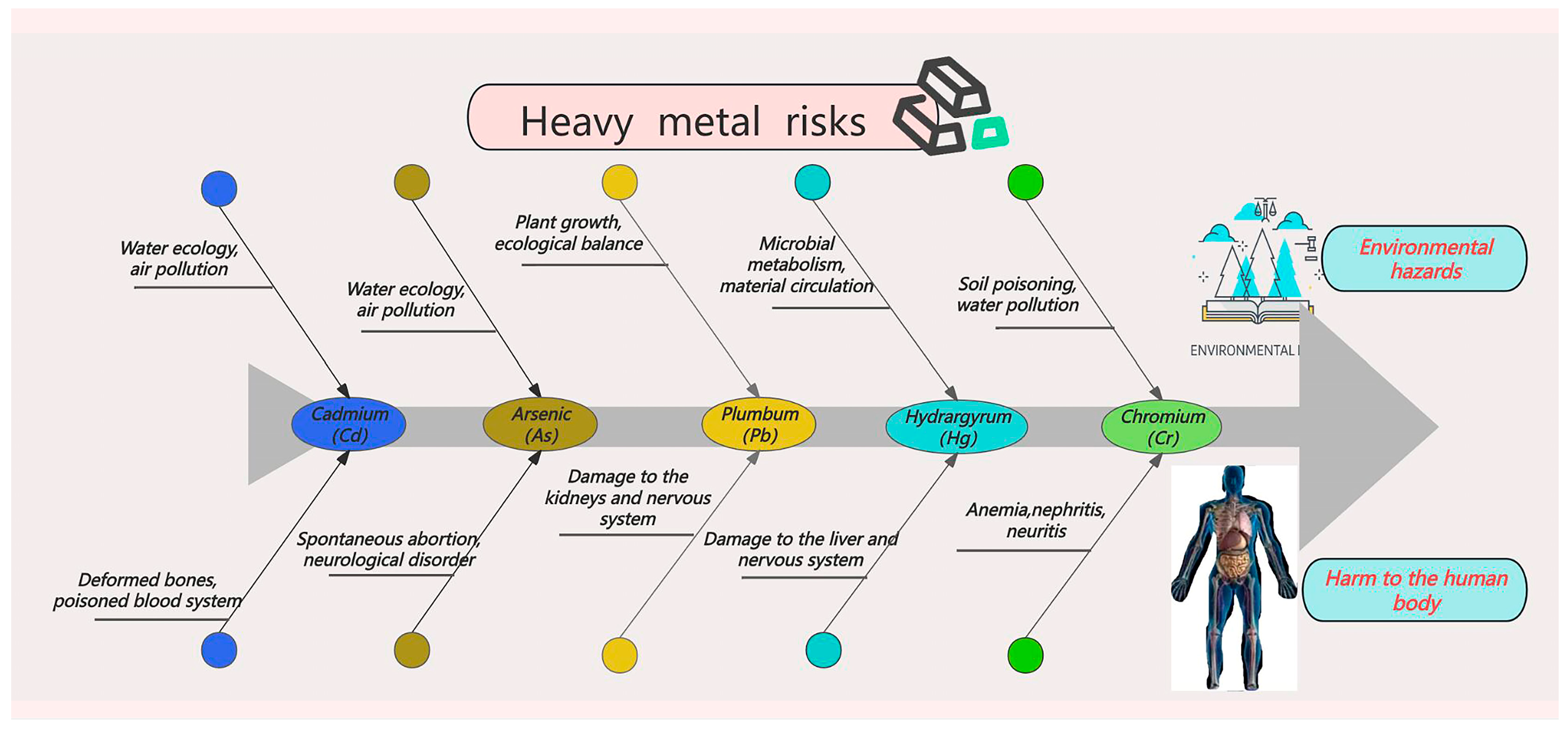
2.4. Risks of Heavy Metals
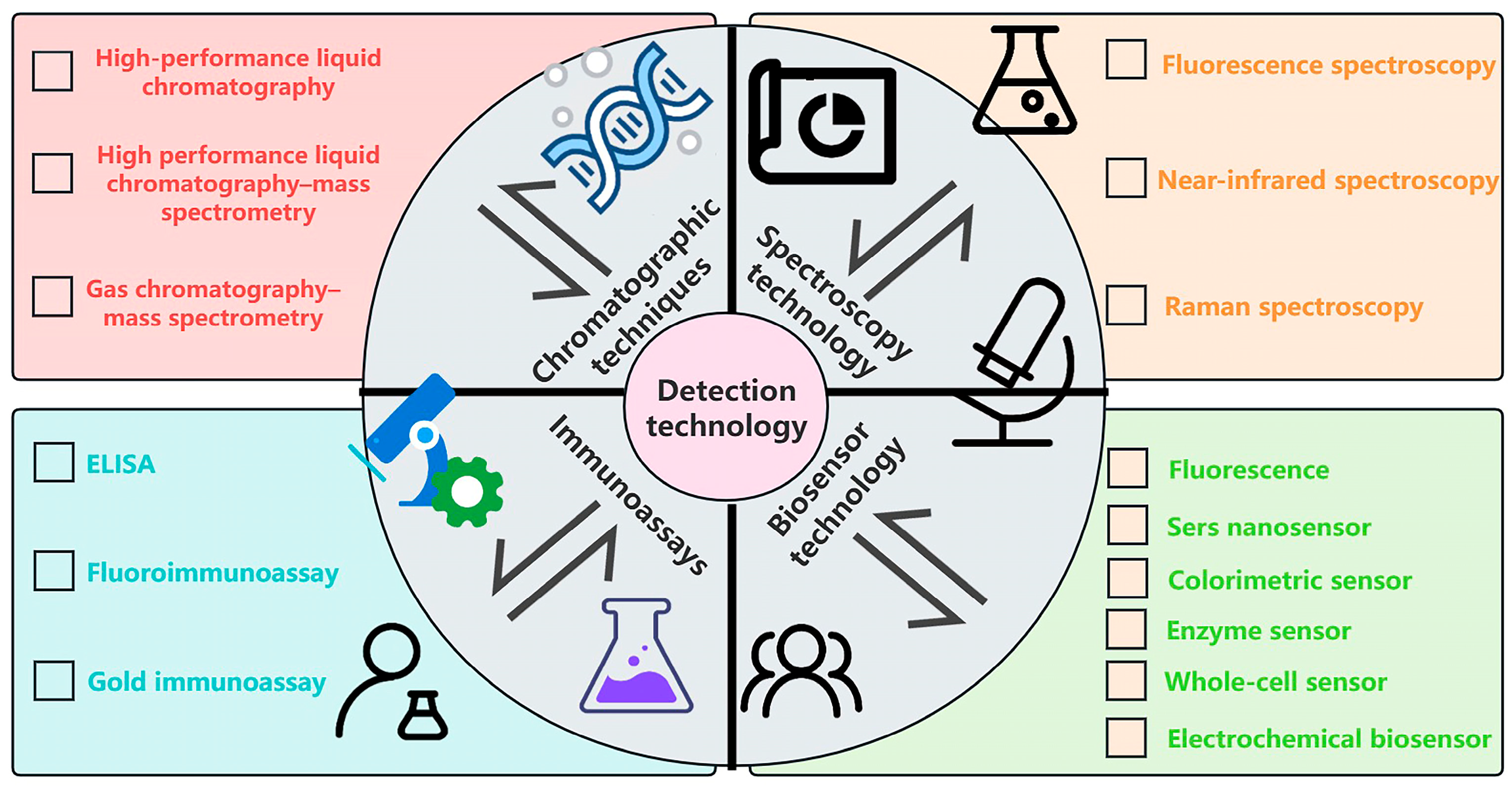
3. Detection Techniques for Pesticide Residues and Heavy Metals
3.1. Spectral Analysis Techniques in Pesticide Residue and Heavy Metal Detection
3.1.1. Fluorescence Spectrometry
3.1.2. Near-Infrared Spectroscopy
3.1.3. Raman Spectroscopy
3.2. Application of Chromatographic Techniques in Pesticide Residue and Heavy Metal Detection in Rice
3.2.1. High-Performance Liquid Chromatography
3.2.2. High-Performance Liquid Chromatography–Mass Spectrometry
3.2.3. Gas Chromatography–Mass Spectrometry
3.3. Immunoassays of Pesticide Residues and Heavy Metals
3.3.1. Enzyme Immunoassay
3.3.2. Fluorescence Immunoassay
3.3.3. Colloidal Gold Immunoassay
3.4. Biosensors in Pesticide Residue and Heavy Metal Detection
3.4.1. Fluorescent Biosensors
3.4.2. Surface-Enhanced Raman Scattering
3.4.3. Colorimetric Biosensors
3.4.4. Enzyme Biosensors
3.4.5. Whole-Cell Biosensors
3.4.6. Electrochemical Biosensors
| Material | Linear Range | LOD | Detection time (T)/Reproducibility (RSD or CV)/Stability (S) | Real Samples | Equipment Cost | Material Cost | Reference |
|---|---|---|---|---|---|---|---|
| Fluorescence spectrometry | 0.5–50 μg/L | 0.05 μg/L−1 | T = 190 s RSD = 1.9% | Rice | USD 80,000 | USD 30 | [101] |
| Near-infrared spectroscopy | 1–100,000 μg/L | ≤1 μg/L | / | Edible oil | USD 120,000 | USD 50 | [102] |
| Raman spectroscopy | 9.99 × 10−3–99.9 μg/L | 0.0999 μg/L | RSD ≤ 8.98% | Apple juice | USD 180,000 | USD 10 | [103] |
| HPLC | 1–100 μg/L | 4.5 μg/L | RDS = 3.8–13.3% | Rice, pea, wheat | USD 70,000 | USD 50 | [104] |
| HPLC-MS/MS | 5–60 μg/L | 0.01–0.2 μg/L | RSD ≤ 10% | Fish, tomato sauce | USD 1,000,000 | USD 500 | [105] |
| GC-MS | 100–10,000 μg/L | 4–780 μg/L | RSD ≤ 8% | Wood vinegar samples | USD 400,000 | USD 150 | [106] |
| ELISA | 0.35–1.0 μg/L | 0.03 μg/L | RSD ≤ 13% | Milk, eggs, honey | USD 10,000 | USD 10 | [107] |
| Fluoroimmunoassay | 5 × 10−5–0.1 μg/L | 1 × 10−5 μg/L | / | Animal-derived foods | USD 15,000 | USD 90 | [108] |
| Gold immunoassay | 0.02–0.75 μg/L | 0.012 μg/L | / | Shellfish samples | USD 5000 | USD 5 | [109] |
| Fluorescent biosensors | 6.6 × 10−3–5.94 μg/L | 1.06 × 10−3 μg/L | RSD ≤ 5.23% | Urine samples | USD 6000 | USD 120 | [110] |
| Surface-enhanced Raman biosensors | 0–8.49 × 107 μg/L | 4.25 × 106 μg/L | / | Xylene | USD 180,000 | USD 10 | [111] |
| Colorimetric biosensors | 10.0–110.0 μg/L | 4.7 μg/L | RDS = 1.90–5.44% | Spiked human serum samples | USD 5000 | USD 20 | [112] |
| Enzyme biosensors | 1.80–1.80 × 105 μg/L | 0.27 μg/L | / | Human serum | USD 10,000 | USD 80 | [113] |
| Whole-cell biosensors | 0.01–1 μg/mL | 0.037 μg/mL | / | Human serum | USD 30,000 | USD 120 | [114] |
| Electrochemical biosensors | 1 × 10−5–8.2 × 10−4 μg/L | 7 × 10−6 μg/L | T = 45 min RSD = 3.6–9.8% | Cereal and feed samples | USD 6000 | USD 30 | [115] |
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abid, J.; Ahmed, S.; Xia, T.; Wang, M. Rice as a Nutritional Grain: Examining Its Role in Healthy Products and Disease Prevention. Food Rev. Int. 2024, 40, 3055–3078. [Google Scholar] [CrossRef]
- Hoang, V.-N.; Nguyen, T.T.; Wilson, C.; Ho, T.Q.; Khanal, U. Scale and Scope Economies in Small Household Rice Farming in Vietnam. J. Integr. Agric. 2021, 20, 3339–3351. [Google Scholar] [CrossRef]
- Kadoglidou, K.; Kalaitzidis, A.; Stavrakoudis, D.; Mygdalia, A.; Katsantonis, D. A Novel Compost for Rice Cultivation Developed by Rice Industrial By-Products to Serve Circular Economy. Agronomy 2019, 9, 553. [Google Scholar] [CrossRef]
- Singh, S.; Rajput, V.D.; Upadhyay, S.K.; Minkina, T. Arsenic Contamination in Rice Agro-ecosystems: Mitigation Strategies for Safer Crop Production. J. Plant Growth Regul. 2023, 42, 6413–6424. [Google Scholar] [CrossRef]
- Akter, M.; Kabir, M.H.; Alam, M.A.; Al Mashuk, H.; Rahman, M.M.; Alam, M.S.; Brodie, G.; Islam, S.M.M.; Gaihre, Y.K.; Rahman, G.K.M.M. Geospatial Visualization and Ecological Risk Assessment of Heavy Metals in Rice Soil of a Newly Developed Industrial Zone in Bangladesh. Sustainability 2023, 15, 7208. [Google Scholar] [CrossRef]
- El Oirdi, M.; Yaseen, M.; Farwa, U.; Raza, M.; Farhan, M.; Sandhu, C.Z.A.; Ali, F.; Aatif, M.; Khan, H.; Nahvi, I. Crops and people: The dangers and potential benefits of pesticides. Cogent Food Agric. 2024, 10, 2334096. [Google Scholar] [CrossRef]
- Asiah, N.; David, W.; Ardiansyah; Madonna, S. Review on pesticide residue on rice. IOP Conf. Ser. Earth Environ. Sci. 2019, 379, 012008. [Google Scholar] [CrossRef]
- Fiankor, D.-D.D.; Curzi, D.; Olper, A. Trade, Price and Quality Upgrading Effects of Agri-Food Standards. Eur. Rev. Agric. Econ. 2021, 48, 835–877. [Google Scholar] [CrossRef]
- Mao, Y.; Tan, H.; Wang, M.; Jiang, T.; Wei, H.; Xu, W.; Jiang, Q.; Bao, H.; Ding, Y.; Wang, F.; et al. Research Progress of Soil Microorganisms in Response to Heavy Metals in Rice. J. Agric. Food Chem. 2022, 70, 8513–8522. [Google Scholar] [CrossRef]
- Vatanpour, N.; Feizy, J.; Hedayati Talouki, H.; Es’haghi, Z.; Scesi, L.; Malvandi, A.M. The High Levels of Heavy Metal Accumulation in Cultivated Rice from the Tajan River Basin: Health and Ecological Risk Assessment. Chemosphere 2020, 245, 125639. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy Metals: Toxicity and Human Health Effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar] [CrossRef] [PubMed]
- Gianì, F.; Masto, R.; Trovato, M.A.; Franco, A.; Pandini, G.; Vigneri, R. Thyroid Stem Cells but Not Differentiated Thyrocytes Are Sensitive to Slightly Increased Concentrations of Heavy Metals. Front. Endocrinol. 2021, 12, 652675. [Google Scholar] [CrossRef]
- Rowland-Jones, R.C.; van den Berg, F.; Racher, A.J.; Martin, E.B.; Jaques, C. Comparison of Spectroscopy Technologies for Improved Monitoring of Cell Culture Processes in Miniature Bioreactors. Biotechnol. Prog. 2017, 33, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, J.; Ren, W.; Feng, Z.; Huang, R.; Yin, Y. Transcriptomic Analysis on Responses of the Liver and Kidney of Finishing Pigs Fed Cd Contaminated Rice. J. Sci. Food Agric. 2018, 98, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Songprasert, N.; Sukaew, T.; Kusreesakul, K.; Swaddiwudhipong, W.; Padungtod, C.; Bundhamcharoen, K. Additional Burden of Diseases Associated with Cd Exposure: A Case Study of Cd Contaminated Rice Fields in Mae Sot District, Tak Province, Thailand. Int. J. Environ. Res. Public Health 2015, 12, 9199–9217. [Google Scholar] [CrossRef]
- Ge, P.; Chen, M.; Cui, Y.; Nie, D. The Research Progress of the Influence of Agricultural Activities on Atmospheric Environment in Recent Ten Years: A Review. Atmosphere 2021, 12, 635. [Google Scholar] [CrossRef]
- Mahboubifar, M.; Zidorn, C.; Farag, M.A.; Zayed, A.; Jassbi, A.R. Chemometric-Based Drug Discovery Approaches from Natural Origins Using Hyphenated Chromatographic Techniques. Phytochem. Anal. 2024, 35, 990–1016. [Google Scholar] [CrossRef]
- Liu, L.; Chang, Y.; Lou, J.; Zhang, S.; Yi, X. Overview on the Development of Alkaline-Phosphatase-Linked Optical Immunoassays. Molecules 2023, 28, 6565. [Google Scholar] [CrossRef]
- Łącki, K.M. High-throughput process development of chromatography steps: Advantages and limitations of different formats used. Biotechnol. J. 2012, 7, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and Microfluidic Biosensors: From Fabrication to Application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef]
- Mou, L.; Jiang, X. Materials for Microfluidic Immunoassays: A Review. Adv. Healthc. Mater. 2017, 6, 1601403. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.; Tang, X.; Sekine, Y.; Egawa, M.; Dwivedi, P.; Kitahama, Y.; Yang, W.; Goda, K. An Ultralow-Cost, Durable, Flexible Substrate for Ultrabroadband Surface-Enhanced Raman Spectroscopy. Adv. Photonics Res. 2023, 5, 2300291. [Google Scholar] [CrossRef]
- Caputo, M.; Lyles, J.; Salazar, M.; Quave, C. Lego mindstorms fraction collector: A low-cost tool for a preparative high performance liquid chromatography system. Anal. Chem. 2019, 92, 1687–1690. [Google Scholar] [CrossRef]
- Carpenter, A.C.; Paulsen, I.T.; Williams, T.C. Blueprints for Biosensors: Design, Limitations, and Applications. Genes 2018, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Brookes, G. Genetically Modified (GM) Crop Use 1996–2020: Environmental impacts associated with pesticide use change. GM Crops Food 2022, 13, 262–289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garud, A.; Pawar, S.; Patil, M.S.; Kale, S.R.; Patil, S. A Scientific Review of Pesticides: Classification, Toxicity, Health Effects, Sustainability, and Environmental Impact. Cureus 2024, 16, e67945. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Liu, T.; Deng, D.; Huang, L.; Min, M.; Xiao, X. Review: Progress towards research on the toxicology of pyrimethanil. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 283, 109940. [Google Scholar] [CrossRef]
- Bianchi, L.; Anunciato, V.M.; Gazola, T.; Perissato, S.M.; Dias, R.d.C.; Tropaldi, L.; Carbonari, C.A.; Velini, E.D. Effects of Glyphosate and Clethodim Alone and in Mixture in Sourgrass (Digitaria insularis). Crop Prot. 2020, 138, 105322. [Google Scholar] [CrossRef]
- Todorov, A.R.; Dryś, M.; Gazagnaire, E.; Podder, M.; Kilpeläinen, I. Cellulose Carbamates via Transcarbamoylation/Transurethanization of Methyl Carbamates in Superbase–Acid Conjugate Ionic Liquids. RSC Adv. 2024, 14, 23118–23128. [Google Scholar] [CrossRef]
- Matsuo, N. Discovery and Development of Pyrethroid Insecticides. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 378–400. [Google Scholar] [CrossRef]
- Hołyńska-Iwan, I.; Szewczyk-Golec, K. Pyrethroids: How They Affect Human and Animal Health? Medicina 2020, 56, 582. [Google Scholar] [CrossRef] [PubMed]
- Drewek, A.; Lubawy, J.; Domek, P.; Polak, J.; Słocińska, M.; Dzięgelewska, A.; Klimaszyk, P. Behavioral and Biochemical Effects of Glyphosate-Based Herbicide Roundup on Unionid Mussels: Are Mussels Good Indicators of Water Pollution with Glyphosate-Based Pesticides? Water 2024, 16, 1882. [Google Scholar] [CrossRef]
- Qian, H.; Yang, Q.; Qu, Y.; Ju, Z.; Zhou, W.; Gao, H. Hydrophobic Deep Eutectic Solvents Based Membrane Emulsification-Assisted Liquid-Phase Microextraction Method for Determination of Pyrethroids in Tea Beverages. J. Chromatogr. A 2020, 1623, 461204. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.J.; Wiberg-Larsen, P.; Baattrup-Pedersen, A.; Friberg, N.; Kronvang, B. Stream Habitat Structure Influences Macroinvertebrate Response to Pesticides. Environ. Pollut. 2012, 164, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kumar, J.; Melo, J.S.; Sandaka, B.P. Progressive Development in Biosensors for Detection of Dichlorvos Pesticide: A Review. J. Environ. Chem. Eng. 2021, 9, 105067. [Google Scholar] [CrossRef]
- Sharma, P.; Mittal, P. Paraquat (Herbicide) as a Cause of Parkinson’s Disease. Park. Relat. Disord. 2024, 119, 105932. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Zeng, C.; Ren, C.; Li, H.; Jagadeesh, R.V.; Yuan, Z.; Li, X. Borylation of Phenols Using Sulfuryl Fluoride Activation. Green Chem. 2023, 25, 7998–8006. [Google Scholar] [CrossRef]
- Magnusson, R.; Rittfeldt, L.; Åstot, C. Evaluation of Sorbent Materials for the Sampling and Analysis of Phosphine, Sulfuryl Fluoride and Methyl Bromide in Air. J. Chromatogr. A 2015, 1375, 17–26. [Google Scholar] [CrossRef]
- Souza-Arroyo, V.; Fabián, J.J.; Bucio-Ortiz, L.; Miranda-Labra, R.U.; Gomez-Quiroz, L.E.; Gutiérrez-Ruiz, M.C. The Mechanism of the Cd-Induced Toxicity and Cellular Response in the Liver. Toxicology 2022, 480, 153339. [Google Scholar] [CrossRef]
- Muvunyi, B.P.; Zou, W.; Zhan, J.; He, S.; Ye, G. Multi-Trait Genomic Prediction Models Enhance the Predictive Ability of Grain Trace Elements in Rice. Front. Genet. 2022, 13, 883853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaur, R.; Garkal, A.; Sarode, L.; Bangar, P.; Mehta, T.; Singh, D.P.; Rawal, R. Understanding Arsenic Toxicity: Implications for Environmental Exposure and Human Health. J. Hazard. Mater. Lett. 2024, 5, 100090. [Google Scholar] [CrossRef]
- Cheng, X.; Sheng, L.; Peng, S.; Thorley, E.; Cao, H.; Li, K. Integrated mechanism of heavy metal bioremediation from soil to rice (Oryza sativa L.) mediated by Enterococcus faecium. Plant Growth Regul. 2022, 97, 523–535. [Google Scholar] [CrossRef]
- Raj, K.; Das, A.P. Lead Pollution: Impact on Environment and Human Health and Approach for a Sustainable Solution. Environ. Chem. Ecotoxicol. 2023, 5, 79–85. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, C.; Malik, R.N.; Chen, J. Human exposure to chromite mining pollution, the toxicity mechanism and health impact. Heliyon 2024, 10, e40083. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Wu, S.; Lu, H.; Xu, S. Ratiometric Fluorescent Probe and Smartphone-Based Visual Recognition for H2O2 and Organophosphorus Pesticide Based on Ce3+/Ce4+ Cascade Enzyme Reaction. Food Chem. 2025, 469, 142577. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, J.; Wang, Y.; Wang, N.; Liu, F.; Chen, Z.; Wang, P.; Wang, S.; Shen, X. Rapid and Accurate Determination of Prohibited Components in Pesticides Based on near Infrared Spectroscopy. Infrared Phys. Technol. 2022, 121, 104038. [Google Scholar] [CrossRef]
- Xiong, Y.; Huang, J.; Wu, R.; Geng, X.; Zuo, H.; Wang, X.; Xu, L.; Ai, S. Exploring Surface-Enhanced Raman Spectroscopy (SERS) Characteristic Peaks Screening Methods for the Rapid Determination of Chlorpyrifos Residues in Rice. Appl. Spectrosc. 2023, 77, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Dastmard, R.; Kaveh, F.; Mehrabian, R.Z.; Ebadi, M.; Lemeski, E.T. Diazinon Removal from Aqueous Solutions Using Rice Husk-Carbonized Macromolecule. Paddy Water Environ. 2023, 21, 377–387. [Google Scholar] [CrossRef]
- Ding, J.; Li, C.; Yin, H.; Zhou, Y.; Wang, S.; Liu, K.; Li, M.; Wang, J. One-Pot Solvothermal Synthesis of Bi/Bi2S3/Bi2WO6 S-Scheme Heterojunction with Enhanced Photoactivity towards Antibiotic Oxytetracycline Degradation under Visible Light. Environ. Pollut. 2023, 327, 121550. [Google Scholar] [CrossRef]
- Medina, M.B.; Munitz, M.S.; Resnik, S.L. Fate and Health Risks Assessment of Some Pesticides Residues during Industrial Rice Processing in Argentina. J. Food Compos. Anal. 2021, 98, 103823. [Google Scholar] [CrossRef]
- Jia, B.Z.; Chen, F.Y.; Yang, X.X.; Hongsibsong, S.; Wang, X.X.; Xu, Z.L.; Luo, L. De Novo Synthesis of a Novel Hapten and Development of a Monoclonal Antibody–Based Immunoassay for the Detection of Dichlorvos and Trichlorfon. Food Chem. 2024, 452, 139580. [Google Scholar] [CrossRef]
- Li, M.; Ma, M.; Hua, X.; Shi, H.; Wang, Q.; Wang, M. Quantum Dots-Based Fluoroimmunoassay for the Simultaneous Detection of Clothianidin and Thiacloprid in Environmental and Agricultural Samples. RSC Adv. 2014, 5, 3039–3044. [Google Scholar] [CrossRef]
- Xie, Y.; Kou, Q.; Sun, Q.; Wang, Y.; Cao, Y.; Le, T. Development and validation of an immunochromatography test strip for rapid detection of pyrimethanil residues. Food Agric. Immunol. 2020, 31, 393–405. [Google Scholar] [CrossRef]
- Liu, J.; Mo, Y.Y.; Zhang, H.; Tang, J.; Bao, H.; Wei, L.; Yang, H. Target-Responsive Metal–Organic Framework Nanosystem with Synergetic Sensitive Detection and Controllable Degradation against the Pesticide Triazophos in Contaminated Samples for Environment Assessment and Food Safety. ACS Appl. Mater. Interfaces 2023, 15, 23783–23791. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Chen, Y.; Zhang, Q.; Kang, C.; Chen, D. Detection of Chlorpyrifos Residue in Apple and Rice Samples Based on Aptamer Sensor: Improving Quantitative Accuracy with Partial Least Squares Model. Microchem. J. 2023, 194, 109352. [Google Scholar] [CrossRef]
- Gogol’, E.V.; Evtyugin, G.A.; Suprun, E.V.; Budnikov, G.K.; Vinter, V.G. Determination of Residual Pesticide in Plant Materials Using Planar Cholinesterase Sensors Modified with Nafion. J. Anal. Chem. 2001, 56, 963–970. [Google Scholar] [CrossRef]
- Xia, Z.; Zhou, Q.; Zhang, Y.; Zhang, T.; Xue, W. N-Succinyl-Chitosan as Ecofriendly Pesticide Carriers: Nano Encapsulation and Synergistic Antifungal Effect on 4-Hydroxyphenyl-2-Propenyl-1-One Derivatives Based on Chalcone Structure. J. Adv. Res. 2024, in press. [CrossRef]
- Wang, X.; Chen, X.; Ji, R.; Wang, T.; He, Y.; Bian, H.; Wang, X.; Hu, W. Mixed Pesticide Recognition Based on Three-Dimensional Fluorescence Spectroscopy and a Convolutional Neural Network. Appl. Opt. 2023, 62, 9018–9027. [Google Scholar] [CrossRef]
- Khan, A.; Khan, M.S.; Hadi, F.; Saddiq, G.; Khan, A.N. Energy-Dispersive X-ray (EDX) Fluorescence Based Analysis of Heavy Metals in Marble Powder, Paddy Soil and Rice (Oryza sativa L.) with Potential Health Risks in District Malakand, Khyber Pakhtunkhwa, Pakistan. Environ. Pollut. Bioavailab. 2021, 33, 301–316. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Li, Y.; Liu, Y.; Li, H.; Jiang, H.; Luo, X.; Chen, L.; Xiong, T.; Guo, H. Identifying the Fate of Cd Passivation during the Long-Term Composting of Rice Straw: Role of Humic Substances. J. Soils Sediments 2024, 24, 525–536. [Google Scholar] [CrossRef]
- Jarruwat, P.; Choomjaihan, P. Feasibility Study on Estimation of Rice Weevil Quantity in Rice Stock Using Near-Infrared Spectroscopy Technique. J. Innov. Opt. Health Sci. 2014, 07, 1450001. [Google Scholar] [CrossRef]
- Bernardes, R.C.; Botina, L.L.; Ribas, A.; Soares, J.M.; Martins, G.F. Artificial Intelligence-Driven Tool for Spectral Analysis: Identifying Pesticide Contamination in Bees from Reflectance Profiling. J. Hazard. Mater. 2024, 480, 136425. [Google Scholar] [CrossRef] [PubMed]
- Logan, N.; Haughey, S.A.; Liu, L.; Burns, D.T.; Quinn, B.; Cao, C.; Elliott, C.T. Handheld SERS Coupled with QuEChERs for the Sensitive Analysis of Multiple Pesticides in Basmati Rice. Npj Sci. Food 2022, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Wang, T.; Li, X.; Li, X. Polylactic Acid–Graphene Oxide-Based Materials for Loading and Sustained Release of Poorly Soluble Pesticides. Langmuir 2020, 36, 12336–12345. [Google Scholar] [CrossRef]
- Banerjee, D.; Adhikary, S.; Bhattacharya, S.; Chakraborty, A.; Dutta, S.; Chatterjee, S.; Ganguly, A.; Nanda, S.; Rajak, P. Breaking Boundaries: Artificial Intelligence for Pesticide Detection and Eco-Friendly Degradation. Environ. Res. 2024, 241, 117601. [Google Scholar] [CrossRef]
- Vieira, L.H.S.; Sicupira, L.C.; Cacique, A.P.; Silvério, F.O. Optimization and validation of LLE-LTP to determine florpyrauxifen-benzyl herbicide in water samples by HPLC-DAD. J. Environ. Sci. Health B 2022, 57, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Choi, E.M.; Lee, Y.; Park, K.S. Study of the Transition Pattern of Heavy Metal Absorption in a Rice-Related Matrix. Anal. Lett. 2021, 54, 2171–2181. [Google Scholar]
- Manca, E.; Noli, B.; Corda, G.; El-Hassani, M.; Manai, A.; Sanna, F.; Argiolas, A.; Melis, M.R.; Manconi, B.; Contini, C.; et al. VGF Modifications Related to Nigrostriatal Dopaminergic Neurodegeneration Induced by the Pesticide Fipronil in Adult Male Rats. Ann. Anat.-Anat. Anz. 2024, 252, 152194. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Jia, H.; Zhou, W.; Li, B.; Huang, H. Residue Analysis of Tetraniliprole in Rice and Related Environmental Samples by HPLC/MS. Microchem. J. 2019, 150, 104168. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Liu, L.; Min, J.; Liu, W.; Li, X.; Pan, X.; Lu, X.; Deng, Q. Comparative Proteomics in Rice Seedlings to Characterize the Resistance to Cd Stress by High-Performance Liquid Chromatography–Tandem Mass Spectrometry (HPLC-MS/MS) with Isobaric Tag for Relative and Absolute Quantitation (iTRAQ). Anal. Lett. 2020, 53, 807–820. [Google Scholar] [CrossRef]
- Nozari, M.; Esmaili-sari, A.; Moradi, A.M.; Bahramifar, N.; Taghavi, L. Contamination, Ecological, and Health Risk Assessment of Heavy Metals and Organophosphorus Pesticides in Single, Double, and Ratoon Cropping of Rice: A Case Study in Mazandaran, North of Iran. Environ. Monit. Assess. 2023, 195, 376. [Google Scholar] [CrossRef]
- Ullah, I.; Mateen, A.; Ahmad, M.A.; Munir, I.; Iqbal, A.; Alghamdi, K.M.S.; Al-Solami, H.M.; Siddiqui, M.F. Heavy Metal ATPase Genes (HMAs) Expression Induced by Endophytic Bacteria, “AI001, and AI002” Mediate Cd Translocation and Phytoremediation. Environ. Pollut. 2022, 293, 118508. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Nakamura, K.; Naito, S. Interlaboratory Study of Immunochromatography for the Rapid Determination of Cd Concentrations in Cereals and Soybeans. J. AOAC Int. 2014, 97, 913–920. [Google Scholar] [CrossRef]
- Sheng, E.; Shi, H.; Zhou, L.; Hua, X.; Feng, L.; Yu, T.; Wang, M. Dual-labeled time-resolved fluoroimmunoassay for simultaneous detection of clothianidin and diniconazole in agricultural samples. Food Chem. 2016, 192, 525–530. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, P.; Shu, Y.; Jing, P.; Xu, L.; Xu, C.H.; Guo, L. A Colloidal Gold Immunoassay Strip Assay for Cadmium Detection in Oilfield Chemicals. Analyst 2023, 148, 4166–4173. [Google Scholar] [CrossRef]
- Abbas, Y.; Deader, F.A.; Qurashi, A.; Al-Qutayri, M.; Chan, V.; Rezeq, M. Gold Nanoparticles Monolayer Based Field-Effect Molecular Sensors. Adv. Electron. Mater. 2024, 11, 2400363. [Google Scholar] [CrossRef]
- Li, Y.; Han, H.; Fei, Y.; Pan, D.; Wang, H. Optimization and validation of a honeycomb-like micro-needle sensor with gold nanoparticles embedded for the determination of hexavalent chromium in seawater. Microchem. J. 2024, 197, 109857. [Google Scholar] [CrossRef]
- Nguyen, N.D.; Nguyen, T.V.; Chu, A.D.; Tran, H.V.; Tran, L.T.; Huynh, C.D. A Label-Free Colorimetric Sensor Based on Silver Nanoparticles Directed to Hydrogen Peroxide and Glucose. Arab. J. Chem. 2018, 11, 1134–1143. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhang, Q.Q.; Yao, S.Y.; Cui, H.W.; Zou, Y.L.; Zhao, L.X. Dual-Recognition “Turn-off-on” Fluorescent Biosensor Triphenylamine-Based Continuous Detection of Copper Ion and Glyphosate Applicated in Environment and Living System. J. Hazard. Mater. 2024, 477, 135216. [Google Scholar] [CrossRef]
- Jin, H.; Liu, R.; Bai, T.; Wei, M.; He, B.; Suo, Z. A Low-Noise Ratiometric Fluorescence Biosensor for Detection of Pb2+ Based on DNAzyme and Exonuclease III–Assisted Cascade Signal Amplification. Anal. Bioanal. Chem. 2022, 414, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Serafinelli, C.; Fantoni, A.; Alegria, E.C.B.A.; Vieira, M. Plasmonic Metal Nanoparticles Hybridized with 2D Nanomaterials for SERS Detection: A Review. Biosensors 2022, 12, 225. [Google Scholar] [CrossRef]
- Li, J.; Shu, Y.; Li, C.; Jiang, Z. Highly Catalytic Nanoenzyme of Covalent Organic Framework Loaded Starch- Surface-Enhanced Raman Scattering/Absorption Bi-Mode Peptide as Biosensor for Ultratrace Determination of Cd. Front. Nutr. 2023, 9, 1075296. [Google Scholar] [CrossRef]
- Nigam, V.K.; Shukla, P. Enzyme Based Biosensors for Detection of Environmental Pollutants—A Review. J. Microbiol. Biotechnol. 2015, 25, 1773–1781. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Wu, Z.; Zhang, F.; Wang, Y.; Wang, X.; Zhang, Z.; Li, C.; Lv, X.; Chen, D.; et al. Universal Method for Label-Free Detection of Pathogens and Biomolecules by Surface-Enhanced Raman Spectroscopy Based on Gold Nanoparticles. Anal. Chem. 2023, 95, 4050–4058. [Google Scholar] [CrossRef]
- Chen, L.; Choo, J. Recent Advances in Surface—Enhanced Raman Scattering Detection Technology for Microfluidic Chips. Electrophoresis 2008, 29, 1815–1828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhong, G.; Kim, D.-E.; Liu, J.; Liu, X. A Portable Lab-on-a-Chip System for Gold-Nanoparticle-Based Colorimetric Detection of Metal Ions in Water. Biomicrofluidics 2014, 8, 052107. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, M.M.; Nguyen, T.A.; Arduini, F.; Bagheri, H. A Paper-Based Colorimetric Sensor Array for Discrimination and Simultaneous Determination of Organophosphate and Carbamate Pesticides in Tap Water, Apple Juice, and Rice. Microchim. Acta 2020, 187, 621. [Google Scholar] [CrossRef]
- Yang, W.; Luo, D.; Xiao, Y.; Zheng, S.; Wang, Z.; Fu, F. Isolation and characterization of the quinclorac-specific aptamer toward a colorimetric sensor for the visual detection of quinclorac in water and soil. Sens. Actuators B Chem. 2023, 393, 134185. [Google Scholar] [CrossRef]
- Ghazy, A.; Nyarku, R.; Faraj, R.; Bentum, K.; Woube, Y.; Williams, M.; Alocilja, E.; Abebe, W. Gold Nanoparticle-Based Plasmonic Detection of Escherichia coli, Salmonella enterica, Campylobacter jejuni, and Listeria monocytogenes from Bovine Fecal Samples. Microorganisms 2024, 12, 1069. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Xue, Z.; Peng, B.; Kou, X.; Gao, Z. Research Progress of Dual-Mode Sensing Technology Strategy Based on SERS and Its Application in the Detection of Harmful Substances in Foods. Trends Food Sci. Technol. 2024, 148, 104487. [Google Scholar] [CrossRef]
- Gogol, E.V.; Evtugyn, G.A.; Marty, J.-L.; Budnikov, H.C.; Winter, V.G. Amperometric biosensors based on nafion coated screen-printed electrodes for the determination of cholinesterase inhibitors. Talanta 2000, 53, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Raveena; Ngasainao, M.R.; Kumari, P. Development of Enzyme-Based Biosensor for Residual Detection of Organophosphate Pesticides Using Functionalized Nanocellulose-Modified Silver Nanoparticles. Microchem. J. 2024, 207, 111856. [Google Scholar] [CrossRef]
- Elsebai, B.; Ghica, M.E.; Abbas, M.N.; Brett, C.M.A. Catalase Based Hydrogen Peroxide Biosensor for Mercury Determination by Inhibition Measurements. J. Hazard. Mater. 2017, 340, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, J.; Kang, X.; Wang, C.; Wang, D.; Liu, J.; Aksay, I.A.; Lin, Y. Glucose Biosensor Based on Immobilization of Glucose Oxidase in Platinum Nanoparticles/Graphene/Chitosan Nanocomposite Film. Talanta 2009, 80, 403–406. [Google Scholar] [CrossRef]
- Francisco, R.; Branco, R.; Schwab, S.; Baldani, J.I.; Morais, P.V. Impact of plant-associated bacteria biosensors on plant growth in the presence of hexavalent chromium. World J. Microbiol. Biotechnol. 2018, 34, 201834. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ali, L.; Yoon, H.; Reid, M.C. Effects of organic sulfur and arsenite/dissolved organic matter ratios on arsenite complexation with dissolved organic matter. Chemosphere 2022, 302, 134770. [Google Scholar] [CrossRef]
- Mulyasuryani, A.; Dofir, M. Enzyme Biosensor for Detection of Organophosphate Pesticide Residues Base on Screen Printed Carbon Electrode (SPCE)-Bovine Serum Albumin (BSA). Engineering 2014, 6, 230–235. [Google Scholar] [CrossRef]
- Cui, X.; Zhou, Y.; Zheng, Y.; Hu, Y.; Wang, Z.; Gao, L.; Cao, L.; Yin, H.; Ai, S. Investigation the Effect of Antibiotics on the Content of N6-Methyladenosine in Rice Seedling Tissue and Heavy Metal on FTO Activity Based on Antibody-Free Photoelectrochemcial Biosensor. Sens. Actuators B Chem. 2022, 364, 131896. [Google Scholar] [CrossRef]
- Tanwar, S.; Mathur, D. Graphene-Based Nanocomposites as Sensing Elements for the Electrochemical Detection of Pesticides: A Review. J. Solid State Electrochem. 2021, 25, 2145–2159. [Google Scholar] [CrossRef]
- Yao, Z.; Liu, M.; Liu, J.; Mao, X.; Na, X.; Ma, Z.; Qian, Y. Sensitivity Enhancement of Inorganic Arsenic Analysis by In Situ Microplasma Preconcentration Coupled with Liquid Chromatography Atomic Fluorescence Spectrometry. J. Anal. At. Spectrom. 2020, 35, 1654–1663. [Google Scholar] [CrossRef]
- Lin, H.; Jiang, H.; He, P.; Haruna, S.A.; Chen, Q.; Xue, Z.; Chan, C.; Ali, S. Non-Destructive Detection of Heavy Metals in Vegetable Oil Based on Nano-Chemoselective Response Dye Combined with near-Infrared Spectroscopy. Sens. Actuators B Chem. 2021, 335, 129716. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, L.; Song, C.; Yuan, H.; Li, X. Quantitative Detection of Dithiocarbamate Pesticides by Surface-Enhanced Raman Spectroscopy Combined with an Exhaustive Peak-Seeking Method. Anal. Methods 2021, 13, 1479–1488. [Google Scholar] [CrossRef]
- Li, Z.; Lu, S.; Jin, J.; Wang, T. Preparation of a New Cellulose Magnetic Molecularly Imprinted Polymer Micro-Spheres to Extract and Analyze the Indole-3-Acetic Acid in Plant Tissues. J. Chromatogr. B 2018, 1092, 343–349. [Google Scholar] [CrossRef]
- Cui, S.; Mao, X.; Zhang, H.; Zeng, H.; Lin, Z.; Zhang, X.; Qi, P. Magnetic Solid-Phase Extraction Based on Magnetic Sulfonated Reduced Graphene Oxide for HPLC–MS/MS Analysis of Illegal Basic Dyes in Foods. Molecules 2021, 26, 7427. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Xun, H.; Yao, X.; Tang, F. Simultaneous Quantification of Twelve Compounds from Bamboo/Wood Vinegar by Gas Chromatography-Mass Spectrometry. Separations 2024, 11, 168. [Google Scholar] [CrossRef]
- Yu, F.; Yu, S.; Yu, L.; Li, Y.; Wu, Y.; Zhang, H.; Qu, L.; de Harrington, P.B. Determination of Residual Enrofloxacin in Food Samples by a Sensitive Method of Chemiluminescence Enzyme Immunoassay. Food Chem. 2014, 149, 71–75. [Google Scholar] [CrossRef]
- Sheng, W.; Huang, N.; Liu, Y.; Zhang, B.; Zhang, W.; Wang, S. An Ultrasensitive Fluorescence Immunoassay Based on Magnetic Separation and Upconversion Nanoparticles as Labels for the Detection of Chloramphenicol in Animal-Derived Foods. Food Anal. Methods 2020, 13, 2039–2049. [Google Scholar] [CrossRef]
- Wang, R.; Zeng, L.; Yang, H.; Zhong, Y.; Wang, J.; Ling, S.; Farhan Saeed, A.; Yuan, J.; Wang, S. Detection of Okadaic Acid (OA) Using ELISA and Colloidal Gold Immunoassay Based on Monoclonal Antibody. J. Hazard. Mater. 2017, 339, 154–160. [Google Scholar] [CrossRef]
- Dwivedi, A.; Srivastava, M.; Srivastava, A.; Kumar, A.; Chaurasia, R.N.; Srivastava, S.K. A Eu3+doped Functional Core-Shell Nanophosphor as Fluorescent Biosensor for Highly Selective and Sensitive Detection of dsDNA. J. Photochem. Photobiol. B 2023, 249, 112802. [Google Scholar] [CrossRef]
- Weber, V.; Brigo, L.; Brusatin, G.; Mattei, G.; Pedron, D.; Pilot, R.; Signorini, R. Hybrid Sol-Gel Surface-Enhanced Raman Sensor for Xylene Detection in Solution. Sensors 2021, 21, 7912. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Lv, Q.; Wang, C.; Liang, J.; Zhou, Z.; Li, G. Colorimetric Biosensor for Visual Determination of Golgi Protein 73 Based on Reduced Graphene Oxide-Carboxymethyl Chitosan-Hemin/Platinum@palladium Nanozyme with Peroxidase-like Activity. Microchim. Acta 2022, 189, 392. [Google Scholar] [CrossRef]
- Huang, J.; Xu, S.; Yan, F.; Liu, J. Electrochemiluminescence Enzyme Biosensors for Ultrasensitive Determination of Glucose Using Glucose Dehydrogenase Immobilized on Vertical Silica Nanochannels. Sens. Actuators B Chem. 2024, 402, 135119. [Google Scholar] [CrossRef]
- He, Y.; Xu, Z.; Kasputis, T.; Zhao, X.; Ibañez, I.; Pavan, F.; Bok, M.; Malito, J.P.; Parreno, V.; Yuan, L.; et al. Development of Nanobody-Displayed Whole-Cell Biosensors for the Colorimetric Detection of SARS-CoV-2. ACS Appl. Mater. Interfaces 2023, 15, 37184–37192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Xie, H.; Sun, R.; Cao, T.; Paudyal, N.; Fang, W.; Song, H. Development of a Magnetic Nanoparticles-Based Screen-Printed Electrodes (MNPs-SPEs) Biosensor for the Quantification of Ochratoxin A in Cereal and Feed Samples. Toxins 2018, 10, 317. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Tian, Y.; Li, Q.; Yao, T.; Yao, J.; Zhang, Z.; Wu, L. Advances in Detection Technologies for Pesticide Residues and Heavy Metals in Rice: A Comprehensive Review of Spectroscopy, Chromatography, and Biosensors. Foods 2025, 14, 1070. https://doi.org/10.3390/foods14061070
Han Y, Tian Y, Li Q, Yao T, Yao J, Zhang Z, Wu L. Advances in Detection Technologies for Pesticide Residues and Heavy Metals in Rice: A Comprehensive Review of Spectroscopy, Chromatography, and Biosensors. Foods. 2025; 14(6):1070. https://doi.org/10.3390/foods14061070
Chicago/Turabian StyleHan, Yu, Ye Tian, Qingqing Li, Tianle Yao, Jie Yao, Zhengmao Zhang, and Long Wu. 2025. "Advances in Detection Technologies for Pesticide Residues and Heavy Metals in Rice: A Comprehensive Review of Spectroscopy, Chromatography, and Biosensors" Foods 14, no. 6: 1070. https://doi.org/10.3390/foods14061070
APA StyleHan, Y., Tian, Y., Li, Q., Yao, T., Yao, J., Zhang, Z., & Wu, L. (2025). Advances in Detection Technologies for Pesticide Residues and Heavy Metals in Rice: A Comprehensive Review of Spectroscopy, Chromatography, and Biosensors. Foods, 14(6), 1070. https://doi.org/10.3390/foods14061070







