Improving Triterpenoid Extraction Efficiency from Inonotus hispidus Using Macroporous Adsorption Resin: An Aim for Functional Food Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Materials
2.2. Culture Media of Fruiting Bodies
2.3. Strain Cultivation
2.4. Cultivation of Fruiting Bodies
2.5. Pretreatment of MAR
2.6. Preparation of the Crude Extract of Triterpenoids
2.7. Determination of Total Triterpenoid Content
2.8. Static Adsorption–Desorption of MAR
2.8.1. Screening of MAR
2.8.2. Adsorption Kinetics
2.8.3. Adsorption Isotherm Evaluation
2.8.4. Screening of Desorption Concentrations
2.9. Dynamic Adsorption and Desorption of MAR HPD-600
2.10. UPLC-Q-TOF-MS Conditions
2.11. Statistical Analysis
3. Results
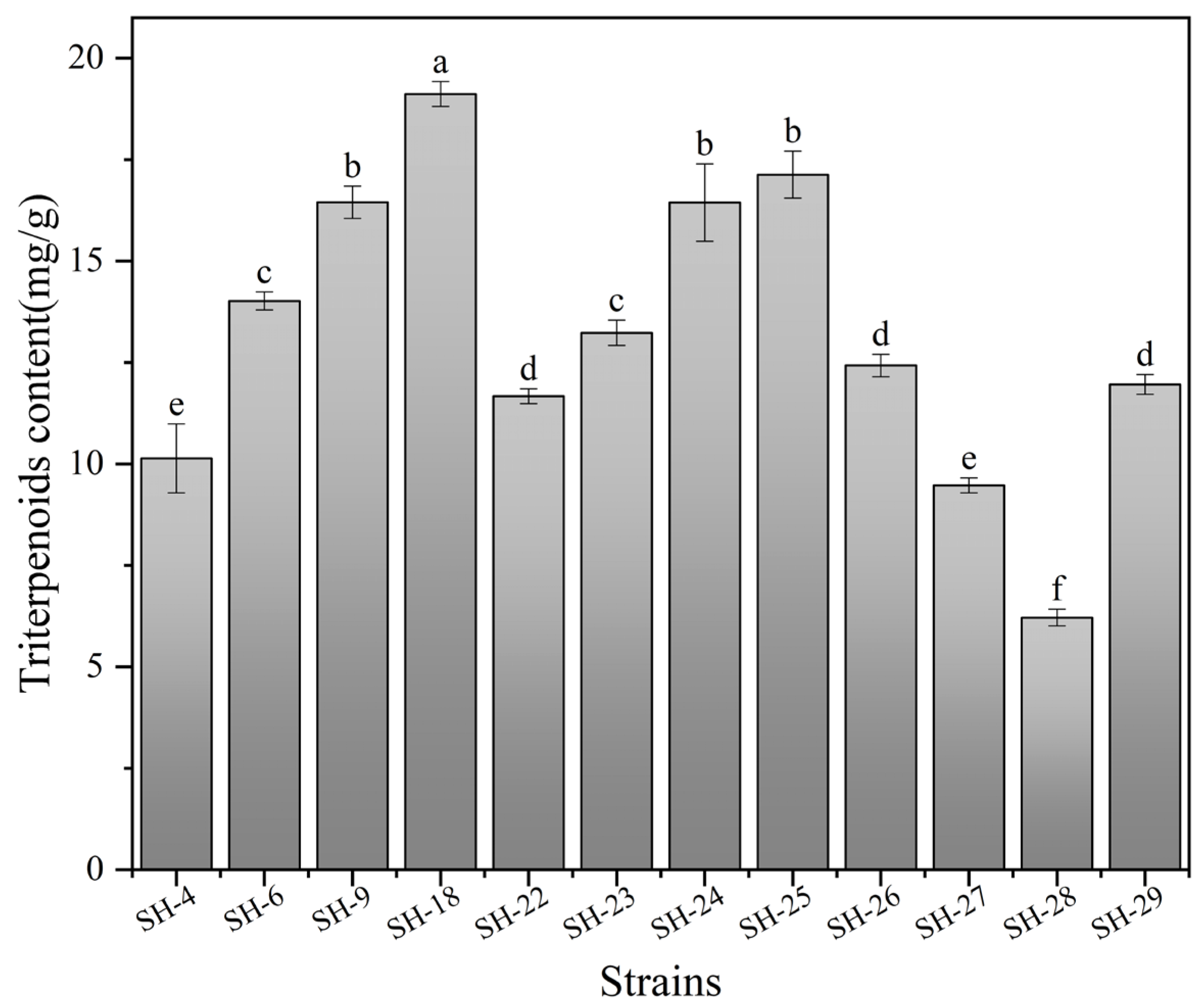
3.1. Screening of I. hispidus Strains with High Triterpenoids Content
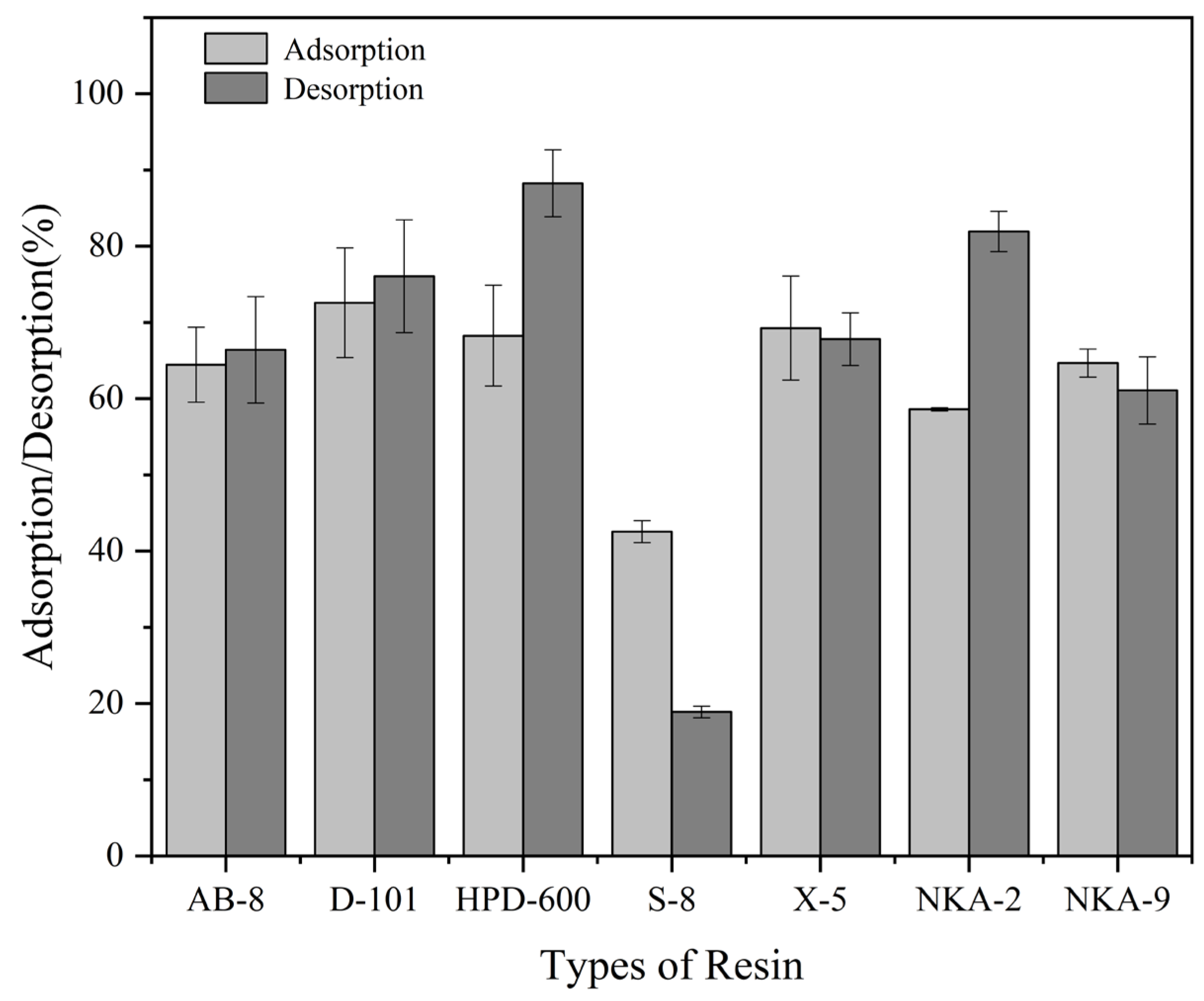
3.2. Screening of the MAR Fitting for Triterpenoid Enrichment
3.3. Static Adsorption and Desorption Tests for Selected MAR
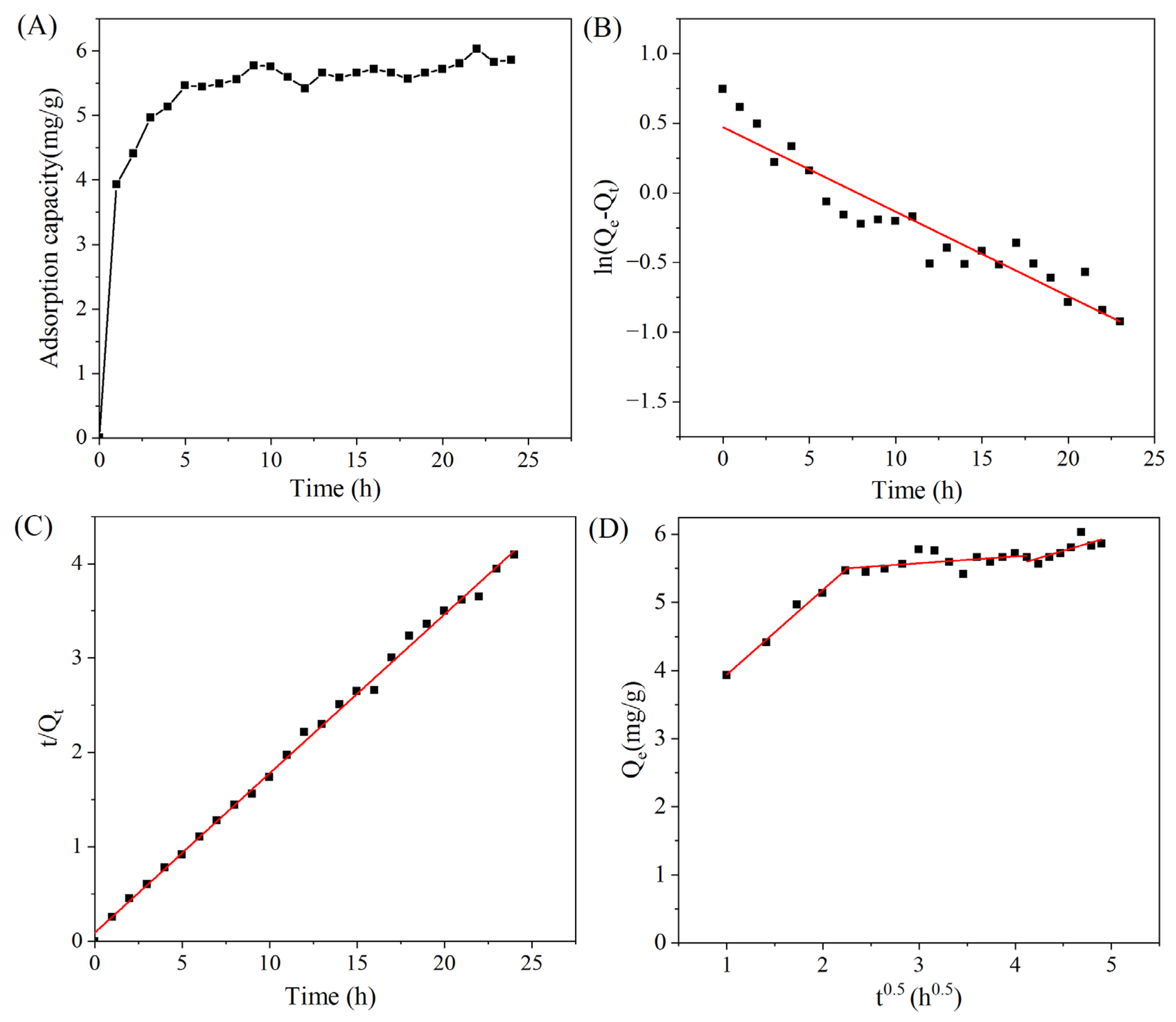
3.3.1. Adsorption Kinetics of MAR HPD-600
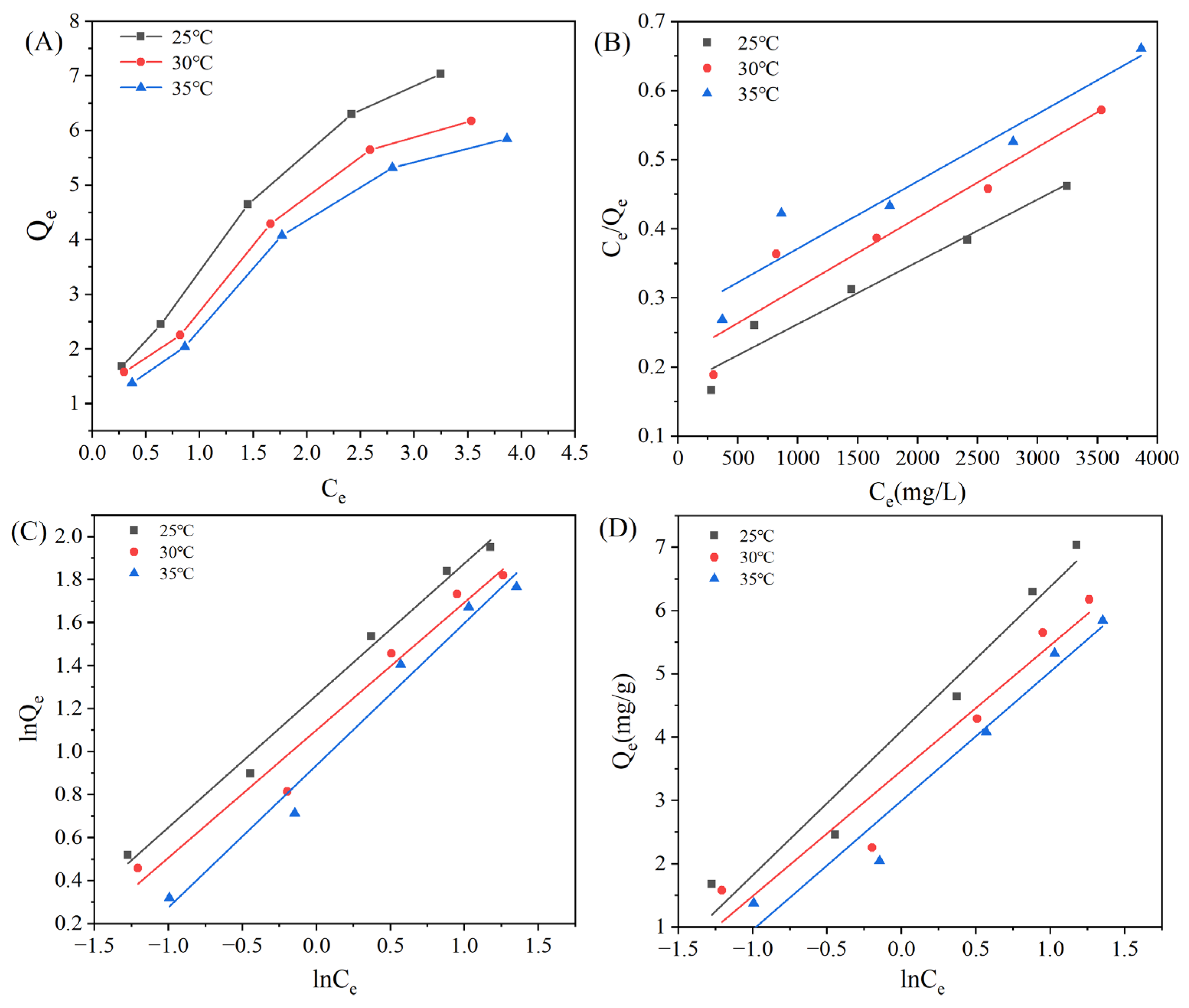
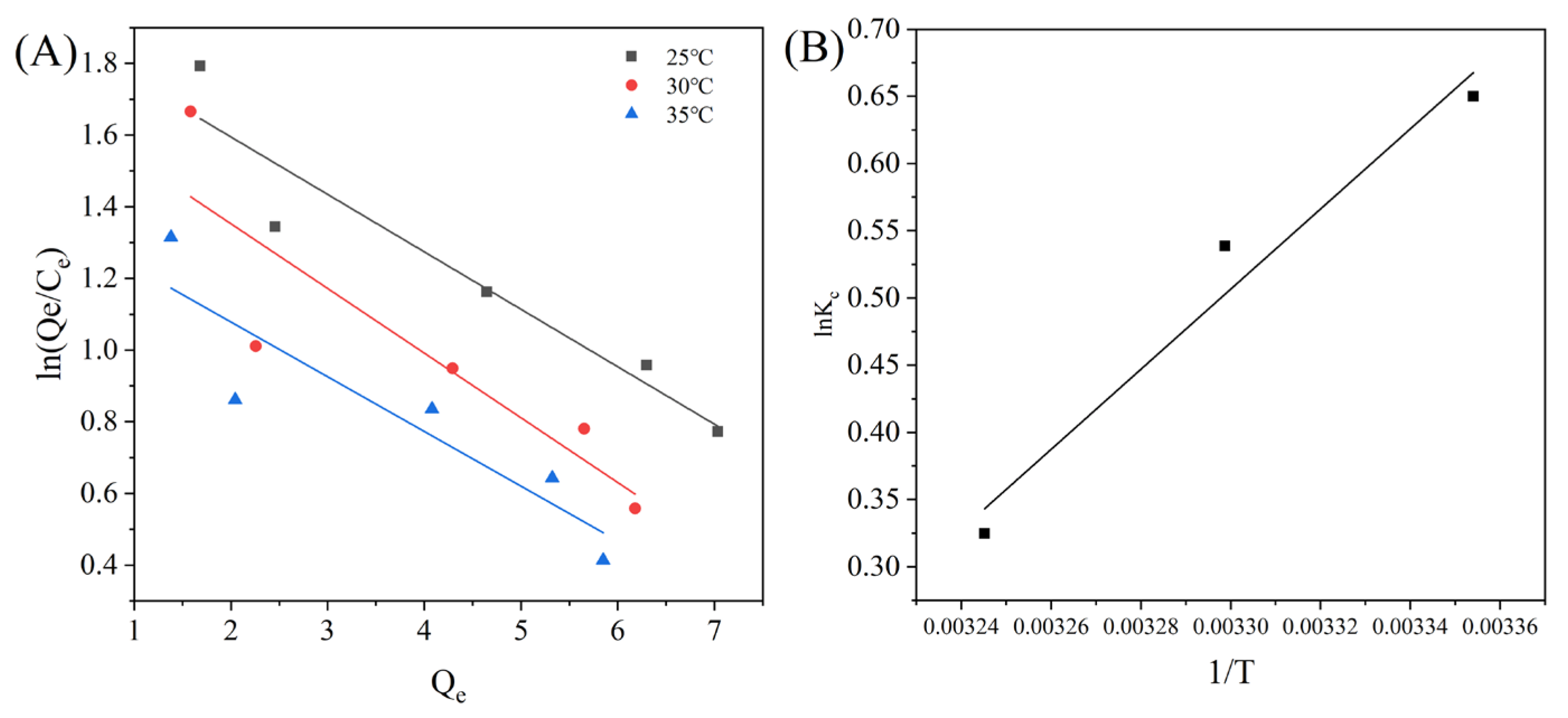
3.3.2. Adsorption Isotherms of MAR HPD-600
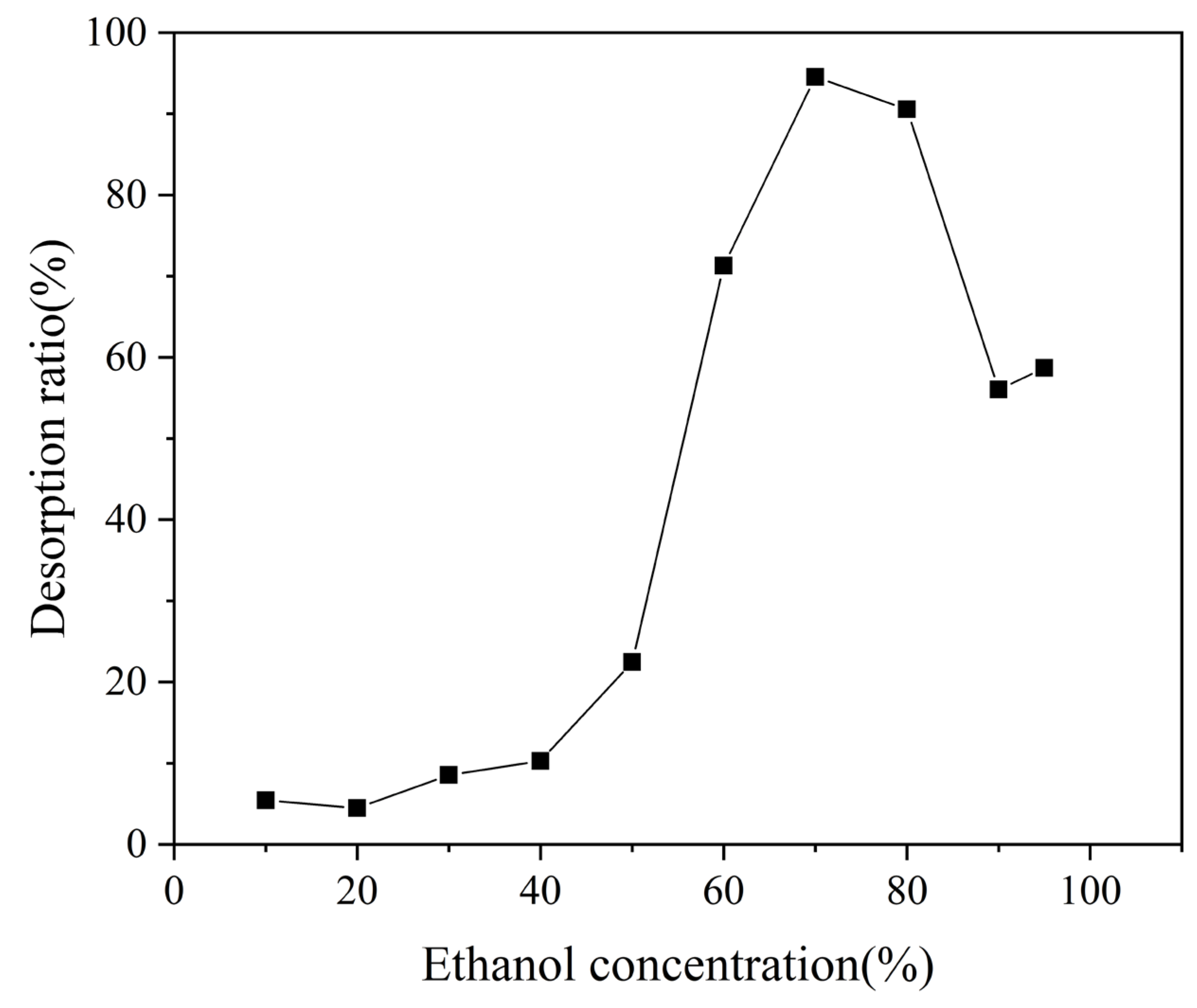
3.3.3. Screening of Desorbent Concentrations of MAR HPD-600
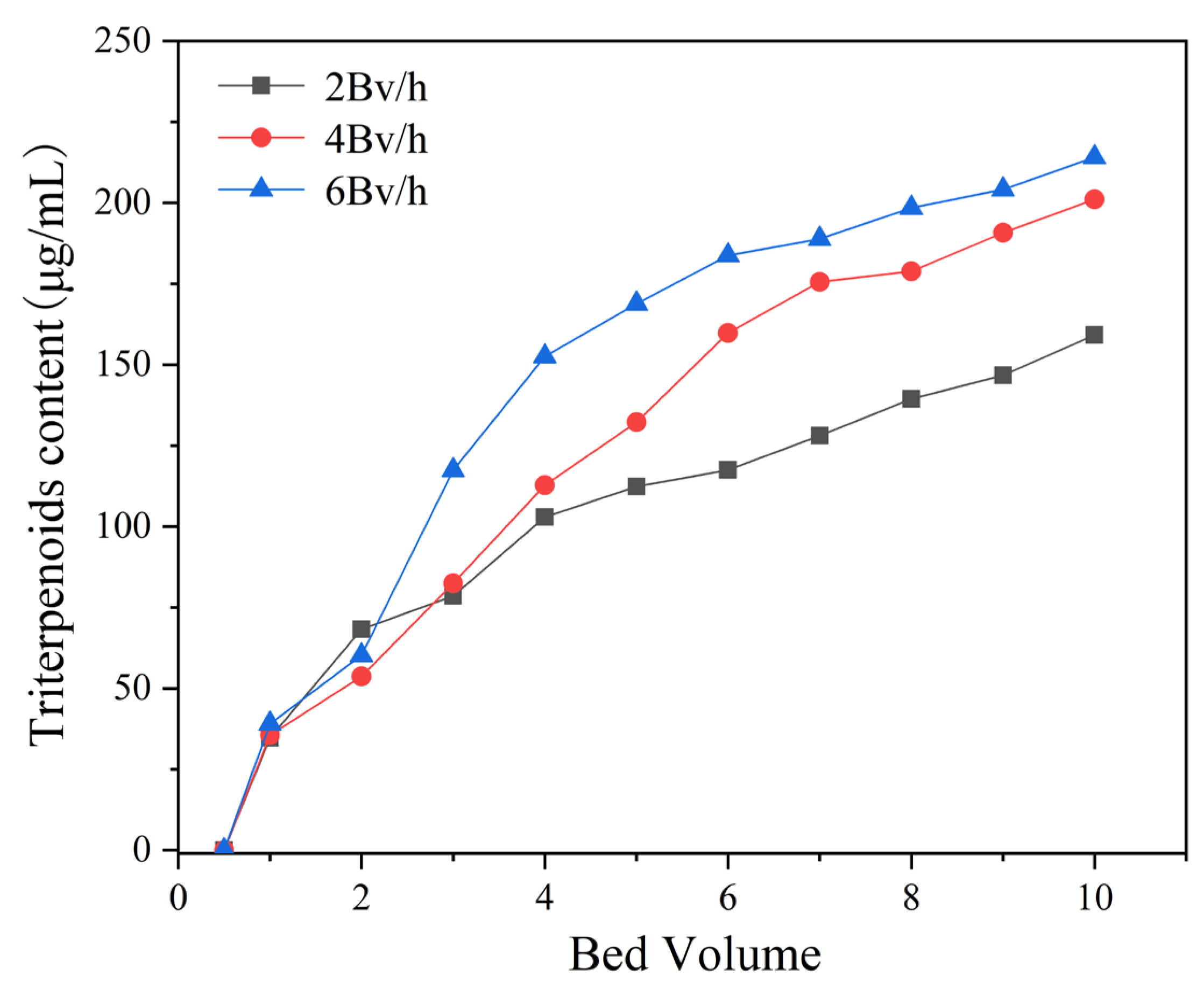
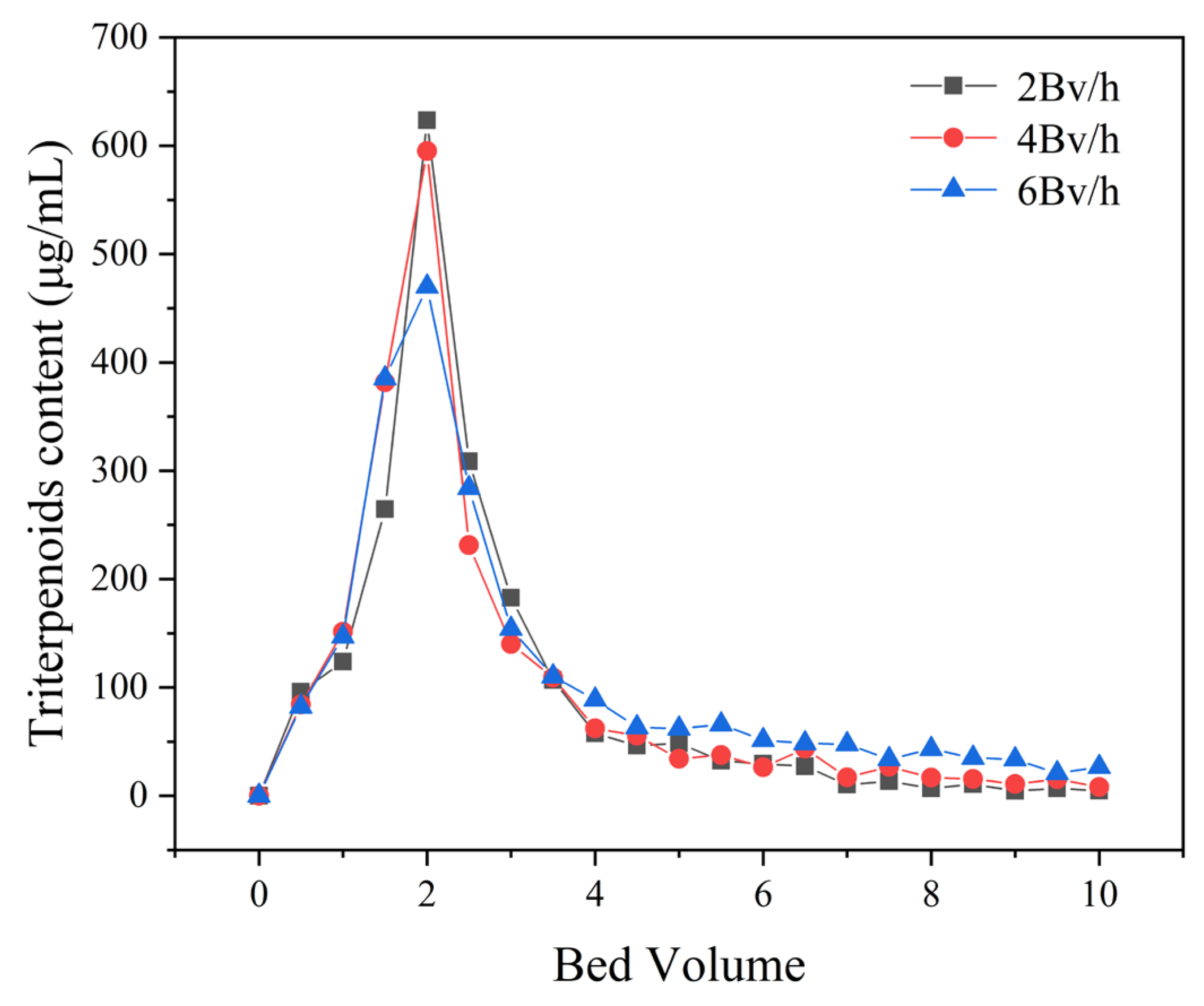
3.4. Dynamic Adsorption and Desorption
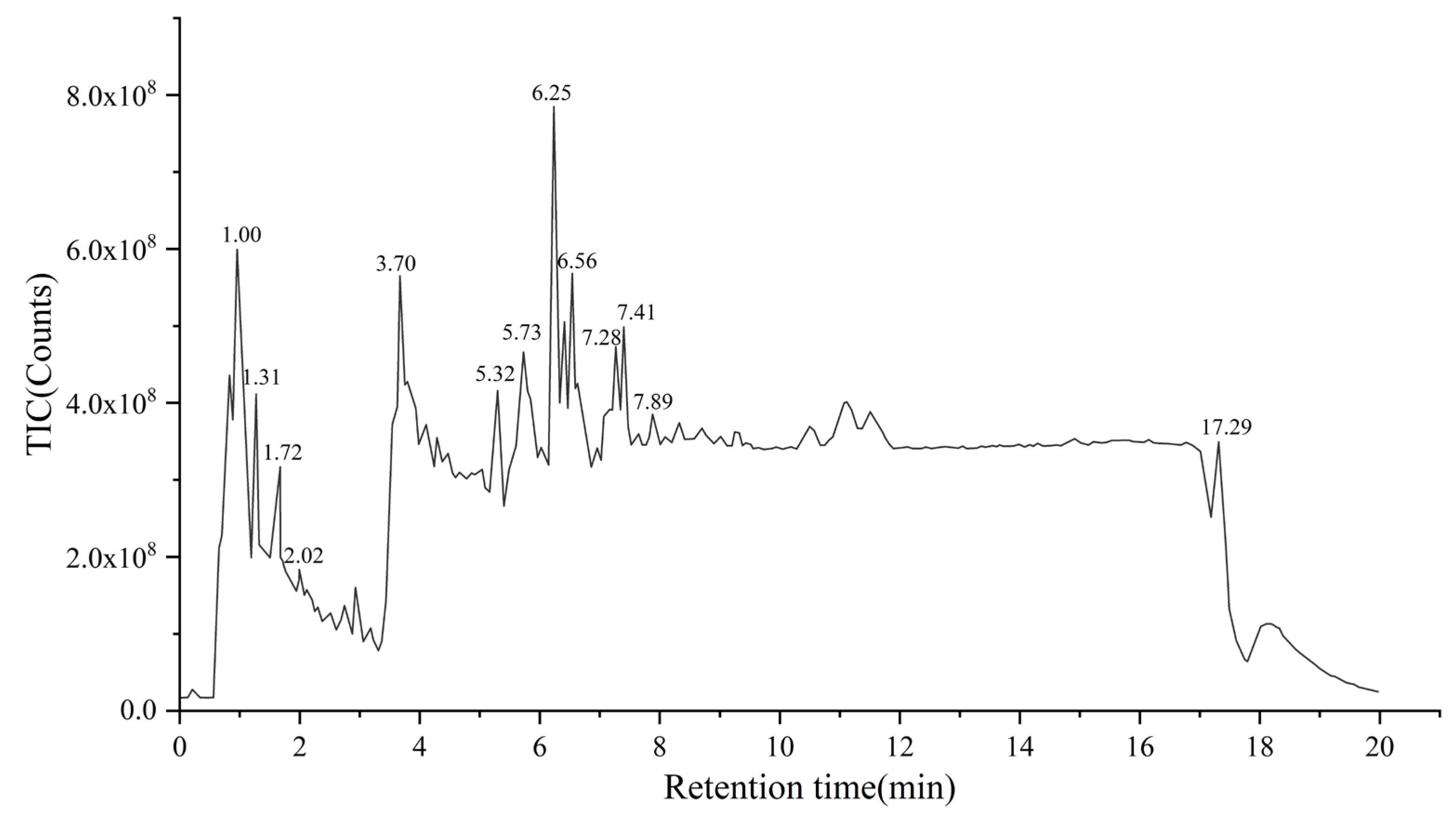
3.5. Compositional Analysis of the Purified Substances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Ma, J.X.; Zhou, M.; Si, J.; Cui, B.K. Current advances and potential trends of the polysaccharides derived from medicinal mushrooms sanghuang. Front. Microbiol. 2022, 13, 965934. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; Silipo, A.; Siciliano, T.; Tebano, M.; Flamini, G.; Braca, A.; Jiménez-Barbero, J. Current analytical methods to study plant water extracts: The example of two mushrooms species, Inonotus hispidus and Sparassis crispa. Phytochem. Anal. 2007, 18, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-J.; Shao, C.-X.; Yang, Y.; Ren, R.; Jin, L.; Hu, D.; Wu, S.-L.; Lei, P.; He, Y.-L.; Xu, J. The antitumor effect of mycelia extract of the medicinal macrofungus Inonotus hispidus on HeLa cells via the mitochondrial-mediated pathway. J. Ethnopharmacol. 2023, 311, 116407. [Google Scholar] [CrossRef]
- Liu, X.; Hou, R.; Xu, K.; Chen, L.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Extraction, characterization and antioxidant activity analysis of the polysaccharide from the solid-state fermentation substrate of Inonotus hispidus. Int. J. Biol. Macromol. 2019, 123, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Awadh Ali, N.A.; Mothana, R.A.A.; Lesnau, A.; Pilgrim, H.; Lindequist, U. Antiviral activity of Inonotus hispidus. Fitoterapia 2003, 74, 483–485. [Google Scholar] [CrossRef]
- Gründemann, C.; Arnhold, M.; Meier, S.; Bäcker, C.; Garcia-Käufer, M.; Grunewald, F.; Steinborn, C.; Huber, R.; Lindequist, U. Influence of Inonotus hispidus on function of human immune cells. Eur. J. Integr. Med. 2016, 8, 54. [Google Scholar] [CrossRef]
- Xue, R.; Han, W.; Gao, X. Inonotus hispidus has development potential in health and future foods. J. Future Foods 2025, 5, 331–341. [Google Scholar] [CrossRef]
- Shao, H.; Li, Y.; Wu, C.; Chen, R.; Kang, J. Triterpenes from antler-shaped fruiting body of Ganoderma lucidum and their hepatoprotective activities. Phytochemistry 2024, 224, 114148. [Google Scholar] [CrossRef]
- Wei, F.-L.; Liu, H.; Zhang, S.-H.; Du, J.-X.; Feng, T.; He, J. Physivitrins I–R, lanostane triterpenoids with anti-inflammatory activities from the fungus Physisporinus vitreus. Phytochemistry 2025, 229, 114314. [Google Scholar] [CrossRef]
- Hoang, M.H.; Nguyen, T.A.T.; Pham, N.K.T.; Dang, V.S.; Vo, T.N. A new oleanane-skeleton triterpene isolated from Coffea canephora. Nat. Prod. Res. 2022, 36, 5161–5167. [Google Scholar] [CrossRef]
- Malyarenko, T.V.; Kicha, A.A.; Kalinovsky, A.I.; Dmitrenok, P.S.; Malyarenko, O.S.; Kuzmich, A.S.; Stonik, V.A.; Ivanchina, N.V. New Triterpene Glycosides from the Far Eastern Starfish Solaster pacificus and Their Biological Activity. Biomolecules 2021, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef]
- Ning, Z.; He, Z.; Yang, G.; Wang, Y.; Zhang, G.; Khan, A.A.; Li, F.; Zhang, J.; Hu, W. Dammarane-type triterpene saponins from the roots of Panax notoginseng (Burk.) F. H. Chen and their neuroprotective effects. Fitoterapia 2023, 168, 105541. [Google Scholar] [CrossRef]
- Wang, Z.X.; Feng, X.L.; Liu, C.; Gao, J.M.; Qi, J. Diverse Metabolites and Pharmacological Effects from the Basidiomycetes Inonotus hispidus. Antibiotics 2022, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Lu, X.-Y.; Han, J.-X.; Aisa, H.A.; Yuan, T. Triterpenoids and phenolics from the fruiting bodies of Inonotus hispidus and their activations of melanogenesis and tyrosinase. Chin. Chem. Lett. 2017, 28, 1052–1056. [Google Scholar] [CrossRef]
- Cai, C.; Ma, J.; Han, C.; Jin, Y.; Zhao, G.; He, X. Extraction and antioxidant activity of total triterpenoids in the mycelium of a medicinal fungus, Sanghuangporus sanghuang. Sci. Rep. 2019, 9, 7418. [Google Scholar] [CrossRef]
- Smina, T.P.; Nitha, B.; Devasagayam, T.P.A.; Janardhanan, K.K. Ganoderma lucidum total triterpenes induce apoptosis in MCF-7 cells and attenuate DMBA induced mammary and skin carcinomas in experimental animals. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2017, 813, 45–51. [Google Scholar] [CrossRef]
- Yingngam, B.; Chiangsom, A.; Brantner, A. Modeling and optimization of microwave-assisted extraction of pentacyclic triterpenes from Centella asiatica leaves using response surface methodology. Ind. Crops Prod. 2020, 147, 112231. [Google Scholar] [CrossRef]
- Issaoui, A.; Ksibi, H.; Ksibi, M. Supercritical fluid extraction of triterpenes and aliphatic hydrocarbons from olive tree derivatives. Arab. J. Chem. 2017, 10, S3967–S3973. [Google Scholar] [CrossRef]
- Xu, R.; Fazio, G.C.; Matsuda, S.P.T. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Wang, X.; Su, J.; Chu, X.; Zhang, X.; Kan, Q.; Liu, R.; Fu, X. Adsorption and Desorption Characteristics of Total Flavonoids from Acanthopanax senticosus on Macroporous Adsorption Resins. Molecules 2021, 26, 4162. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Li, G.L.; Chen, D.L.; Zhang, L.W. A green and simple method for enrichment of major diterpenoids from the buds of Wikstroemia chamaedaphne with macroporous resins and their activation of latent human immunodeficiency virus activity. Int. J. Biol. Macromol. 2024, 272, 132932. [Google Scholar] [CrossRef]
- Alizadeh, P.; Alizadeh, P.; Rahimi, M.; Amjadi, S.; Bayati, M.; Nejad Ebrahimi, S. Enrichment of rosmarinic acid from comfrey (Symphytum officinale L.) root extract by macroporous adsorption resins and molecular docking studies. Ind. Crops Prod. 2024, 214, 118541. [Google Scholar] [CrossRef]
- Cheng, J.; Song, J.; Wei, H.; Wang, Y.; Huang, X.; Liu, Y.; Lu, N.; He, L.; Lv, G.; Ding, H.; et al. Structural characterization and hypoglycemic activity of an intracellular polysaccharide from Sanghuangporus sanghuang mycelia. Int. J. Biol. Macromol. 2020, 164, 3305–3314. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, R.; Zhang, Z.; Chen, J.; Yang, N.; Li, K.; Liu, X.; Li, B.; Chen, X.; Wang, Y.; et al. Enhanced pigment removal from intracellular polysaccharides of Arthrospira platensis using macroporous resin NKA-II. Algal Res. 2024, 80, 103502. [Google Scholar] [CrossRef]
- NY/T 3676-2020; Determination of Total Triterpene in Ganoderma—Spectrophotometric Method. Ministry of Agriculture and Rural Affairs of the People’s Republic of China, Agriculture Press of China: Beijing, China, 2020.
- Leyton, A.; Vergara-Salinas, J.R.; Pérez-Correa, J.R.; Lienqueo, M.E. Purification of phlorotannins from Macrocystis pyrifera using macroporous resins. Food Chem. 2017, 237, 312–319. [Google Scholar] [CrossRef]
- Zhao, P.; Qi, C.; Wang, G.; Dai, X.; Hou, X. Enrichment and purification of total flavonoids from Cortex Juglandis Mandshuricae extracts and their suppressive effect on carbon tetrachloride-induced hepatic injury in Mice. J. Chromatogr. B 2015, 1007, 8–17. [Google Scholar] [CrossRef]
- Pang, X.; Guo, Z.; Ao, L.; Yang, Y.; Liu, C.; Gu, Z.; Xin, Y.; Li, M.; Zhang, L. Integrated cell metabolomics and serum metabolomics to reveal the mechanism of hypouricemic effect of Inonotus hispidus. J. Funct. Foods 2023, 105, 105572. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Q.; Zhang, B.; Zhang, J.; Fan, L.; Kang, J.; Lin, Y.; Huang, Y.; Tan, T.-C.; Ho, L.-H. Adsorption and desorption characteristics of flavonoids from white tea using macroporous adsorption resin. J. Chromatogr. A 2024, 1715, 464621. [Google Scholar] [CrossRef]
- Chang, X.-L.; Wang, D.; Chen, B.-Y.; Feng, Y.-M.; Wen, S.-H.; Zhan, P.-Y. Adsorption and Desorption Properties of Macroporous Resins for Anthocyanins from the Calyx Extract of Roselle (Hibiscus sabdariffa L.). J. Agric. Food Chem. 2012, 60, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, S.; Zhou, H.; Wu, H.; Wang, S.; Yong, X.; Zhou, J. Separation and purification of glabridin from a deep eutectic solvent extract of Glycyrrhiza glabra residue by macroporous resin and its mechanism. Sep. Purif. Technol. 2023, 315, 123731. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Adsorption properties of macroporous adsorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, S.; Bunia, I.; Racovita, S.; Neagu, V. Adsorption of cefotaxime sodium salt on polymer coated ion exchange resin microparticles: Kinetics, equilibrium and thermodynamic studies. Carbohydr. Polym. 2011, 85, 376–387. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Léon, J.J.L.; de Matos, T.N. Elucidation of mechanism involved in adsorption of Pb(II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Umukoro, E.H.; Oyekunle, J.A.O.; Owoyomi, O.; Ogunfowokan, A.O.; Oke, I.A. Adsorption characteristics and mechanisms of plantain peel charcoal in removal of Cu (II) and Zn (II) ions from wastewaters. Ife J. Sci. 2014, 16, 365–376. [Google Scholar]
- Wang, W.; Dong, S.; Zhang, W.; Qi, Z.; Liu, X. Study on adsorption/resolution properties, enrichment and purification of phenolic substances of Inonotus hispidus by macroporous adsorption resin. Ind. Crops Prod. 2024, 216, 118661. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Liu, C.; Zhang, Y.; Li, S. Total Triterpenoid Extraction from Inonotus Obliquus Using Ionic Liquids and Separation of Potential Lactate Dehydrogenase Inhibitors via Ultrafiltration High-Speed Countercurrent Chromatography. Molecules 2021, 26, 2467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gong, X.; Qu, H. An effective and comprehensive optimization strategy for preparing Ginkgo biloba leaf extract enriched in shikimic acid by macroporous resin column chromatography. Phytochem. Anal. 2024, 35, 1428–1442. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Q.; Yan, X.; Zhou, Z.; Zhao, J.; Xia, J.; Gao, X.; Ye, M.; Deng, L.; Zeng, Z.; et al. Enrichment of polyphenols from Cinnamomum camphora seed kernel by macroporous adsorption resins and evaluation of its antioxidant and enzyme inhibitory activities. Ind. Crops Prod. 2024, 222, 119486. [Google Scholar] [CrossRef]
- Huang, J.; Guo, Z.-h.; Cheng, P.; Sun, B.-h.; Gao, H.-Y. Three new triterpenoids from Salacia hainanensis Chun et How showed effective anti-α-glucosidase activity. Phytochem. Lett. 2012, 5, 432–437. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Merzenich, G.; Panek, D.; Zeitler, H.; Vetter, H.; Wagner, H. Drug development from natural products: Exploiting synergistic effects. Indian. J. Exp. Biol. 2010, 48, 208–219. [Google Scholar]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Tsai, R.T.; Liu, S.P.; Chen, C.S.; Tsai, M.C.; Chien, S.H.; Hung, H.S.; Lin, S.Z.; Shyu, W.C.; Fu, R.H. Neuroprotective Effects of Betulin in Pharmacological and Transgenic Caenorhabditis elegans Models of Parkinson’s Disease. Cell Transpl. 2017, 26, 1903–1918. [Google Scholar] [CrossRef]
- Chen, L.; Yao, H.; Chen, X.; Wang, Z.; Xiang, Y.; Xia, J.; Liu, Y.; Wang, Y. Ginsenoside Rg1 Decreases Oxidative Stress and Down-Regulates Akt/mTOR Signalling to Attenuate Cognitive Impairment in Mice and Senescence of Neural Stem Cells Induced by d-Galactose. Neurochem. Res. 2018, 43, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Proshkina, E.; Plyusnin, S.; Babak, T.; Lashmanova, E.; Maganova, F.; Koval, L.; Platonova, E.; Shaposhnikov, M.; Moskalev, A. Terpenoids as Potential Geroprotectors. Antioxidants 2020, 9, 529. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, S.-H.; Kwon, O.-S.; Shin, T.-J.; Lee, J.-H.; Lee, B.-H.; Yoon, I.-S.; Pyo, M.K.; Rhim, H.; Lim, Y.-H.; et al. Effects of Ginsenosides, Active Ingredients of Panax ginseng, on Development, Growth, and Life Span of Caenorhabditis elegans. Biol. Pharm. Bull. 2007, 30, 2126–2134. [Google Scholar] [CrossRef]
- Fang, C.; Yang, H.; Fan, D.; Deng, J. Dietary Triterpenoids in Functional Food and Drug Ingredients: A review of structure-activity relationships, biosynthesis, applications, and AI-driven strategies. Trends Food Sci. Technol. 2025, 159, 104961. [Google Scholar] [CrossRef]
- Kou, R.-W.; Du, S.-T.; Xia, B.; Zhang, Q.; Yin, X.; Gao, J.-M. Phenolic and Steroidal Metabolites from the Cultivated Edible Inonotus hispidus Mushroom and Their Bioactivities. J. Agric. Food Chem. 2021, 69, 668–675. [Google Scholar] [CrossRef]
- Ying, Y.-M.; Zhang, L.-Y.; Zhang, X.; Bai, H.-B.; Liang, D.-E.; Ma, L.-F.; Shan, W.-G.; Zhan, Z.-J. Terpenoids with alpha-glucosidase inhibitory activity from the submerged culture of Inonotus obliquus. Phytochemistry 2014, 108, 171–176. [Google Scholar] [CrossRef] [PubMed]

| MAR | Surface Area (m2/g) | Average Pore Diameter (nm) | Particle Diameter (mm) | Polarity |
|---|---|---|---|---|
| AB-8 | 480–520 | 13–14 | 0.3–1.25 | Weak polar |
| D-101 | 500–550 | 9–11 | 0.3–1.25 | Non-polar |
| HPD-600 | 550–600 | 8–9 | 0.3–1.25 | Weak polar |
| S-8 | 100–120 | 28–30 | 0.3–1.25 | Polar |
| X-5 | 500–600 | 29–30 | 0.3–1.25 | Weak polar |
| NKA-2 | 160–200 | 14.5–15.5 | 0.3–1.25 | Polar |
| NKA-9 | 250–290 | 15.5–16.5 | 0.3–1.25 | Polar |
| Models | Equations | Parameters |
|---|---|---|
| Pseudo-first-order | K1 = 0.0265 h−1 | |
| R2 = 0.8960 | ||
| Qe = 2.96 mg/g | ||
| Pseudo-second-order | K2 = 0.0026 h−1 | |
| R2 = 0.9976 | ||
| Qe = 5.92 mg/g | ||
| Intraparticle diffusion | R2 = 0.9880 | |
| R2 = 0.2575 | ||
| R2 = 0.6167 |
| Models | T (°C) | Equations | Parameters | ||
|---|---|---|---|---|---|
| Qm (mg/g) | KL (L/mg) | R2 | |||
| Langmuir | 25 | 11.10 | 0.5232 | 0.9602 | |
| 30 | 98.33 | 0.0478 | 0.90031 | ||
| 35 | 10.26 | 0.3558 | 0.92091 | ||
| n | KF [(mg/g) (mL/mg)1/n] | R2 | |||
| Freundlich | 25 | 1.6299 | 3.5310 | 0.99081 | |
| 30 | 1.6857 | 3.0051 | 0.96987 | ||
| 35 | 1.5129 | 2.5511 | 0.97918 | ||
| BT | KT (mL/mg) | R2 | |||
| Temkin | 25 | 2.2812 | 6.0280 | 0.96245 | |
| 30 | 1.9822 | 5.7582 | 0.93338 | ||
| 35 | 2.0408 | 4.3391 | 0.95733 | ||
| ΔG (KJ/mol) | ΔH (KJ/mol) | ΔS (J/mol) | ||
|---|---|---|---|---|
| 25 °C | 30 °C | 35 °C | −0.36 | −1.12 |
| −4.75 | −4.32 | −3.54 | ||
| Number | tR (min) | Observed (m/z) | Formula | Error (mDa) | Name |
|---|---|---|---|---|---|
| 1 | 5.6 | 544.381 | C32H52O5 | −2.7 | 16β-Methoxyalisol B 23-acetate |
| 2 | 5.72 | 461.3996 | C30H52O3 | 0.6 | Olibanumols J |
| 3 | 5.87 | 503.409 | C24H38O4 | −0.9 | (20S,24R)-3β-O-Acetyl-20,25-epoxydammarane-24-ol |
| 4 | 6.51 | 503.4084 | C24H38O4 | −1.1 | Ocotillol acetate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Liu, S.; Wang, S.; Qi, Z.; Zhuang, Q.; Liu, X. Improving Triterpenoid Extraction Efficiency from Inonotus hispidus Using Macroporous Adsorption Resin: An Aim for Functional Food Development. Foods 2025, 14, 1069. https://doi.org/10.3390/foods14061069
Dong S, Liu S, Wang S, Qi Z, Zhuang Q, Liu X. Improving Triterpenoid Extraction Efficiency from Inonotus hispidus Using Macroporous Adsorption Resin: An Aim for Functional Food Development. Foods. 2025; 14(6):1069. https://doi.org/10.3390/foods14061069
Chicago/Turabian StyleDong, Shuhan, Shuliang Liu, Shilong Wang, Zhengliang Qi, Qianqian Zhuang, and Xinli Liu. 2025. "Improving Triterpenoid Extraction Efficiency from Inonotus hispidus Using Macroporous Adsorption Resin: An Aim for Functional Food Development" Foods 14, no. 6: 1069. https://doi.org/10.3390/foods14061069
APA StyleDong, S., Liu, S., Wang, S., Qi, Z., Zhuang, Q., & Liu, X. (2025). Improving Triterpenoid Extraction Efficiency from Inonotus hispidus Using Macroporous Adsorption Resin: An Aim for Functional Food Development. Foods, 14(6), 1069. https://doi.org/10.3390/foods14061069





