Characteristics of Porous Starch from Lotus Seeds Using Dextranase: Protection and Sustained Release of Proanthocyanidins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation and Characterizations of LPS
- Preparation of LPS
- Determination of dextranase activity
- Observation of morphological characteristics of LPS
- Particle size distribution
- Determination of oil/water absorption rate
- Differential scanning calorimetry (DSC)
2.2.2. Adsorption of PC by LPS
- Plotting of the PC standard curve
- Determination of loading capacity
2.2.3. PC Adsorption Conditions
- Adsorption affected by PC concentration
- Adsorption affected by temperature
2.2.4. Fourier Transform Infrared Spectroscopy (FTIR) of Samples
2.2.5. X-Ray Diffraction (XRD) of Samples
2.2.6. UV Radiation Stability of PC-LPS
2.2.7. Adsorption Kinetics of PC
2.2.8. In Vitro Slow Release of PCs
- Preparation of artificial gastric and intestinal fluids
- Simulated gastric phase digestion
- Simulated enteric phase digestion
- Calculation of cumulative drug release rate
2.2.9. Data Analysis
3. Results and Discussion
3.1. Characterization of (LPS)
3.1.1. Scanning Electron Microscopy (SEM) of LPS
3.1.2. Particle Size Distribution
3.1.3. Oil/Water Absorption Rate of LPS
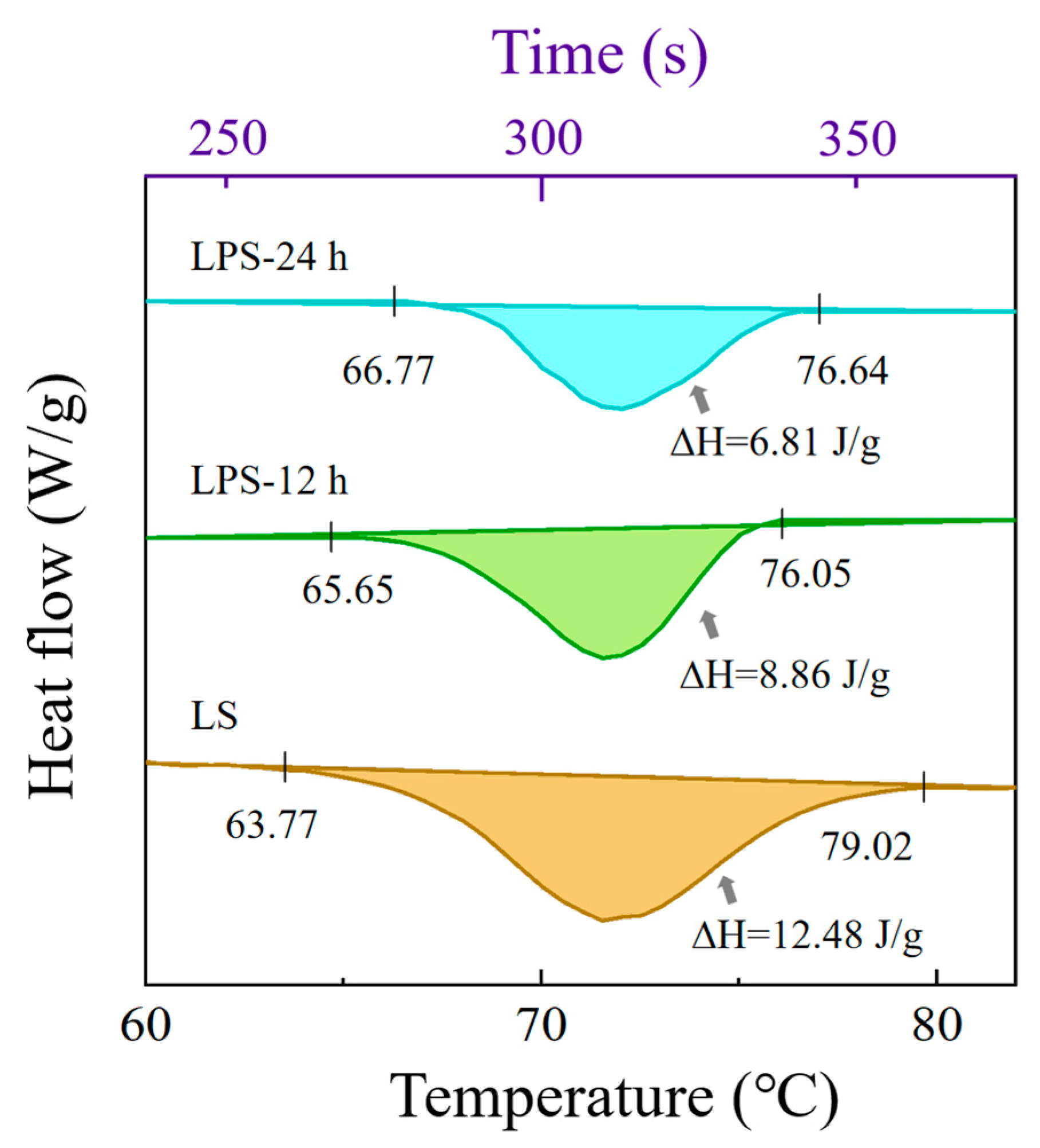
3.1.4. Thermal Properties of Samples
3.2. Adsorption of PC by LPS
3.2.1. Effect of PC Concentration on Adsorption Amount
3.2.2. Effect of Different Temperatures on Adsorption Capacity
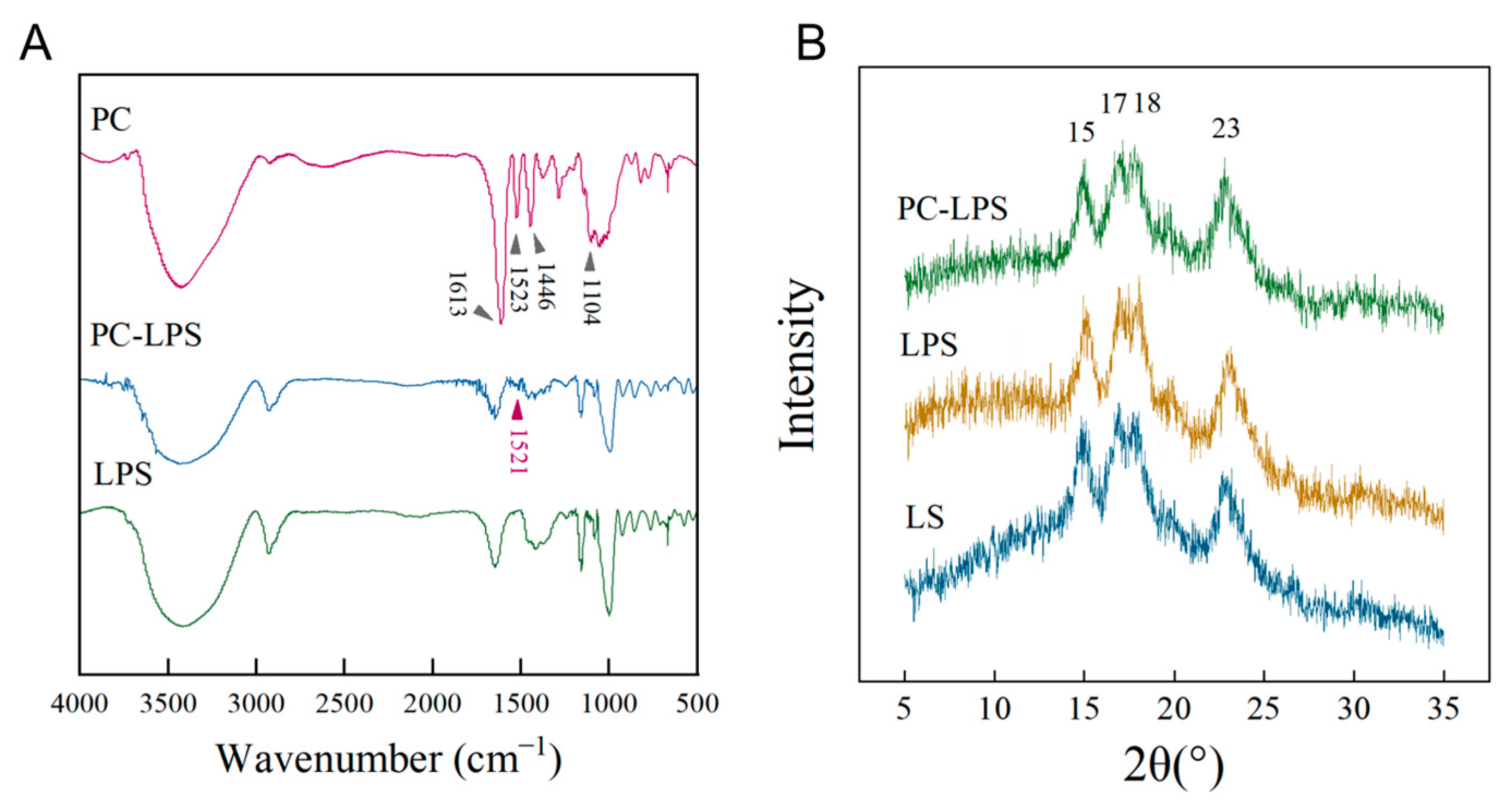
3.3. Characteristics of Samples
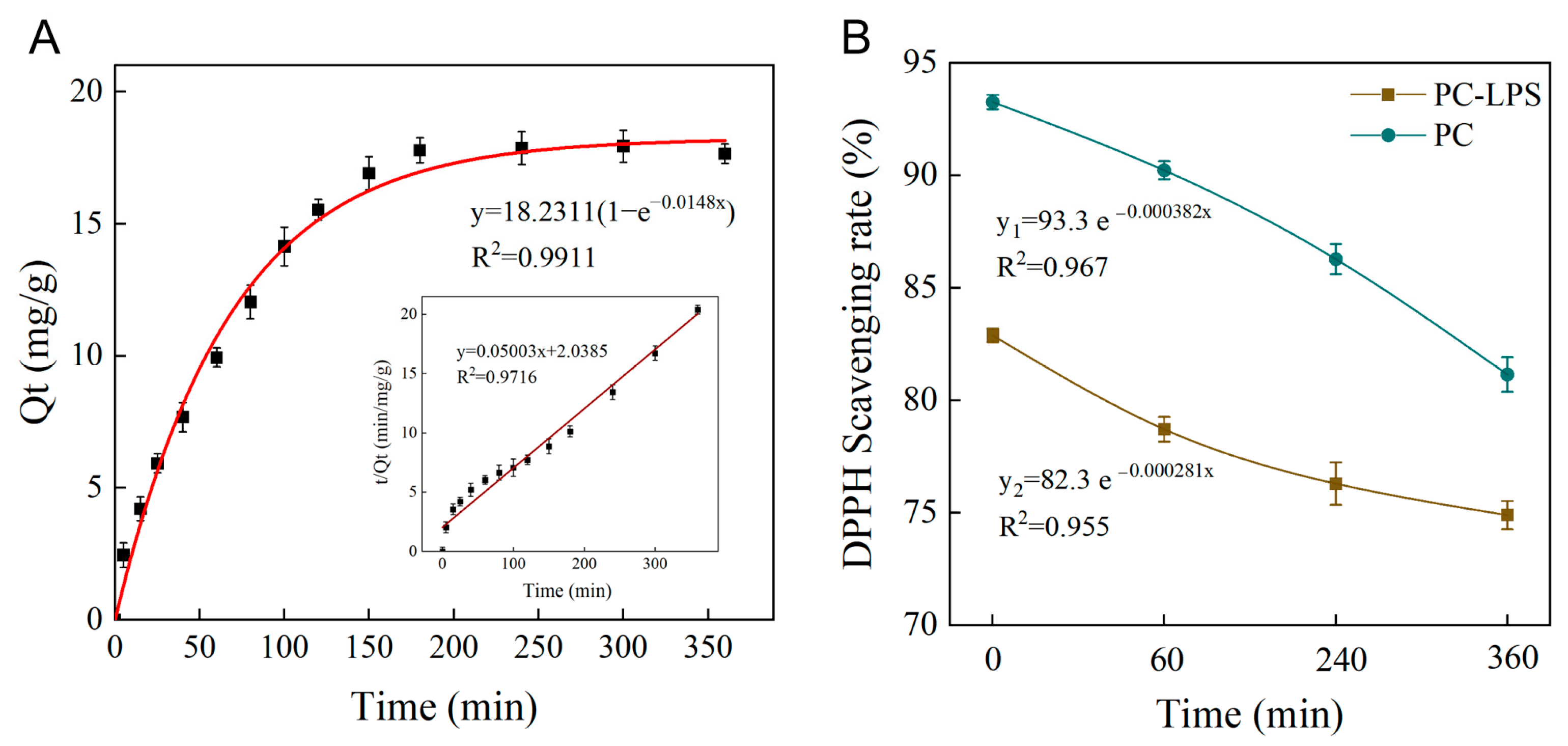
3.4. Adsorption Kinetics of PC
3.5. UV Radiation Stability of PC-LPS
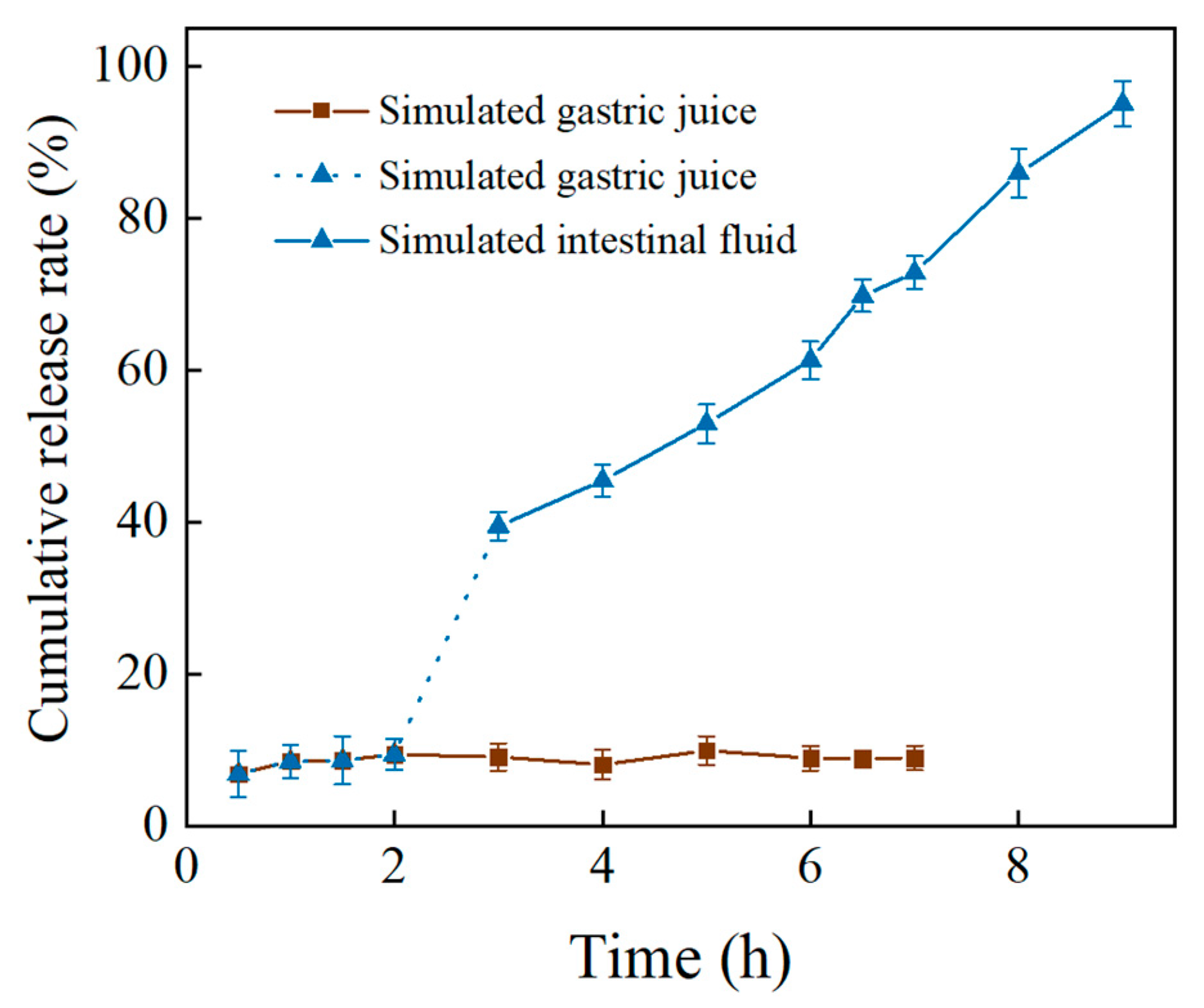
3.6. Slow Release of PC In Vitro
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Fan, Z. Molecular structure in relation to swelling, gelatinization, and rheological properties of lotus seed starch. Food Hydrocoll. 2023, 146, 109259. [Google Scholar] [CrossRef]
- Piloni, R.V.; Bordón, M.G.; Barrera, G.N.; Martínez, M.L.; Ribotta, P.D. Porous Microparticles of Corn Starch as Bio-Carriers for Chia Oil. Foods 2022, 11, 4022. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Liu, J.; Xu, X. Preparation, characterization, physicochemical property and potential application of porous starch: A review. Int. J. Biol. Macromol. 2020, 148, 1169–1181. [Google Scholar] [CrossRef]
- Sujka, M.; Wiącek, A. Physicochemical Characteristics of Porous Starch Obtained by Combined Physical and Enzymatic Methods, Part 1: Structure, Adsorption, and Functional Properties. Int. J. Mol. Sci. 2024, 25, 1662. [Google Scholar] [CrossRef]
- Cao, F.; Lu, S. OSA modified porous starch acts as an efficient carrier for loading and sustainedly releasing naringin. Food Chem. 2025, 463 Pt 2, 141176. [Google Scholar] [CrossRef]
- Li, J.; Fan, J.; Hu, F. Ultrasound-assisted acid/enzymatic hydrolysis preparation of loquat kernel porous starch: A carrier with efficient palladium loading capacity. Int. J. Biol. Macromol. 2023, 247, 125676. [Google Scholar] [CrossRef]
- Zhou, X.; Chang, Q.; Li, J.; Jiang, L.; Xing, Y.; Jin, Z. Preparation of V-type porous starch by amylase hydrolysis of V-type granular starch in aqueous ethanol solution. Int. J. Biol. Macromol. 2021, 183, 890–897. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, Y.; Lu, P. Novel active starch films incorporating tea polyphenols-loaded porous starch as food packaging materials. Int. J. Biol. Macromol. 2021, 192, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Jiang, F.; Cai, J.; Hu, S.; Zhou, R.; Liu, G.; Wang, Y.; Wang, H.; He, J.; Xiong, X. Facile microencapsulation of olive oil in porous starch granules: Fabrication, characterization, and oxidative stability. Int. J. Biol. Macromol. 2018, 111, 755–761. [Google Scholar] [CrossRef]
- Lai, R.; Xian, D.; Xiong, X.; Yang, L.; Song, J.; Zhong, J. Proanthocyanidins: Novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cells. Redox Rep. 2018, 23, 130–135. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Jamuna, S.; Ashokkumar, R.; Raja, I.S.; Devaraj, S.N. Anti-Atherogenic Protection by Oligomeric Proanthocyanidins via Regulating Collagen Crosslinking Against CC Diet-Induced Atherosclerosis in Rats. Appl. Biochem. Biotechnol. 2023, 195, 4881–4892. [Google Scholar] [CrossRef]
- Andersen, C.; Audrey, I.; Leppä, M.; Thamsborg, S.M.; Salminen, J.; Williams, A. Structure-function analysis of purified proanthocyanidins reveals a role for polymer size in suppressing inflammatory responses. Commun. Biol. 2021, 4, 896. [Google Scholar] [CrossRef]
- Lee, Y.A.; Cho, E.J.; Yokozawa, T. Oligomeric proanthocyanidins improve memory and enhance phosphorylation of vascular endothelial growth factor receptor-2 in senescence-accelerated mouse prone/8. Br. J. Nutr. 2010, 103, 479–489. [Google Scholar] [CrossRef]
- Tao, W.; Pan, H.; Jiang, H.; Wang, M.; Ye, X.; Chen, S. Extraction and identification of proanthocyanidins from the leaves of persimmon and loquat. Food Chem. 2022, 372, 130780. [Google Scholar] [CrossRef]
- Ding, Z.; Mo, M.; Zhang, K.; Bi, Y.; Kong, F. Preparation, characterization and biological activity of proanthocyanidin-chitosan nanoparticles. Int. J. Biol. Macromol. 2021, 188, 43–51. [Google Scholar] [CrossRef]
- Purwitasari, L.; Wulanjati, M.P.; Pranoto, Y.; Witasari, L.D. Characterization of porous starch from edible canna (Canna edulis Kerr.) produced by enzymatic hydrolysis using thermostable α-amylase. Food Chem. Adv. 2023, 2, 100152. [Google Scholar] [CrossRef]
- Luo, X.; Wang, R.; Wang, J.; Li, Y.; Luo, H.; Zeng, X.; Woo, M.; Han, Z. Combining pulsed electric field and cross-linking to enhance the structural and physicochemical properties of corn porous starch. Food Chem. 2023, 418, 135971. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tsuji, S.; Tonogai, Y. Analysis of Proanthocyanidins in Grape Seed Extracts, Health Foods and Grape Seed Oils. J. Health Sci. 2003, 49, 45–54. [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, L.; Wang, P.; Zhan, L.; Yang, Y.; He, T. Structural and Functional Properties of Porous Corn Starch Obtained by Treating Raw Starch with AmyM. Foods 2023, 12, 3157. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, M.; Ni, H.; Bai, Y.; Hao, Q.; Zhang, L.; Kang, X.; Lyu, M.; Wang, S. Preparation of Sweet Potato Porous Starch by Marine Dextranase and Its Adsorption Characteristics. Foods 2024, 13, 549. [Google Scholar] [CrossRef]
- Wahab, M.; Janaswamy, S. Porous corn starch granules as effective host matrices for encapsulation and sustained release of curcumin and resveratrol. Carbohydr. Polym. 2024, 333, 121967. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hu, H. Characteristics of catechin loading rice porous starch/chitosan functional microsphere and its adsorption towards Pb2+. Heliyon 2022, 8, e10048. [Google Scholar] [CrossRef]
- Govindaraju, I.; Pallen, S.; Umashankar, S.; Mal, S.; Kaniyala, M.S.; Mahato, D.R.; Zhuo, G.; Mahato, K.; Mazumder, N. Microscopic and spectroscopic characterization of rice and corn starch. Microsc. Res. Tech. 2020, 83, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Keeratiburana, T.; Hansen, A.R.; Soontaranon, S.; Blennow, A.; Tongta, S. Porous high amylose rice starch modified by amyloglucosidase and maltogenic α-amylase. Carbohydr. Polym. 2020, 230, 115611. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, G.; Li, J.; Wang, W.; Hu, A.; Zheng, J. Structural and physicochemical properties of resistant starch under combined treatments of ultrasound, microwave, and enzyme. Int. J. Biol. Macromol. 2023, 232, 123331. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, W.; Ma, Q.; Wang, J.; Sun, J. Microwave-assisted enzymatic hydrolysis as a novel efficient way to prepare porous starch. Carbohydr. Polym. 2023, 301, 120306. [Google Scholar] [CrossRef]
- Li, H.; Jiao, A.; Wei, B.; Wang, Y.; Wu, C.; Jin, Z.; Tian, Y. Porous starch extracted from Chinese rice wine vinasse: Characterization and adsorption properties. Int. J. Biol. Macromol. 2013, 61, 156–159. [Google Scholar] [CrossRef]
- Mario, E.R.; Martin, A.H.; Jose, M.D.; Cristian, F.R.; Marius, R.; Beatriz, M.; Sandra, M.L. Crystalline structures of the main components of starch. Curr. Opin. Food Sci. 2021, 37, 107–111. [Google Scholar]
- Xie, Y.; Li, M.N.; Chen, H.; Zhang, B. Effects of the combination of repeated heat-moisture treatment and compound enzymes hydrolysis on the structural and physicochemical properties of porous wheat starch. Food Chem. 2019, 274, 351–359. [Google Scholar] [CrossRef]
- Zhang, B.; Cui, D.; Liu, M.; Gong, H.; Huang, Y.; Han, F. Corn porous starch: Preparation, characterization and adsorption property. Int. J. Biol. Macromol. 2012, 50, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Dura, A.; Błaszczak, W.; Rosell, C.M. Functionality of porous starch obtained by amylase or amyloglucosidase treatments. Carbohydr. Polym. 2014, 101, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Benavent-Gil, Y.; Rosell, C.M. Morphological and physicochemical characterization of porous starches obtained from different botanical sources and amylolytic enzymes. Int. J. Biol. Macromol. 2017, 103, 587–595. [Google Scholar] [CrossRef]
- Davoudi, Z.; Azizi, M.H.; Barzegar, M. Porous corn starch obtained from combined cold plasma and enzymatic hydrolysis: Microstructure and physicochemical properties. Int. J. Biol. Macromol. 2022, 223, 790–797. [Google Scholar] [CrossRef]
- Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Krueger, C.G.; Reed, J.D. Antimicrobial proanthocyanidin-chitosan composite nanoparticles loaded with gentamicin. Int. J. Biol. Macromol. 2020, 162, 1500–1508. [Google Scholar] [CrossRef]
- Dou, Y.; Mei, M.; Kettunen, T.; Mäkinen, M.; Jänis, J. Chemical fingerprinting of phenolic compounds in Finnish berry wines using Fourier transform ion cyclotron resonance mass spectrometry. Food Chem. 2022, 383, 132303. [Google Scholar] [CrossRef] [PubMed]
- Pasieczna-Patkowska, S.; Cichy, M.; Flieger, J. Application of Fourier Transform Infrared (FTIR) Spectroscopy in Characterization of Green Synthesized Nanoparticles. Molecules 2025, 30, 684. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Chen, Y.; Ma, X.; Xia, M. Chitosan and procyanidin composite films with high antioxidant activity and pH responsivity for cheese packaging. Food Chem. 2021, 338, 128013. [Google Scholar] [CrossRef]
- Han, X.; Ma, P.; Shen, M.; Wen, H.; Xie, J. Modified porous starches loading curcumin and improving the free radical scavenging ability and release properties of curcumin. Food Res. Int. 2023, 168, 112770. [Google Scholar] [CrossRef]
- Guo, L.; Li, J.; Gui, Y.; Zhu, Y.; Yu, B.; Tan, C.; Fang, Y.; Cui, B. Porous starches modified with double enzymes: Structure and adsorption properties. Int. J. Biol. Macromol. 2020, 164, 1758–1765. [Google Scholar] [CrossRef]
- Niu, B.; Li, Z.; Luan, C.; Zhao, B. The dissolution and bioavailability of curcumin reinforced by loading into porous starch under solvent evaporation. Int. J. Biol. Macromol. 2025, 287, 138611. [Google Scholar] [CrossRef]
- Zheng, X.; Qiu, C.; Long, J.; Jiao, A.; Xu, X.; Jin, Z.; Wang, J. Preparation and characterization of porous starch/β-cyclodextrin microsphere for loading curcumin: Equilibrium, kinetics and mechanism of adsorption. Food Biosci. 2021, 41, 101081. [Google Scholar] [CrossRef]
- Lv, J.; Jiang, S.; Niu, B.; Pang, M.; Jiang, S. Preparation and characterization of porous corn starch and its adsorption toward grape seed proanthocyanidins. Starch-Stärke 2016, 68, 1254–1263. [Google Scholar]
- Li, Y.; Zhang, H.; Zhao, Y.; Lv, H.; Liu, K. Encapsulation and Characterization of Proanthocyanidin Microcapsules by Sodium Alginate and Carboxymethyl Cellulose. Foods 2024, 13, 740. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Du, J.; Zhang, Y.; Gu, Z.; Li, Z.; Cheng, L.; Li, C.; Hong, Y. Polysaccharide-coated porous starch-based oral carrier for paclitaxel: Adsorption and sustained release in colon. Carbohydr. Polym. 2022, 291, 119571. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jia, R.; Liu, L.; Lin, W.; Guo, Z. Comparative study on dynamic in vitro digestion characteristics of lotus seed starch-EGCG complex prepared by different processing methods. Food Chem. 2024, 455, 139849. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Liu, Y.; Kong, M.; Wang, J.; Li, Y.; Zhao, Y. Study on the extraction, purification, stability, free radical scavenging kinetics, polymerization degree and characterization of proanthocyanidins from Pinus koraiensis seed scales. Food Chem. 2024, 454, 139776. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Comitato, R.; Ginés, I.; Ardévol, A.; Pinent, M.; Virgili, F.; Terra, X.; Blay, M. Protective Effect of Proanthocyanidins in a Rat Model of Mild Intestinal Inflammation and Impaired Intestinal Permeability Induced by LPS. Mol. Nutr. Food Res. 2019, 63, e1800720. [Google Scholar] [CrossRef]
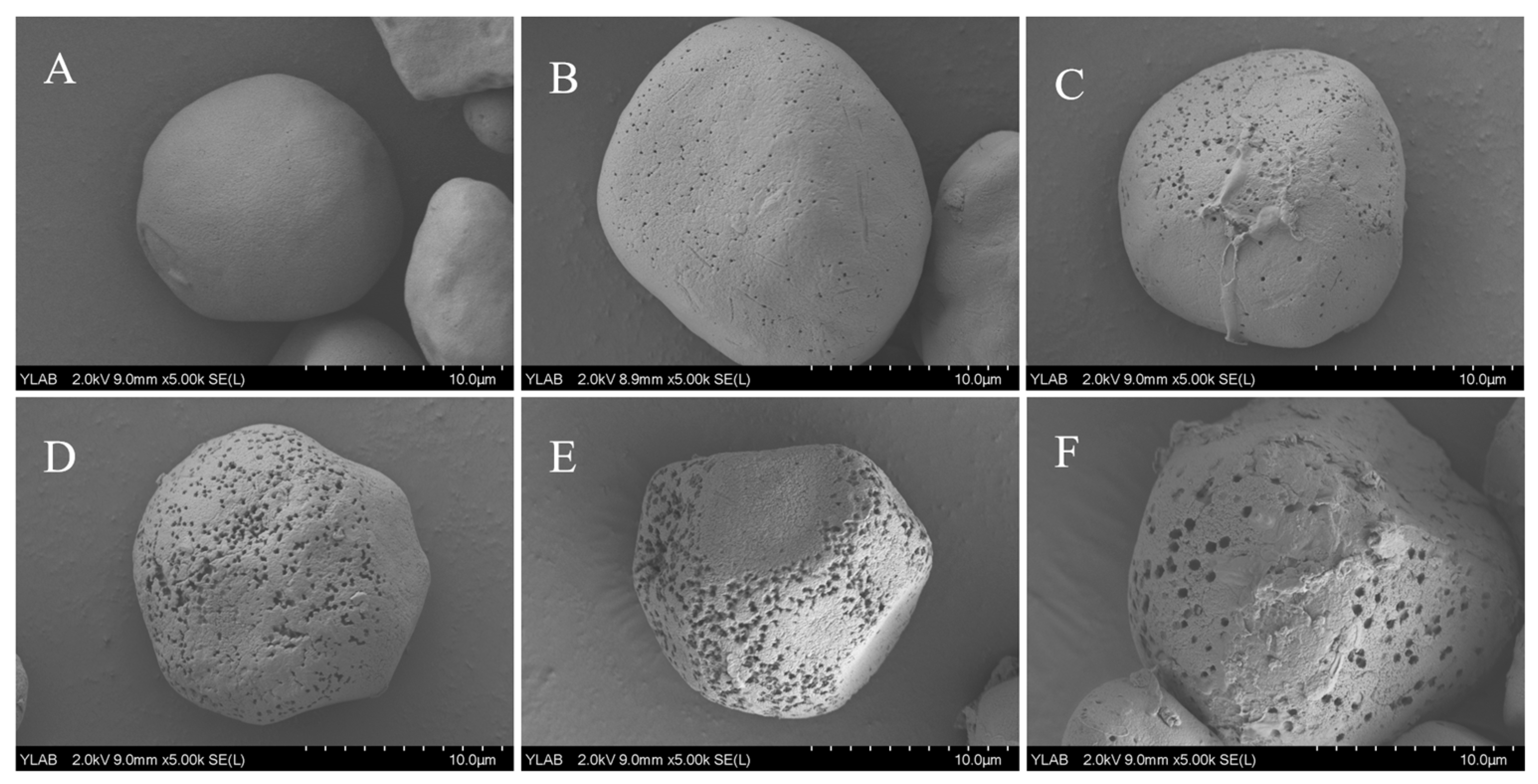
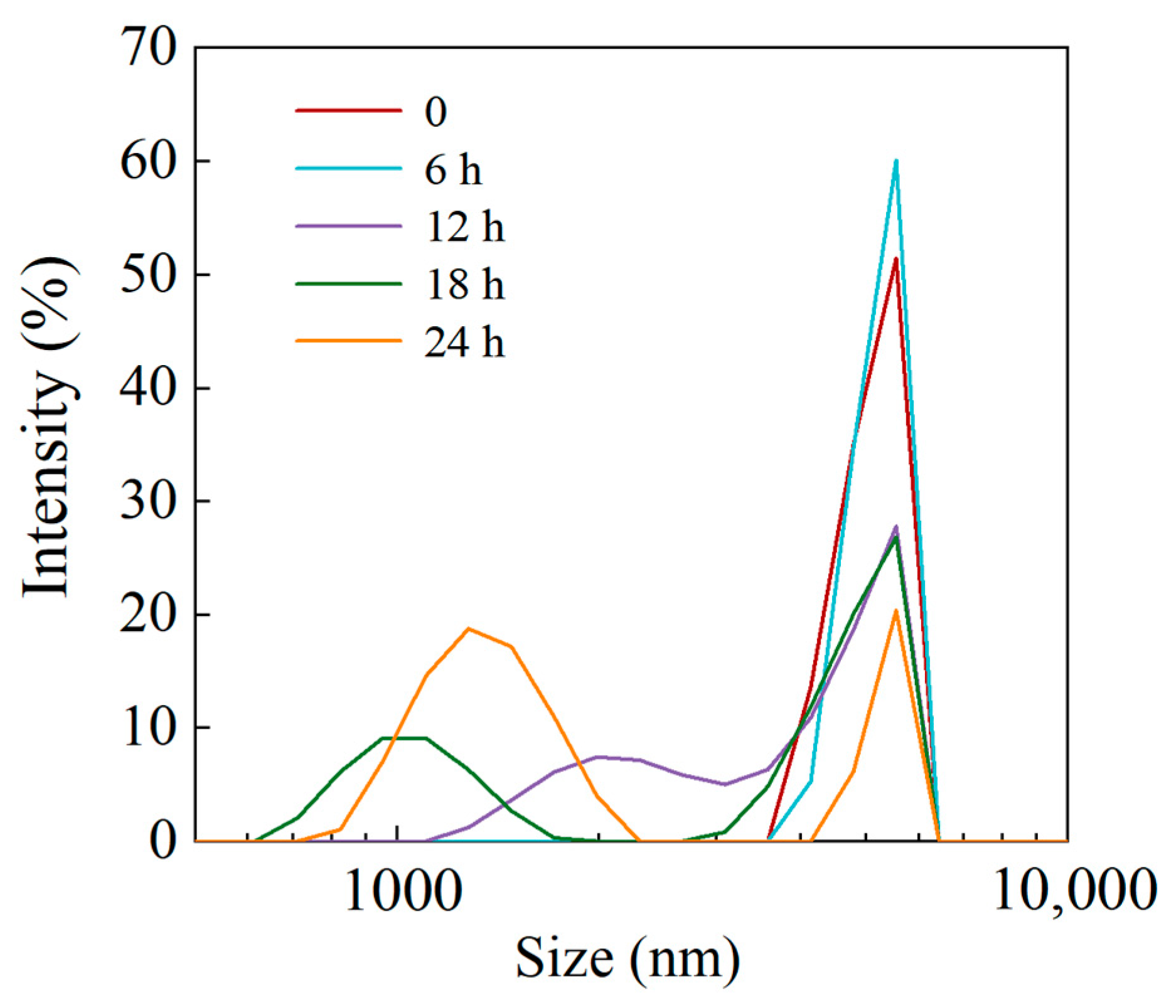
| Enzyme Digestion Time (h) | Oil Absorption (%) | Water Absorption (%) |
|---|---|---|
| 0 | 78.44 ± 1.34 b | 81.92 ± 1.56 b |
| 24 | 92.68 ± 1.09 a | 108.49 ± 1.06 a |
| Samples | To (°C) | Tp (°C) | Tc (°C) | ∆H (J/g) |
|---|---|---|---|---|
| LS | 63.77 ± 0.28 c | 71.59 ± 0.38 a | 79.02 ± 0.40 a | 12.48 ± 0.47 a |
| LPS-20 h | 65.65 ± 0.25 b | 71.84 ± 0.21 a | 76.05 ± 0.33 b | 8.86 ± 0.35 b |
| LPS-24 h | 66.77 ± 0.24 a | 71.98 ± 0.57 a | 76.64 ± 0.38 ab | 6.81 ± 0.50 c |
| PC Concentration (mg/mL) | PC Loading (mg/g) |
|---|---|
| 0.4 | 5.28 ± 2.31 d |
| 0.8 | 7.17 ± 1.18 c |
| 1.2 | 8.62 ± 1.36 b |
| 1.6 | 10.24 ± 5.27 a |
| 2.0 | 10.02 ± 3.15 a |
| Temperature (°C) | PC Loading (mg/g) |
|---|---|
| 30 | 9.11 ± 2.45 d |
| 40 | 15.94 ± 1.57 a |
| 50 | 12.82 ± 3.12 b |
| 55 | 10.37 ± 1.29 c |
| Starch Type | Crystallinity (%) |
|---|---|
| LS | 36.30 |
| LPS | 49.32 |
| PC-LPS | 43.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, M.; Jiang, W.; Li, S.; Liu, S.; Liu, M.; Lyu, M.; Wang, S. Characteristics of Porous Starch from Lotus Seeds Using Dextranase: Protection and Sustained Release of Proanthocyanidins. Foods 2025, 14, 1050. https://doi.org/10.3390/foods14061050
Wang Y, Wang M, Jiang W, Li S, Liu S, Liu M, Lyu M, Wang S. Characteristics of Porous Starch from Lotus Seeds Using Dextranase: Protection and Sustained Release of Proanthocyanidins. Foods. 2025; 14(6):1050. https://doi.org/10.3390/foods14061050
Chicago/Turabian StyleWang, Yuying, Ming’ao Wang, Weihong Jiang, Siying Li, Siyu Liu, Mingwang Liu, Mingsheng Lyu, and Shujun Wang. 2025. "Characteristics of Porous Starch from Lotus Seeds Using Dextranase: Protection and Sustained Release of Proanthocyanidins" Foods 14, no. 6: 1050. https://doi.org/10.3390/foods14061050
APA StyleWang, Y., Wang, M., Jiang, W., Li, S., Liu, S., Liu, M., Lyu, M., & Wang, S. (2025). Characteristics of Porous Starch from Lotus Seeds Using Dextranase: Protection and Sustained Release of Proanthocyanidins. Foods, 14(6), 1050. https://doi.org/10.3390/foods14061050







