Next-Generation Edible Packaging: Development of Water-Soluble, Oil-Resistant, and Antioxidant-Loaded Pouches for Use in Noodle Sauces
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of Different Formulation Films
- Monolayer Films: Solutions of 5% PVA and 2% CS were prepared by stirring in a heated water bath (95 °C) for 1 h. In addition, 2% HPMC and 2% SA solutions were prepared by stirring at room temperature overnight.
- Composite Films: Mixtures of 50:50 (v/v) of the following combinations were prepared: CS + PVA, CS + HPMC, SA + PVA, and SA + HPMC.
- Bilayer Films: The inner and outer layers were formed using a 50:50 (v/v) ratio of polymer solutions, producing the following combinations: PVA/CS, PVA/SA, HPMC/CS, and HPMC/SA. Bilayer films involved the outer layer being poured over the pre-dried inner layer and are referred to as “Inner/Outer”. Here, there is an expression from inside to outside, that is, the first mentioned layer is the inner surface.
2.3. Determination of Optimum Film Casting Volume
2.4. Characterization of Films Prepared at Ideal Casting Volume
2.4.1. Color
2.4.2. Barrier Properties
2.4.3. Chemical Characteristics
2.4.4. Thermal Properties
2.4.5. Morphological Characteristics
2.4.6. Water Contact Angle Measurement
2.4.7. Water Solubility
2.5. Preparation and Characterization of Antioxidant-Enriched Films
2.5.1. Curcumin Diffusion Analysis
2.5.2. Release Study to Different Food Simulants
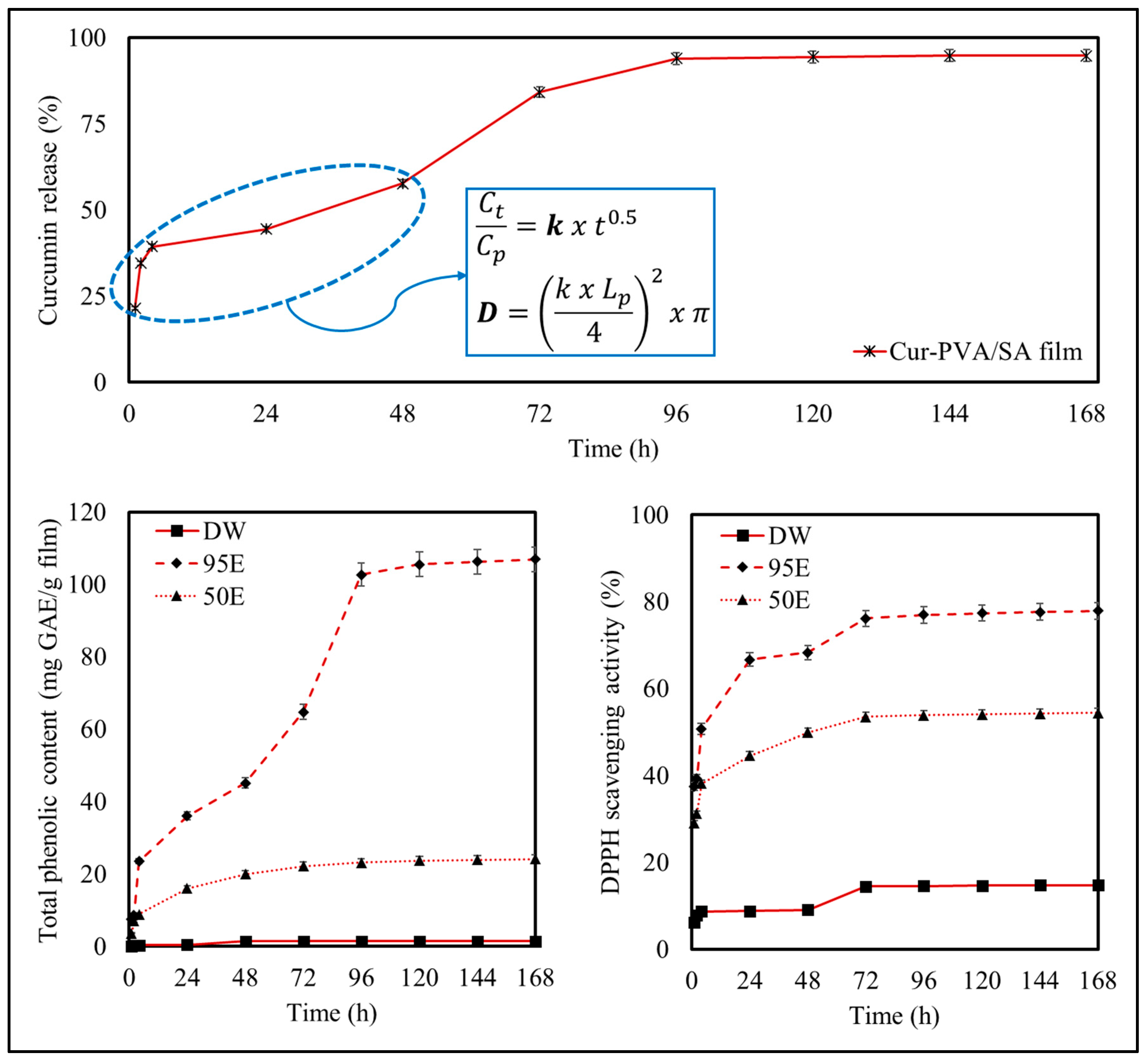
2.5.3. Total Phenolic Content and Antioxidant Activity
2.6. Production of Film Pouches and Their Usage for Single-Serving Seasoning-Oil Packaging
2.7. Storage of the Seasoning Oil-Containing Pouches in Contact with the Noodles
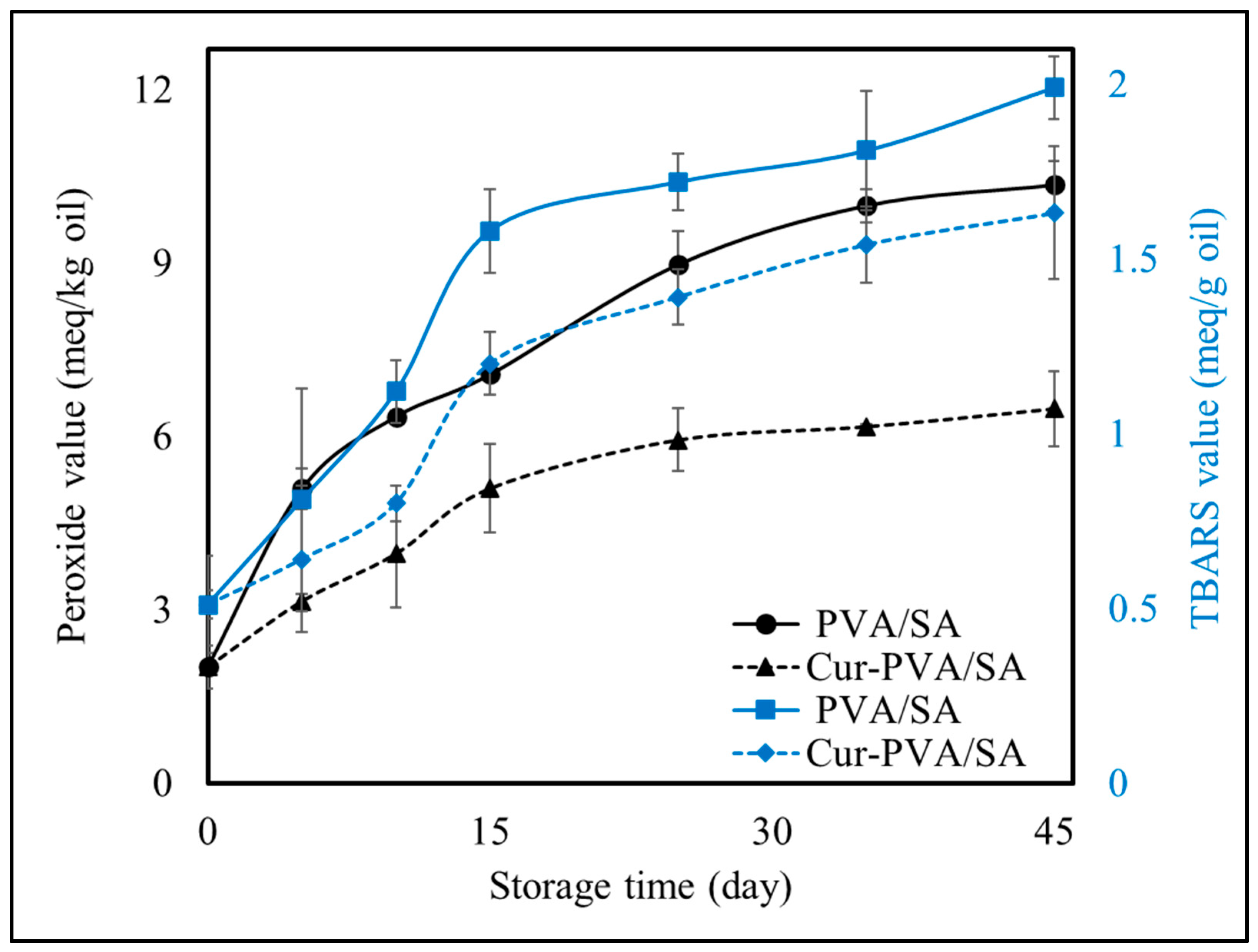
2.8. Quality Changes in Oil Packaged in Pouches During Storage
2.9. Statistical Analysis
3. Results and Discussion
3.1. Determination of Optimum Film Casting Volume
3.2. Determination of Optimum Physicochemical Characteristics of Films
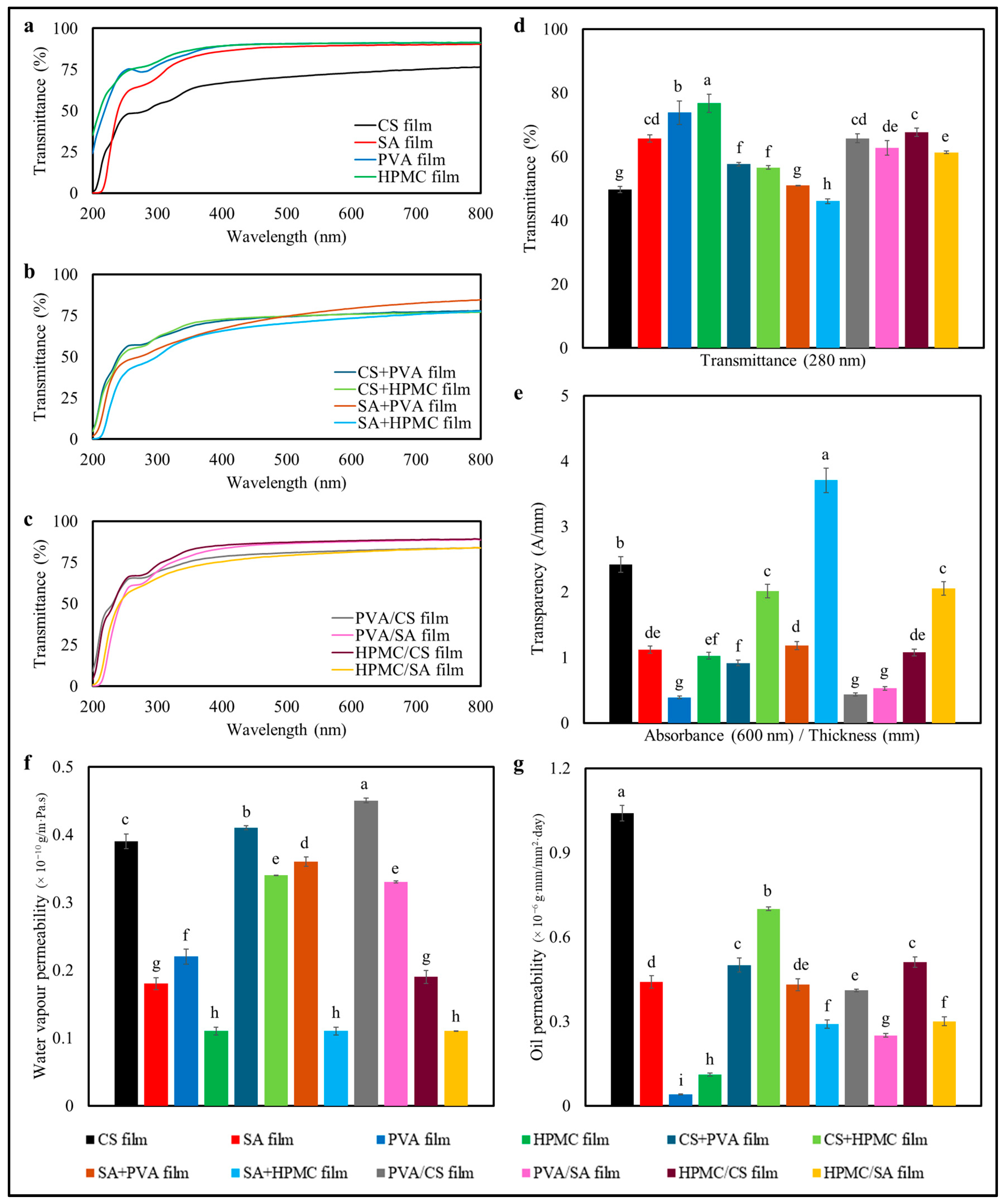
3.2.1. Color
3.2.2. Barrier Properties
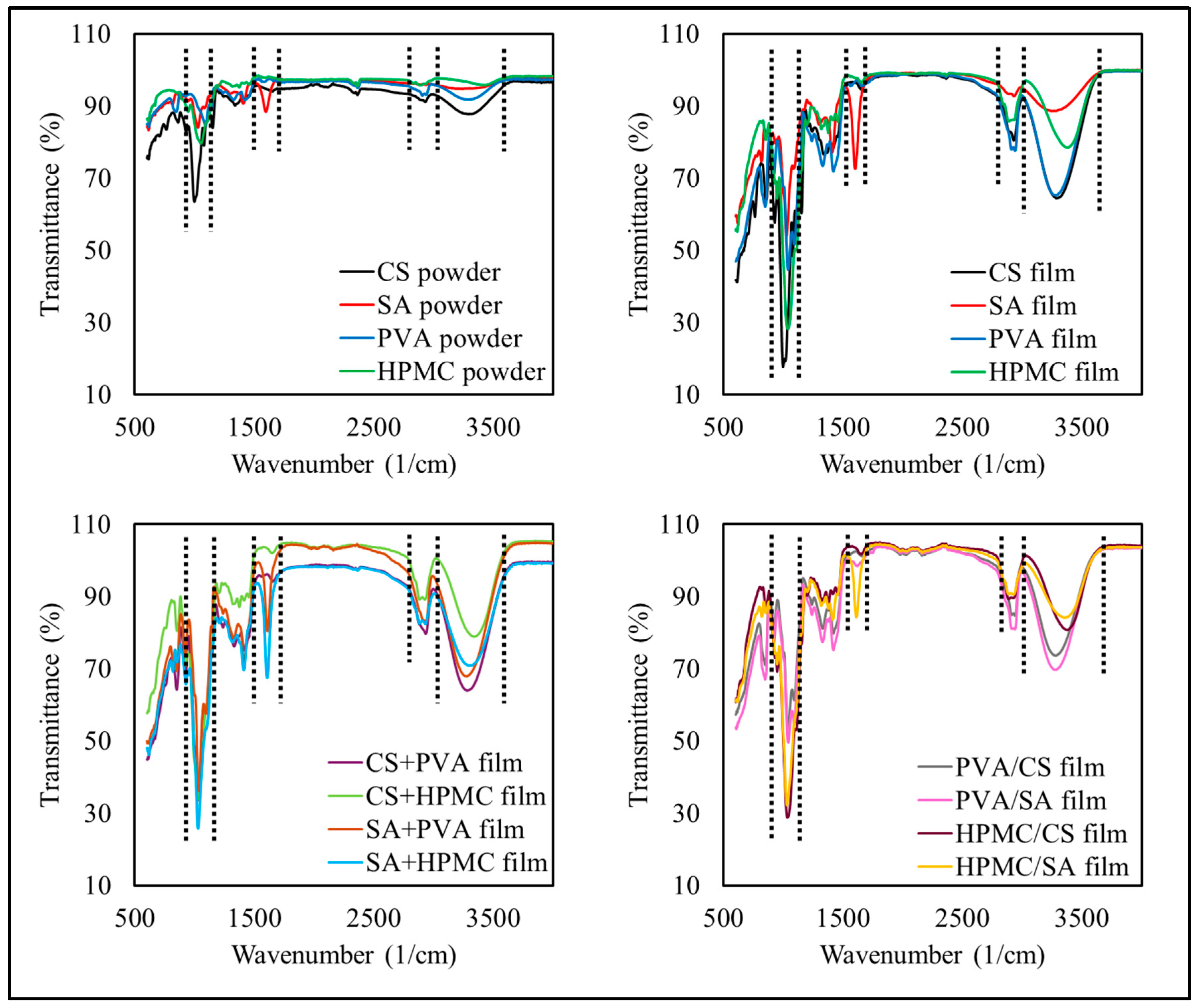
3.2.3. Chemical Characteristics
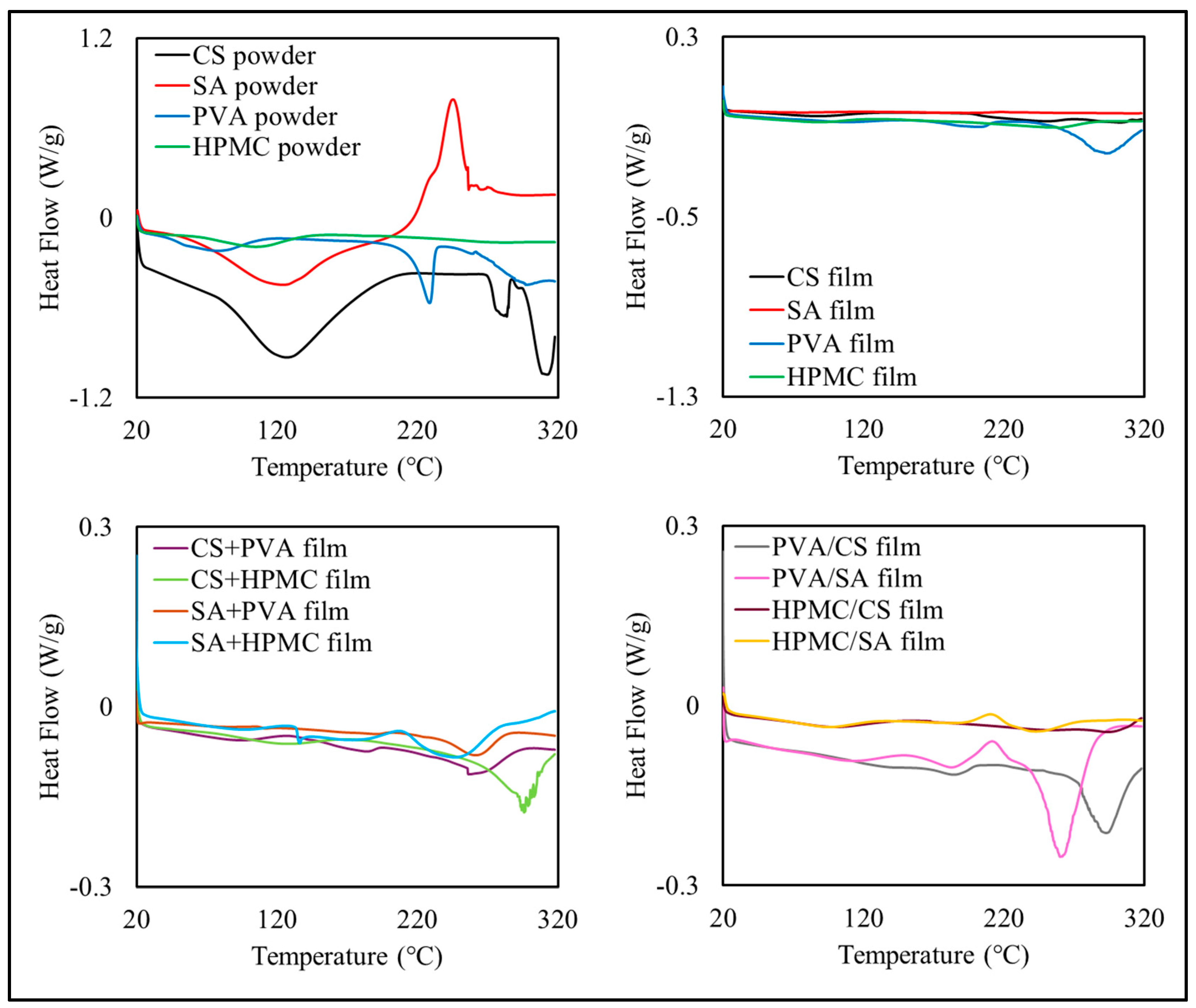
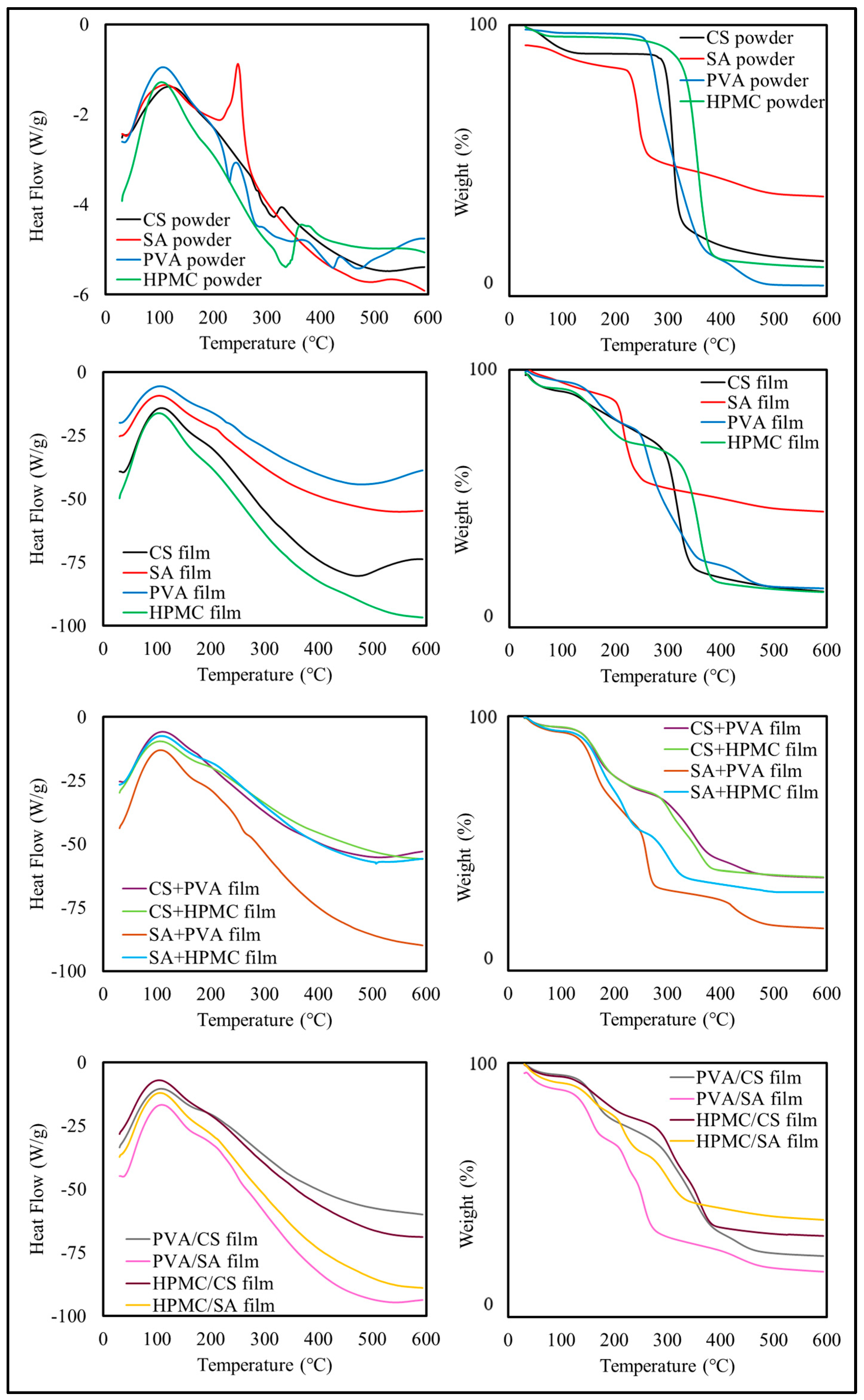
3.2.4. Thermal Properties
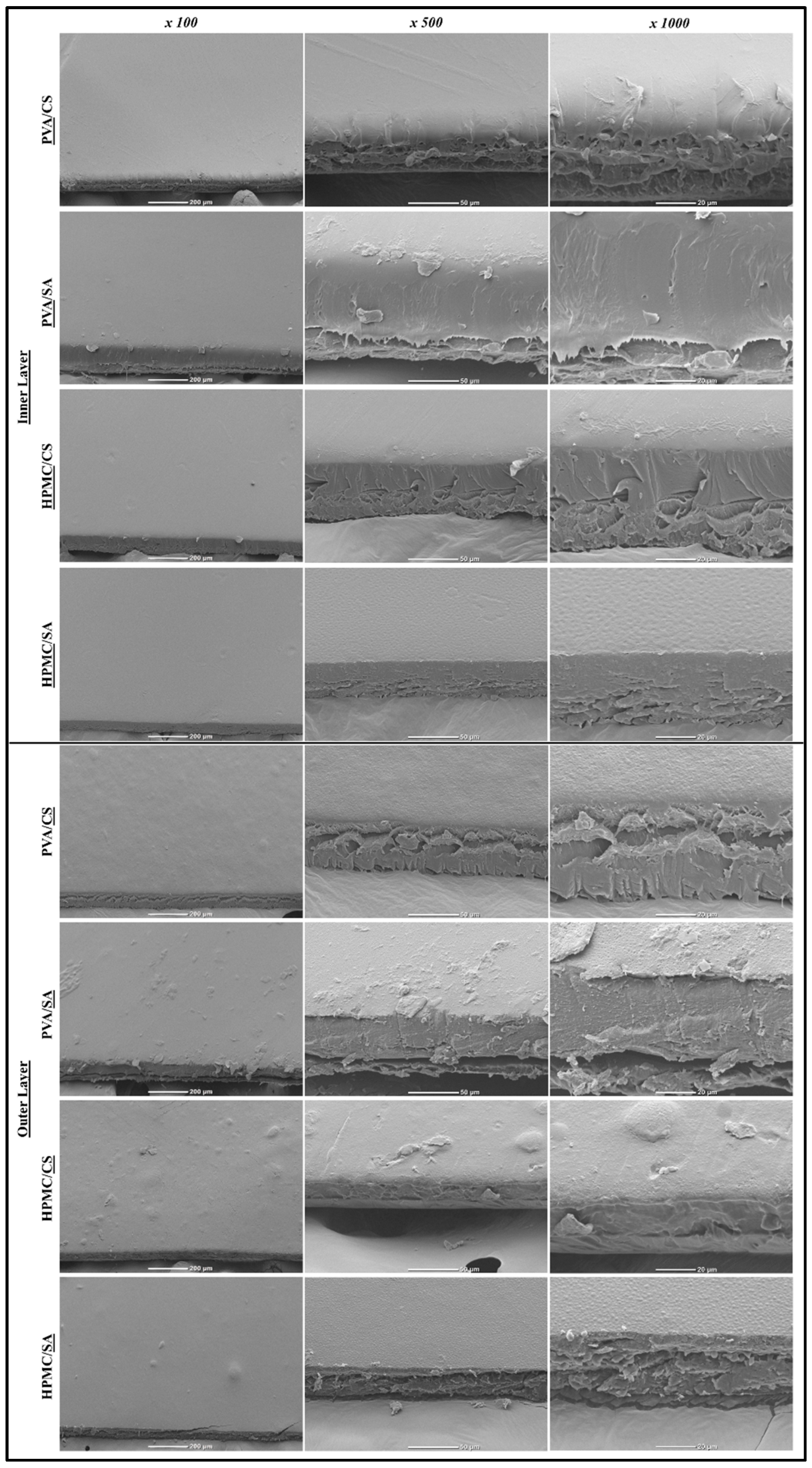
3.2.5. Morphological Characteristics
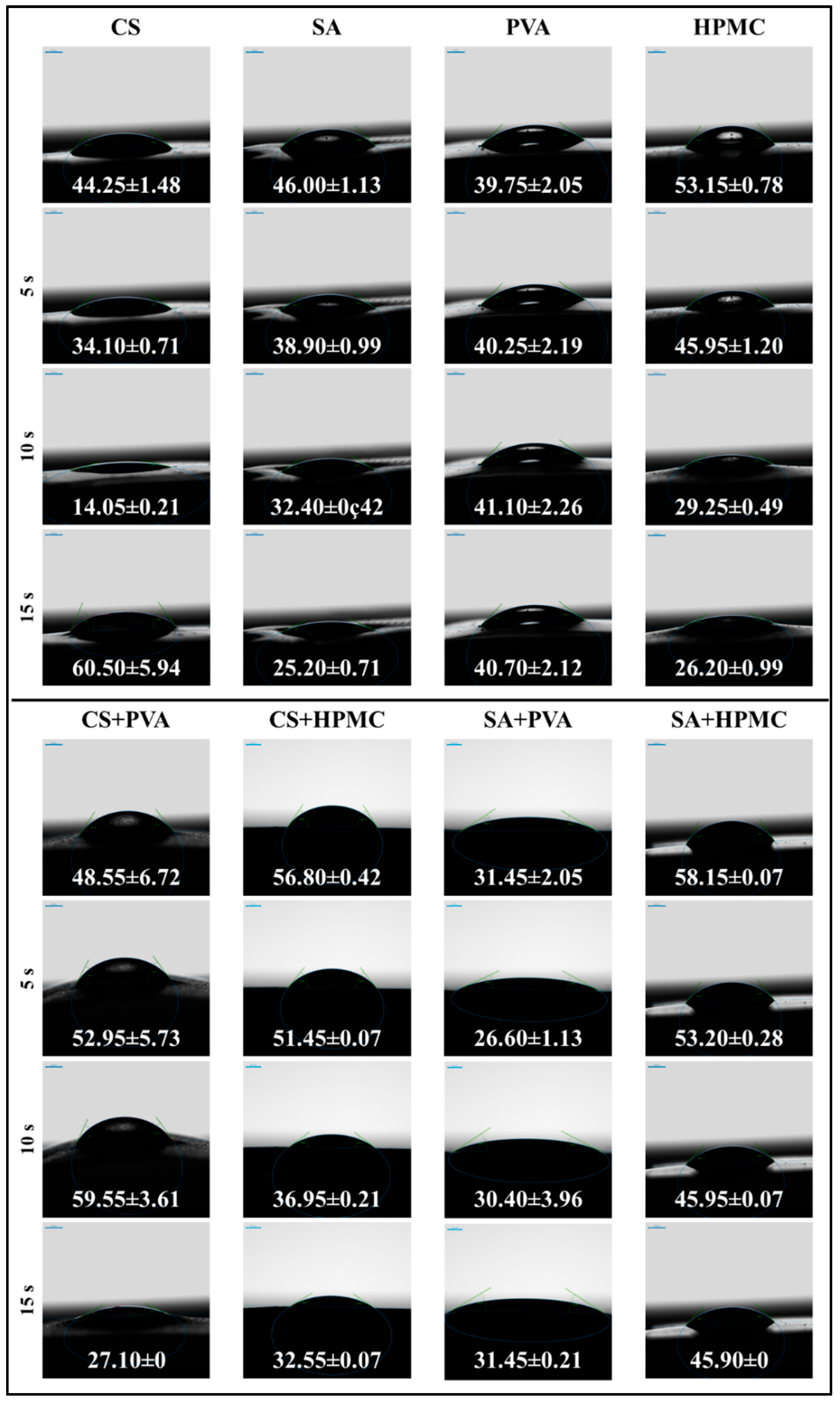
3.2.6. Water Contact Angle Measurements
3.2.7. Water Dissolution Properties
3.3. Identification of Optimum Biodegradable Films
3.4. Characterization of Antioxidant-Enriched Film
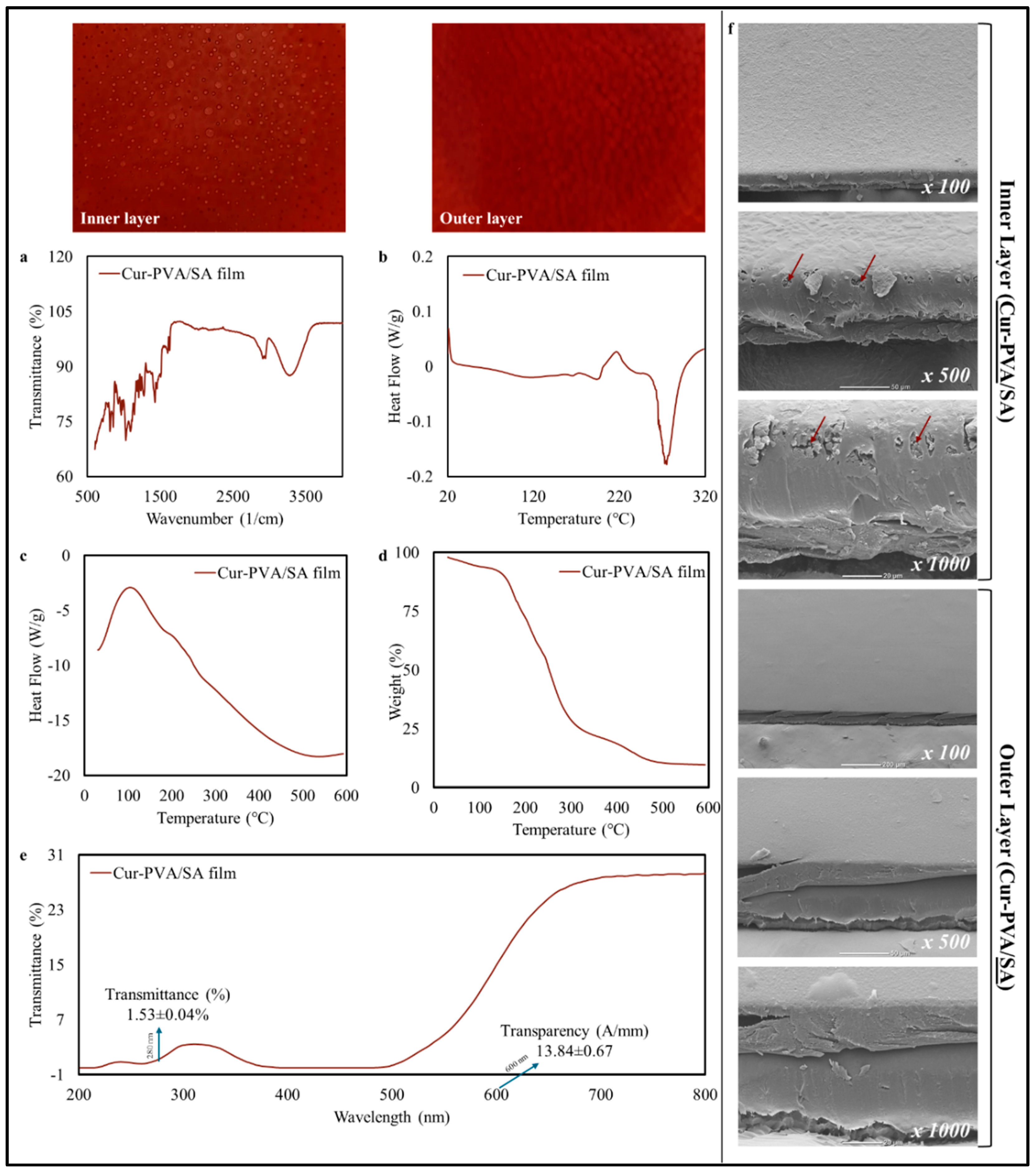
3.4.1. Physicochemical Characteristics
3.4.2. Mathematical Diffusion Analysis
3.4.3. Release Study to Different Food Simulants
3.5. Solubility Behavior of Pouches Containing Single-Serving Seasoning Oil
3.6. Storage of Seasoning Oil-Containing Pouches in the Presence of Noodles
3.7. Quality Changes in Oil Packaged in Pouches During Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demircan, B.; Velioglu, Y.S. Revolutionizing single-use food packaging: A comprehensive review of heat-sealable, water-soluble, and edible pouches, sachets, bags, or packets. Crit. Rev. Food Sci. Nutr. 2023, 65, 1497–1517. [Google Scholar] [CrossRef] [PubMed]
- Gamboni, J.E.; Bonfiglio, G.V.; Slavutsky, A.M.; Bertuzzi, M.A. Evaluation of edible films as single-serve pouches for a sustainable packaging system. Food Chem. Adv. 2023, 3, 100547. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Mukherjee, C.; Varghese, D.; Krishna, J.S.; Boominathan, T.; Rakeshkumar, R.; Dineshkumar, S.; Sivaramakrishna, A. Recent advances in biodegradable polymers–properties, applications and future prospects. Eur. Polym. J. 2023, 192, 112068. [Google Scholar] [CrossRef]
- Ozdestan, Ö.; Demircan, B. Transportation of flavorings and bioactive substances in food systems with edible films and coatings and their effects on functionality. Pamukkale Univ. J. Eng. Sci. 2020, 26, 1245–1256. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Gautam, G.; Mishra, P. Development and characterization of copper nanocomposite containing bilayer film for coconut oil packaging. J. Food Process. Preserv. 2017, 41, e13243. [Google Scholar] [CrossRef]
- Dong, Y.; Rao, Z.; Liu, Y.; Zheng, X.; Tang, K.; Liu, J. Soluble soybean polysaccharide/gelatin active edible films incorporated with curcumin for oil packaging. Food Packag. Shelf Life 2023, 35, 101039. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, J.; Yang, L.; Zhang, X. Optimized dynamic monitoring and quality management system for post-harvest matsutake of different preservation packaging in cold chain. Foods 2022, 11, 2646. [Google Scholar] [CrossRef]
- Hromiš, N.; Lazić, V.; Popović, S.; Šuput, D.; Bulut, S.; Kravić, S.; Romanić, R. The possible application of edible pumpkin oil cake film as pouches for flaxseed oil protection. Food Chem. 2022, 371, 131197. [Google Scholar] [CrossRef]
- Rosenbloom, R.A.; Zhao, Y. Hydroxypropyl methylcellulose or soy protein isolate-based edible, water-soluble, and antioxidant films for safflower oil packaging. J. Food Sci. 2021, 86, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Physical/thermal properties and heat seal ability of bilayer films based on fish gelatin and poly (lactic acid). Food Hydrocoll. 2018, 77, 248–256. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T.; de la Caba, K. Fish gelatin monolayer and bilayer films incorporated with epigallocatechin gallate: Properties and their use as pouches for storage of chicken skin oil. Food Hydrocoll. 2019, 89, 783–791. [Google Scholar] [CrossRef]
- Patil, S.; Bharimalla, A.K.; Mahapatra, A.; Dhakane-Lad, J.; Arputharaj, A.; Kumar, M.; Raja, A.; Kambli, N. Effect of polymer blending on mechanical and barrier properties of starch-polyvinyl alcohol based biodegradable composite films. Food Biosci. 2021, 44, 101352. [Google Scholar] [CrossRef]
- Kader, A.; Shahruzzaman, M.; Parvin, N.; Shams, K.; Kamruzzaman, M.; Haque, P.; Khan, M.A. Development of biocompatible packaging material: Starch/PVA-based bio-composite enhanced with hydroxypropyl methylcellulose. Polym. Technol. Mater. 2024, 63, 2418–2432. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, P.; Wu, Y.; Ouyang, J. Chitosan-hydroxypropyl methylcellulose and sodium alginate bilayer edible films for chestnut preservation. Food Chem. 2025, 466, 142254. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Influence of hydroxypropylmethylcellulose addition and homogenization conditions on properties and aging of corn starch based films. Carbohydr. Polym. 2012, 89, 676–686. [Google Scholar] [CrossRef]
- Qiao, J.; Dong, Y.; Chen, C.; Xie, J. Development and characterization of starch/PVA antimicrobial active films with controlled release property by utilizing electrostatic interactions between nanocellulose and lauroyl arginate ethyl ester. Int. J. Biol. Macromol. 2024, 261, 129415. [Google Scholar] [CrossRef]
- Zhuang, C.; Jiang, Y.; Zhong, Y.; Zhao, Y.; Deng, Y.; Yue, J.; Wang, D.; Jiao, S.; Gao, H.; Chen, H.; et al. Development and characterization of nano-bilayer films composed of polyvinyl alcohol, chitosan and alginate. Food Control 2018, 86, 191–199. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Deng, Y.; Chu, Y.; Zhong, Y.; Wang, G.; Li, H. Curcumin-based waterborne polyurethane-gelatin composite bioactive films for effective UV shielding and inhibition of oil oxidation. Food Control 2022, 141, 109199. [Google Scholar] [CrossRef]
- Ningrum, A.; Perdani, A.W.; Supriyadi; Munawaroh, H.S.H.; Aisyah, S.; Susanto, E. Characterization of tuna skin gelatin edible films with various plasticizers-essential oils and their effect on beef appearance. J. Food Process. Preserv. 2021, 45, e15701. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Guo, X.; Ji, M.; Wang, J.; Cheng, C.; Zhang, Q. Physicochemical, antioxidant, in vitro release, and heat sealing properties of fish gelatin films incorporated with β-cyclodextrin/curcumin complexes for apple juice preservation. Food Bioprocess Technol. 2018, 11, 447–461. [Google Scholar] [CrossRef]
- Demircan, B.; Ozdestan-Ocak, O. Effects of lemon essential oil and ethyl lauroyl arginate on the physico-chemical and mechanical properties of chitosan films for mackerel fillet coating application. J. Food Meas. Charact. 2021, 15, 1499–1508. [Google Scholar] [CrossRef]
- Ma, Q.; Ren, Y.; Wang, L. Investigation of antioxidant activity and release kinetics of curcumin from tara gum/polyvinyl alcohol active film. Food Hydrocoll. 2017, 70, 286–292. [Google Scholar] [CrossRef]
- Liu, F.; Avena-Bustillos, R.J.; Chiou, B.-S.; Li, Y.; Ma, Y.; Williams, T.G.; Wood, D.F.; McHugh, T.H.; Zhong, F. Controlled-release of tea polyphenol from gelatin films incorporated with different ratios of free/nanoencapsulated tea polyphenols into fatty food simulants. Food Hydrocoll. 2017, 62, 212–221. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Vicente, A.A.; Pinheiro, A.C. Incorporation of curcumin-loaded lipid-based nano delivery systems into food: Release behavior in food simulants and a case study of application in a beverage. Food Chem. 2023, 405, 134740. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.; Zasada, K.; Galus, S. An attempt to develop fast dissolving biopolymer-based pouches for instant coffee®. Postępy Tech. Przetwórstwa Spożywczego 2022, 2, 70–78. [Google Scholar]
- Kchaou, H.; Jridi, M.; Nasri, M.; Debeaufort, F. Design of gelatin pouches for the preservation of flaxseed oil during storage. Coatings 2020, 10, 150. [Google Scholar] [CrossRef]
- Prakoso, F.A.H.; Indiarto, R.; Utama, G.L. Edible film casting techniques and materials and their utilization for meat-based product packaging. Polymers 2023, 15, 2800. [Google Scholar] [CrossRef]
- Cai, J.; Xiao, J.; Chen, X.; Liu, H. Essential oil loaded edible films prepared by continuous casting method: Effects of casting cycle and loading position on the release properties. Food Packag. Shelf Life 2020, 26, 100555. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2021, 20, 875–900. [Google Scholar] [CrossRef]
- Channa, I.A.; Ashfaq, J.; Siddiqui, M.A.; Chandio, A.D.; Shar, M.A.; Alhazaa, A. Multi-shaded edible films based on gelatin and starch for the packaging applications. Polymers 2022, 14, 5020. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Singh, S.; Lee, Y.S. Characterization of edible film containing essential oils in hydroxypropyl methyl-cellulose and its effect on quality attributes of ‘Formosa’ plum (Prunus salicina L.). LWT 2016, 70, 213–222. [Google Scholar] [CrossRef]
- De Paola, M.G.; Paletta, R.; Lopresto, C.G.; Lio, G.E.; De Luca, A.; Chakraborty, S.; Calabrò, V. Stability of film-forming dispersions: Affects the morphology and optical properties of polymeric films. Polymers 2021, 13, 1464. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhu, Q.; Guo, J.; Gu, J.; Guo, J.; Wu, Y.; Ren, L.; Yang, S.; Jiang, J. Preparation and characterization of edible films from gelatin and hydroxypropyl methyl cellulose/sodium carboxymethyl cellulose. Heliyon 2024, 11, e41613. [Google Scholar] [CrossRef]
- Thakur, R.; Gupta, V.; Ghosh, T.; Das, A.B. Effect of anthocyanin-natural deep eutectic solvent (lactic acid/fructose) on mechanical, thermal, barrier, and pH-sensitive properties of polyvinyl alcohol based edible films. Food Packag. Shelf Life 2022, 33. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Vazquez, M. Characterization of mechanical and barrier properties of bacterial cellulose, glycerol and polyvinyl alcohol (PVOH) composite films with eco-friendly UV-protective properties. Food Hydrocoll. 2020, 99, 105323. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.; Sun, J.; Lv, S. Bioactive edible sodium alginate films incorporated with tannic acid as antimicrobial and antioxidative food packaging. Foods 2022, 11, 3044. [Google Scholar] [CrossRef]
- Xu, X.; Liu, H.; Duan, S.; Liu, X.; Zhang, K.; Tu, J. A novel pumpkin seeds protein-pea starch edible film: Mechanical, moisture distribution, surface hydrophobicity, UV-barrier properties and potential application. Mater. Res. Express 2020, 6, 125355. [Google Scholar] [CrossRef]
- Khater, E.-S.; Bahnasawy, A.; Abu Gabal, B.; Abbas, W.; Morsy, O. Effect of adding nano-materials on the properties of hydroxypropyl methylcellulose (HPMC) edible films. Sci. Rep. 2023, 13, 5063. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef]
- Badita, C.R.; Aranghel, D.; Burducea, C.; Mereuta, P. Characterization of sodium alginate based films. Rom. J. Phys. 2020, 65, 1–8. [Google Scholar]
- Tedesco, M.P.; Garcia, V.A.d.S.; Borges, J.G.; Osiro, D.; Vanin, F.M.; Yoshida, C.M.P.; de Carvalho, R.A. Production of oral films based on pre-gelatinized starch, CMC and HPMC for delivery of bioactive compounds extract from acerola industrial waste. Ind. Crop. Prod. 2021, 170. [Google Scholar] [CrossRef]
- Shivangi, S.; Dorairaj, D.; Negi, P.S.; Shetty, N.P. Development and characterization of a pectin-based edible film that contains mulberry leaf extract and its bio-active components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Shan, P.; Wang, K.; Yu, F.; Yi, L.; Sun, L.; Li, H. Gelatin/sodium alginate multilayer composite film crosslinked with green tea extract for active food packaging application. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 131013. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Qi, G.; Li, C.; Xu, P.; Zheng, T.; Zhu, X.; Kenny, J.M.; Puglia, D.; Ma, P. Highly transparent PVA/nanolignin composite films with excellent UV shielding, antibacterial and antioxidant performance. React. Funct. Polym. 2021, 162. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, Q.; Yuan, C.; Guo, L.; Li, Z.; Zhang, J.; Nishinari, K.; Zhao, M.; Cui, B. Characterizations of corn starch edible films reinforced with whey protein isolate fibrils. Food Hydrocoll. 2024, 147, 109412. [Google Scholar] [CrossRef]
- Lin, D.; Zheng, Y.; Huang, Y.; Ni, L.; Zhao, J.; Huang, C.; Xing, B. Investigation of the structural, physical properties, antioxidant, and antimicrobial activity of chitosan-nano-silicon aerogel composite edible films incorporated with okara powder. Carbohydr. Polym. 2020, 250, 116842. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Yang, J.; Zhong, F.; Liu, F. Properties of sodium alginate-based nanocomposite films: Effects of aspect ratio and surface charge of cellulose nanocrystals. Int. J. Biol. Macromol. 2024, 256, 128420. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liu, Q.; Ding, Y.; Zhang, W.; Ma, M.-G. Multi-performance sodium alginate-based composite films for sensing and electromagnetic shielding. Int. J. Biol. Macromol. 2025, 287, 138557. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; He, L.; Liu, Y. Preparation and properties of sodium carboxymethyl cellulose/sodium alginate/chitosan composite film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef]
- Trinh, B.M.; Chang, B.P.; Mekonnen, T.H. The barrier properties of sustainable multiphase and multi-component packaging materials: A review. Prog. Mater. Sci. 2023, 133, 101071. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. GRAS Notification for Monosol’s Use of PVA as a Component in Edible Film; GRAS Notice No. 767, 1–24; U.S. Food and Drug Administration: Washington, DC, USA, 2018. Available online: https://www.fda.gov/media/128920/download#:~:text=In%20these%20comprehensive%20safety%20evaluations,levels%20described%20in%20those%20assessments (accessed on 30 December 2024).
- U.S. Food and Drug Administration. Code for Federal Regulations Title 21 Part 184-Direct Food Substances Affirmed as Generally Recognized as Safe. 2024. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1724 (accessed on 5 January 2025).
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; He, J.; Luo, J.; Cai, J.; Wei, J.; Li, P.; Zhong, H. Preparation of curcumin-loaded chitosan/polyvinyl alcohol intelligent active films for food packaging and freshness monitoring. Int. J. Biol. Macromol. 2024, 276, 133807. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart edible films based on gelatin and curcumin. Food Hydrocoll. 2017, 66, 8–15. [Google Scholar] [CrossRef]
- Amani, F.; Rezaei, A.; Akbari, H.; Dima, C.; Jafari, S.M. Active packaging films made by Complex Coacervation of Tragacanth gum and gelatin loaded with curcumin; characterization and antioxidant activity. Foods 2022, 11, 3168. [Google Scholar] [CrossRef]
- Xie, Q.; Zheng, X.; Li, L.; Ma, L.; Zhao, Q.; Chang, S.; You, L. Effect of curcumin addition on the properties of bio-degradable pectin/chitosan films. Molecules 2021, 26, 2152. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Characterization of chitosan film incorporated with curcumin extract. Polymers 2021, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Kalaycıoglu, Z.; Torlak, E.; Akın-Evingur, G.; Ozen, I.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, Y.; Yang, J.; Pang, R.; Chen, Y.; Mo, L.; Jiang, Q.; Qin, Z. Preparation of curcumin-chitosan composite film with high antioxidant and antibacterial capacity: Improving the solubility of curcumin by encapsulation of biopolymers. Food Hydrocoll. 2023, 145, 109150. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.-W. Curcumin and its uses in active and smart food packaging applications—A comprehensive review. Food Chem. 2022, 375, 131885. [Google Scholar] [CrossRef]
- Oliveira Filho, J.G.; Egea, M.B. Edible bioactive film with curcumin: A potential “functional” packaging? Int. J. Mol. Sci. 2022, 23, 5638. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Costa, M.J.; Maciel, L.C.; Pastrana, L.; Vicente, A.A.; Cerqueira, M.A. Active carboxymethyl-cellulose-based edible films: Influence of free and encapsulated curcumin on films’ properties. Foods 2021, 10, 1512. [Google Scholar] [CrossRef]
- Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of active edible films made from banana starch and curcumin-loaded nanoemulsions. Food Chem. 2020, 371, 131121. [Google Scholar] [CrossRef]
- Taghinia, P.; Abdolshahi, A.; Sedaghati, S.; Shokrollahi, B. Smart edible films based on mucilage of lallemantia iberica seed incorporated with curcumin for freshness monitoring. Food Sci. Nutr. 2021, 9, 1222–1231. [Google Scholar] [CrossRef]
- Badillo, P.A.; García, P.; Rodríguez, J.L.; García, M.A.; Casariego, A. Controlled release of phenols from biode gradable chitosan films enriched with turmeric (Curcuma longa) extract. Rev. San Gregor. 2024, 1, 82–89. [Google Scholar]
- Hong, Z.; Zhou, W.; Deng, H.; Huang, Q. Fabrication, performance and curcumin-controlled release of electrospun sarcoplasmic protein nanofiber films via layer-by-layer self-assembly. Colloids Surf. A Physicochem. Eng. Asp. 2023, 672, 131731. [Google Scholar] [CrossRef]
- Thorat, A.A.; Dalvi, S.V. Particle formation pathways and polymorphism of curcumin induced by ultrasound and additives during liquid antisolvent precipitation. CrystEngComm 2014, 16, 11102–11114. [Google Scholar] [CrossRef]
- Codex Alimentarius: Standard for Named Vegetable Oils. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B210-1999%252FCXS_210e.pdf (accessed on 30 December 2024).
- Chen, J.; Zhang, L.; Li, Y.; Zhang, N.; Gao, Y.; Yu, X. The formation, determination and health implications of polar compounds in edible oils: Current status, challenges and perspectives. Food Chem. 2021, 364, 130451. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Lee, S.Y.; Rhee, C. Edible oxygen barrier bilayer film pouches from corn zein and soy protein isolate for olive oil packaging. LWT 2010, 43, 1234–1239. [Google Scholar] [CrossRef]
- Carpiné, D.; Dagostin, J.L.A.; Bertan, L.C.; Mafra, M.R. Development and characterization of soy protein isolate emulsion-based edible films with added coconut oil for olive oil packaging: Barrier, mechanical, and thermal properties. Food Bioprocess Technol. 2015, 8, 1811–1823. [Google Scholar] [CrossRef]




| Films 1* | Thickness (mm) | Weight (g) | ||||
|---|---|---|---|---|---|---|
| 10 mL | 15 mL | 20 mL | 10 mL | 15 mL | 20 mL | |
| CS | 0.031 ± 0.001 d | 0.055 ± 0.005 e | 0.077 ± 0.006 fg | 0.289 ± 0.001 g | 0.335 ± 0.005 j | 0.483 ± 0.004 e |
| SA | 0.030 ± 0.004 d | 0.043 ± 0.004 ef | 0.060 ± 0.002 g | 0.236 ± 0.002 h | 0.328 ± 0.003 j | 0.485 ± 0.013 e |
| PVA | 0.064 ± 0.002 b | 0.105 ± 0.007 c | 0.206 ± 0.010 b | 0.631 ± 0.006 a | 0.995 ± 0.001 a | 1.329 ± 0.008 b |
| HPMC | 0.025 ± 0.004 d | 0.039 ± 0.003 g | 0.068 ± 0.006 g | 0.278 ± 0.001 g | 0.363 ± 0.003 i | 0.531 ± 0.006 e |
| CS+PVA | 0.075 ± 0.004 ab | 0.132 ± 0.007 b | 0.179 ± 0.004 c | 0.624 ± 0.005 a | 0.879 ± 0.005 b | 1.467 ± 0.261 a |
| CS+HPMC | 0.047 ± 0.007 c | 0.080 ± 0.005 d | 0.113 ± 0.014 d | 0.336 ± 0.003 e | 0.494 ± 0.001 f | 0.673 ± 0.005 d |
| SA+PVA | 0.071 ± 0.008 ab | 0.093 ± 0.004 cd | 0.110 ± 0.007 de | 0.517 ± 0.008 b | 0.684 ± 0.009 d | 1.212 ± 0.017 b |
| SA+HPMC | 0.026 ± 0.004 d | 0.034 ± 0.003 fg | 0.068 ± 0.004 g | 0.322 ± 0.003 f | 0.477 ± 0.004 g | 0.621 ± 0.001 de |
| PVA/CS | 0.072 ± 0.003 ab | 0.182 ± 0.029 a | 0.264 ± 0.025 a | 0.467 ± 0.003 c | 0.702 ± 0.006 c | 0.907 ± 0.008 c |
| PVA/SA | 0.084 ± 0.007 a | 0.104 ± 0.004 c | 0.186 ± 0.014 c | 0.425 ± 0.004 d | 0.587 ± 0.010 e | 0.813 ± 0.005 c |
| HPMC/CS | 0.034 ± 0.003 d | 0.052 ± 0.004 ef | 0.088 ± 0.002 ef | 0.225 ± 0.008 i | 0.336 ± 0.004 j | 0.490 ± 0.003 e |
| HPMC/SA | 0.029 ± 0.002 d | 0.040 ± 0.003 efg | 0.055 ± 0.002 g | 0.284 ± 0.008 g | 0.405 ± 0.005 h | 0.550 ± 0.001 de |
| Films 1* | L* | a* | b* | ΔE* | YI | WI |
|---|---|---|---|---|---|---|
| CS | 31.96 ± 0.74 c | 0.39 ± 0.01 b | −0.33 ± 0.03 bc | 64.40 ± 0.72 j | −1.49 ± 0.17 b | 31.96 ± 0.74 c |
| SA | 4.75 ± 0.03 l | 0.23 ± 0.04 d | −0.53 ± 0.03 e | 91.22 ± 0.03 a | −15.84 ± 0.86 g | 4.75 ± 0.03 l |
| PVA | 11.54 ± 0.04 j | 0.35 ± 0.01 c | −0.83 ± 0.03 f | 84.45 ± 0.03 c | −10.23 ± 0.34 f | 11.54 ± 0.04 j |
| HPMC | 37.09 ± 0.03 a | 0.64 ± 0.02 a | −0.44 ± 0.02 d | 59.36 ± 0.03 l | −1.71 ± 0.08 b | 37.08 ± 0.03 a |
| CS+PVA | 14.85 ± 0.14 g | 0.12 ± 0.02 e | −0.52 ± 0.02 e | 81.23 ± 0.13 f | −4.97 ± 0.23 d | 14.85 ± 0.14 g |
| CS+HPMC | 15.83 ± 0.04 f | 0.22 ± 0.02 d | −0.45 ± 0.02 d | 80.27 ± 0.03 g | −4.06 ± 0.19 c | 15.83 ± 0.04 f |
| SA+PVA | 10.09 ± 0.12 k | 0.36 ± 0.03 bc | −1.07 ± 0.07 h | 85.85 ± 0.13 b | −15.19 ± 0.96 g | 10.08 ± 0.12 k |
| SA+HPMC | 14.31 ± 0.04 h | 0.37 ± 0.01 bc | 0.07 ± 0.01 a | 81.82 ± 0.03 e | 0.73 ± 0.11 a | 14.34 ± 0.04 h |
| PVA/CS | 22.05 ± 0.02 e | 0.07 ± 0.01 f | −0.32 ± 0.03 b | 74.16 ± 0.02 h | −2.05 ± 0.19 b | 22.05 ± 0.02 e |
| PVA/SA | 34.58 ± 0.33 b | 0.11 ± 0.02 e | −0.38 ± 0.01 c | 61.84 ± 0.32 k | −1.56 ± 0.01 b | 34.58 ± 0.33 b |
| HPMC/CS | 24.05 ± 0.07 d | 0.25 ± 0.01 d | −0.97 ± 0.02 g | 72.07 ± 0.07 i | −5.76 ± 0.10 e | 24.05 ± 0.07 d |
| HPMC/SA | 13.51 ± 0.45 i | 0.12 ± 0.01 e | −0.35 ± 0.01 bc | 82.58 ± 0.44 d | −3.70 ± 0.17 c | 13.51 ± 0.45 i |
| Films 1* | Test at 25 ± 2 °C | Test at 87 ± 2 °C | ||
|---|---|---|---|---|
| Dissolution Time (s) | Solubility (%) | Dissolution Time (s) | Solubility (%) | |
| CS | >60 | 11.73 ± 2.22 f | >60 | 72.44 ± 3.33 c |
| SA | 47.28 ± 1.97 b | 100.00 ± 0 a | 13.42 ± 1.83 d | 100.00 ± 0 a |
| PVA | >60 | 5.03 ± 0.76 g | 30.57 ± 5.25 b | 97.98 ± 0.56 a |
| HPMC | >60 | 59.89 ± 2.10 c | >60 | 5.30 ± 0.70 f |
| CS+PVA | >60 | 4.62 ± 1.41 g | 39.85 ± 1.85 a | 99.54 ± 0.38 a |
| CS+HPMC | >60 | 29.97 ± 3.20 d | >60 | 42.94 ± 1.71 e |
| SA+PVA | >60 | 58.48 ± 2.69 c | 21.44 ± 1.54 c | 100.00 ± 0 a |
| SA+HPMC | >60 | 72.26 ± 3.96 b | >60 | 2.79 ± 0.17 f |
| PVA/CS | >60 | 10.63 ± 1.28 f | >60 | 87.59 ± 2.33 b |
| PVA/SA | >60 | 56.36 ± 5.60 c | 30.67 ± 2.00 b | 99.45 ± 0.51 a |
| HPMC/CS | >60 | 22.48 ± 3.15 e | >60 | 3.46 ± 0.48 f |
| HPMC/SA | 52.45 ± 1.99 a | 100.00 ± 0 a | >60 | 55.20 ± 4.48 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demircan, B.; McClements, D.J.; Velioglu, Y.S. Next-Generation Edible Packaging: Development of Water-Soluble, Oil-Resistant, and Antioxidant-Loaded Pouches for Use in Noodle Sauces. Foods 2025, 14, 1061. https://doi.org/10.3390/foods14061061
Demircan B, McClements DJ, Velioglu YS. Next-Generation Edible Packaging: Development of Water-Soluble, Oil-Resistant, and Antioxidant-Loaded Pouches for Use in Noodle Sauces. Foods. 2025; 14(6):1061. https://doi.org/10.3390/foods14061061
Chicago/Turabian StyleDemircan, Bahar, David Julian McClements, and Yakup Sedat Velioglu. 2025. "Next-Generation Edible Packaging: Development of Water-Soluble, Oil-Resistant, and Antioxidant-Loaded Pouches for Use in Noodle Sauces" Foods 14, no. 6: 1061. https://doi.org/10.3390/foods14061061
APA StyleDemircan, B., McClements, D. J., & Velioglu, Y. S. (2025). Next-Generation Edible Packaging: Development of Water-Soluble, Oil-Resistant, and Antioxidant-Loaded Pouches for Use in Noodle Sauces. Foods, 14(6), 1061. https://doi.org/10.3390/foods14061061







