Revealing Volatile Odor Compounds in Watermelon Juice to Enhance Fructose Sweetness Perception: Sensory Evaluation and Molecular Docking Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Concentrations of Sweet VOCs in Watermelon Juice
2.3. Determination of Odor Activity Values (OAVs)
2.4. Sensory Evaluation Team
2.5. Determination of VOCs Thresholds in Fructose Solution
2.6. TI Evaluation for Sweetness Intensity of Fructose Solutions Enhanced by Selected VOCs
2.7. Sensory Evaluation of Sweetness Intensity of Fructose Solutions Enhanced by Selected VOCs
2.8. Preference Evaluation of Fructose Solution with Selected VOCs
2.9. Molecular Docking
2.10. Statistical Analysis
3. Results and Discussion
3.1. Threshold and OAV of Sweet VOCs in Fructose Solution
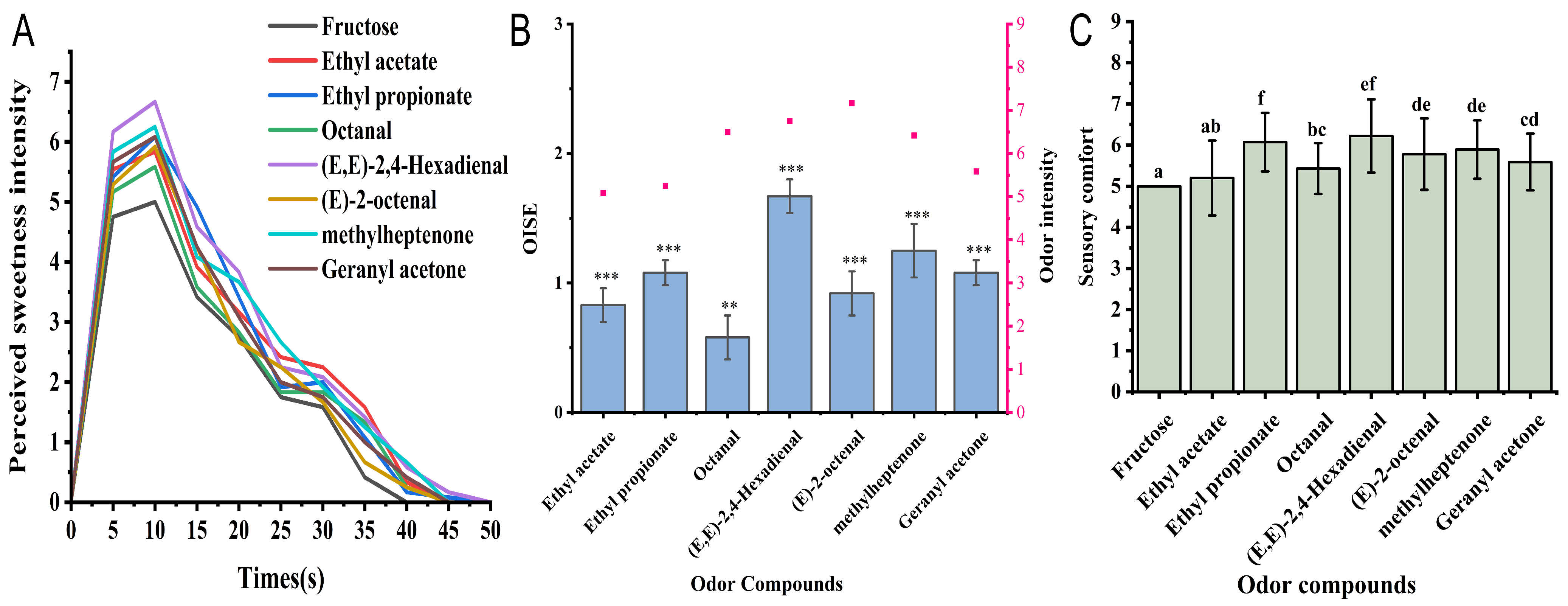
3.2. Dynamic Perception of Sweetness Intensity of Fructose Solution Induced by Sweet VOCs
3.3. Evaluation of Sweetness Enhancement on Fructose Solutions by Sweet VOCs
3.4. Evaluation of Sensory Preference of Fructose Solutions After Adding Sweet VOCs
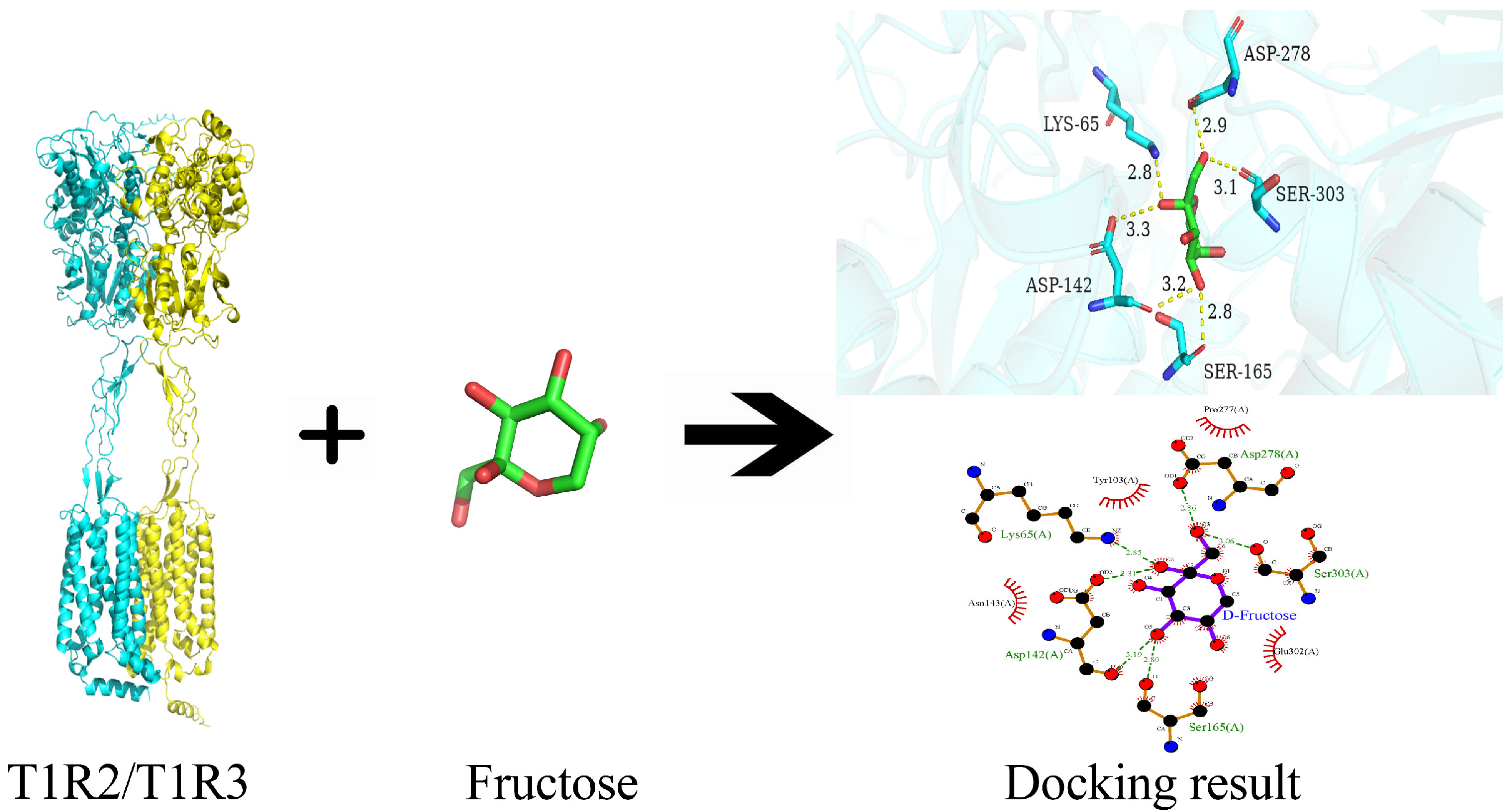
3.5. Binary Interaction Between Fructose and T1R2/T1R3
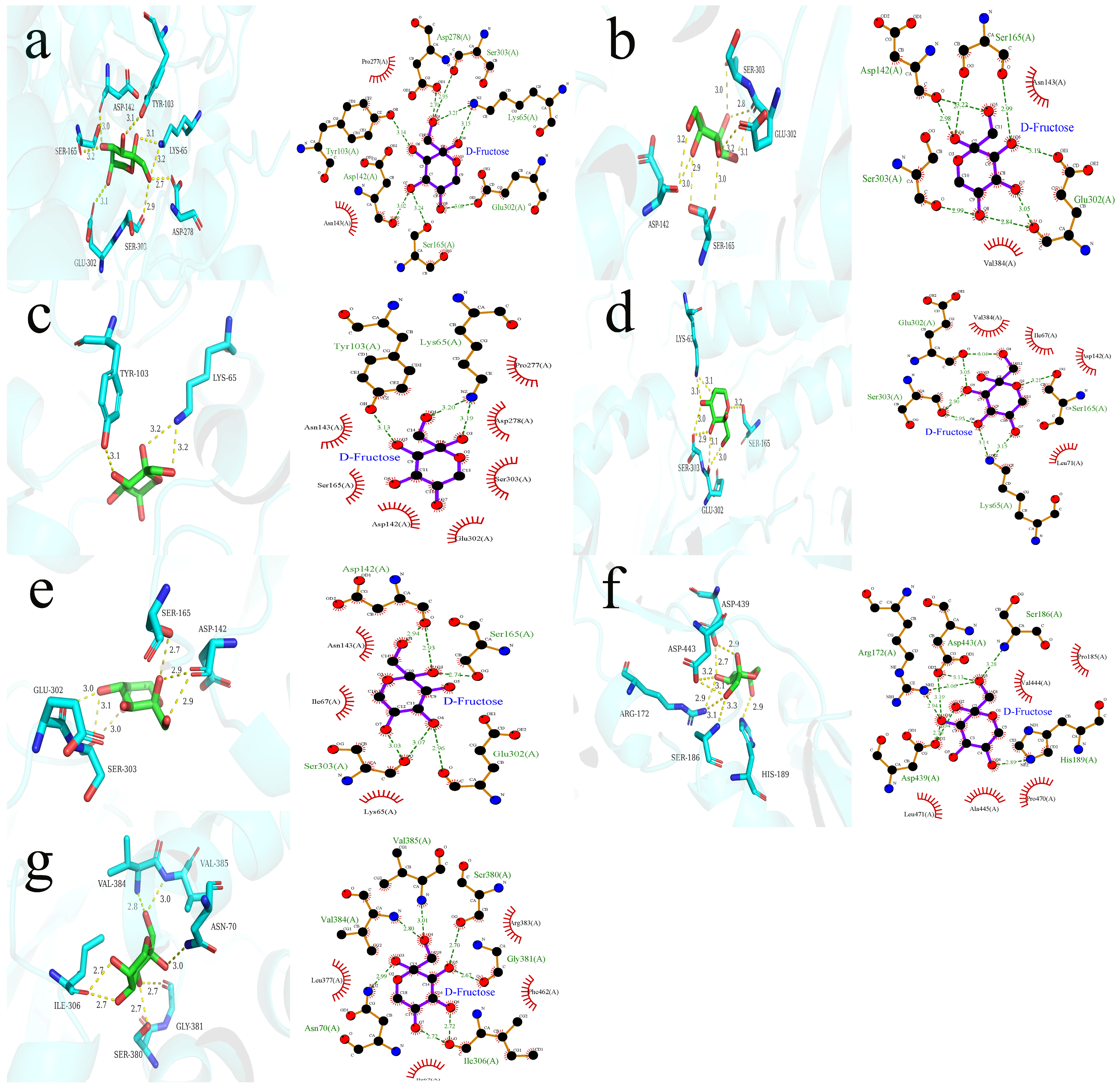
3.6. Ternary Interaction of VOCs, Fructose, and T1R2/T1R3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OISE | Odor-induced sweetness enhancement |
| VOCs | Volatile odor compounds |
| TI | Time-intensity |
| LD | Dynamic quantitative descriptive analysis |
| SIM | Selective ion monitoring |
| OT | odor threshold |
| VFD | Venus flytrap domain |
References
- Arshad, S.; Rehman, T.; Saif, S.; Rajoka, M.S.R.; Ranjha, M.M.A.N.; Hassoun, A.; Cropotova, J.; Trif, M.; Younas, A.; Aadil, R.M. Replacement of refined sugar by natural sweeteners: Focus on potential health benefits. Heliyon 2022, 8, e10711. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhang, D.; Xu, Y.; Wu, J.; Lao, F. The effect of mango aroma in low-sugar beverage: A sensory study on odor induced sweetness enhancement. Food Res. Int. 2024, 188, 114451. [Google Scholar] [CrossRef]
- Mansoor, S.; Jain, P.; Hassan, N.; Farooq, U.; Mirza, M.A.; Pandith, A.A.; Iqbal, Z. Role of Genetic and Dietary Implications in the Pathogenesis of Global Obesity. Food Rev. Int. 2022, 38, 434–455. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Zhou, K.; Zhang, P.; Ren, X.; Mei, X. Toxicity of food sweetener-sodium cyclamate on osteoblasts cells. Biochem. Biophys. Res. Commun. 2019, 508, 507–511. [Google Scholar] [CrossRef]
- Christofides, E.A. POINT: Artificial Sweeteners and Obesity—Not the Solution and Potentially a Problem. Endocr. Pract. 2021, 27, 1052–1055. [Google Scholar] [CrossRef]
- Witkowski, M.; Nemet, I.; Alamri, H.; Wilcox, J.; Gupta, N.; Nimer, N.; Haghikia, A.; Li, X.S.; Wu, Y.; Saha, P.P.; et al. The artificial sweetener erythritol and cardiovascular event risk. Nat. Med. 2023, 29, 710–718. [Google Scholar] [CrossRef]
- Niu, Y.; Wei, J.; Zhu, J.; Feng, H.; Ren, Y.; Guo, Z.; Zhang, J.; Zhou, R.; She, Y.; Wang, Z.; et al. Study on the mechanism of glucose-lowering and sweetening of key sweet aroma compounds in sweet orange. Food Res. Int. 2024, 196, 114874. [Google Scholar] [CrossRef]
- Verhagen, J.V. The neurocognitive bases of human multimodal food perception: Consciousness. Brain Res. Rev. 2007, 53, 271–286. [Google Scholar] [CrossRef]
- Oliveira, A.A.A.; Andrade, A.C.; Bastos, S.C.; Condino, J.P.F.; Curzi Júnior, A.; Pinheiro, A.C.M. Use of strawberry and vanilla natural flavors for sugar reduction: A dynamic sensory study with yogurt. Food Res. Int. 2021, 139, 109972. [Google Scholar] [CrossRef]
- Bertelsen, A.S.; Zeng, Y.; Mielby, L.A.; Sun, Y.; Byrne, D.V.; Kidmose, U. Cross-modal Effect of Vanilla Aroma on Sweetness of Different Sweeteners among Chinese and Danish Consumers. Food. Qual. Prefer. 2021, 87, 104036. [Google Scholar] [CrossRef]
- Barba, C.; Beno, N.; Guichard, E.; Thomas-Danguin, T. Selecting odorant compounds to enhance sweet flavor perception by gas chromatography/olfactometry-associated taste (GC/O-AT). Food Chem. 2018, 257, 172–181. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, H.; Niu, Y.; Zhu, J.; Yu, Y.; She, Y.; Zhou, R.; Wang, Z.; Zhang, J. Effect and mechanism of green and aldehyde aroma compounds from sweet orange on sucrose sweetness perception. Food Chem. X 2024, 24, 101853. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Gao, J.; Niu, Y.; Wang, Z.; Zhou, R.; Zhang, J.; Zhu, J. Elucidation of the sweetening mechanism of sweet orange fruit aroma compounds on sucrose solution using sensory evaluation, electronic tongue, molecular docking, and molecular dynamics simulation. LWT 2024, 205, 116555. [Google Scholar] [CrossRef]
- Xiao, Z.; Hu, Y.; Niu, Y.; Zhang, J.; Yang, B. Five representative esters and aldehydes from fruits can enhance sweet perception. LWT 2024, 194, 115804. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Wang, B.; Song, H.; Zou, T. Screening of the volatile compounds in fresh and thermally treated watermelon juice via headspace-gas chromatography-ion mobility spectrometry and comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry analysis. LWT 2021, 137, 110478. [Google Scholar] [CrossRef]
- Yang, F.; Shi, C.; Yan, L.; Xu, Y.; Dai, Y.; Bi, S.; Liu, Y. Low-frequency ultrasonic treatment: A potential strategy to improve the flavor of fresh watermelon juice. Ultrason. Sonochem. 2022, 91, 106238. [Google Scholar] [CrossRef]
- Acree, T.E.; Barnard, J.; Cunningham, D.G. A procedure for the sensory analysis of gas chromatographic effluents. Food Chem. 1984, 14, 273–286. [Google Scholar] [CrossRef]
- Tan, F.; Wang, P.; Zhan, P.; Tian, H. Characterization of key aroma compounds in flat peach juice based on gas chromatography-mass spectrometry-olfactometry (GC-MS-O), odor activity value (OAV), aroma recombination, and omission experiments. Food Chem. 2022, 366, 130604. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zeng, C.; Ma, T.; Fang, Y.; Sun, X. Analysis of the Flavor Code of Newly Cultivated Excellent Apple Varieties in China Using the Sensomics. J. Agric. Food. Chem. 2025, 73, 4840–4849. [Google Scholar] [CrossRef]
- Yang, F.; Meng, H.; Fu, A.; Liu, Y.; Bi, S. Quantification- and structural-taste intensity of umami peptides from Agrocybe aegerita through quantitative structure-activity relationship. Food Chem. 2024, 455, 139919. [Google Scholar] [CrossRef] [PubMed]
- GB/T 22366-2022; Sensory Analysis—Methodology—General Guidance for Measuring Odour, Flavour and Taste Detection Thresholds by a Three-Alternative Forced-Choice (3-AFC) Procedure. Standardization Administration of the People’s Republic of China, National Standardization Administration: Beijing, China, 2022.
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. European Committee for Standardization: Brussels, Belgium, 2007.
- Chen, Y.P.; Wang, M.; Fang, X.; Liya, A.; Zhang, H.; Blank, I.; Zhu, H.; Liu, Y. Odorants Identified in Chinese Dry-Cured Ham Contribute to Salty Taste Enhancement. J. Agric. Food. Chem. 2024, 72, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Yang, F.; Chen, E.; Dai, Y.; Xu, Y.; Liu, Y.; Bi, S. Elucidation of the interaction between fructose and key aroma compounds in watermelon juice via Raman spectroscopy and nuclear magnetic resonance. Food Res. Int. 2022, 159, 111613. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, H.; Niu, Y.; Zhu, J. Characterization of the Aroma-Active Compounds in Banana (Musa AAA Red green) and Their Contributions to the Enhancement of Sweetness Perception. J. Agric. Food. Chem. 2021, 69, 15301–15313. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.S.; Kim, K.H.; Kim, K.O. Sensory Characteristics and Consumer Acceptability of Decaffeinated Green Teas. J. Food Sci. 2009, 74, S135–S141. [Google Scholar] [CrossRef] [PubMed]
- Songsamoe, S.; Saengwong-ngam, R.; Koomhin, P.; Matan, N. Understanding consumer physiological and emotional responses to food products using electroencephalography (EEG). Trends Food Sci. Technol. 2019, 93, 167–173. [Google Scholar] [CrossRef]
- Toda, Y.; Nakagita, T.; Hayakawa, T.; Okada, S.; Narukawa, M.; Imai, H.; Ishimaru, Y.; Misaka, T. Two Distinct Determinants of Ligand Specificity in T1R1/T1R3 (the Umami Taste Receptor). J. Biol. Chem. 2013, 288, 36863–36877. [Google Scholar] [CrossRef]
- Mahalapbutr, P.; Darai, N.; Panman, W.; Opasmahakul, A.; Kungwan, N.; Hannongbua, S.; Rungrotmongkol, T. Atomistic mechanisms underlying the activation of the G protein-coupled sweet receptor heterodimer by sugar alcohol recognition. Sci. Rep. 2019, 9, 10205. [Google Scholar]
- Chen, W.; Li, W.; Wu, D.; Zhang, Z.; Chen, H.; Zhang, J.; Wang, C.; Wu, T.; Yang, Y. Characterization of novel umami-active peptides from Stropharia rugoso-annulata mushroom and in silico study on action mechanism. J. Food Compos. Anal. 2022, 110, 104530. [Google Scholar] [CrossRef]
- Zhu, L.; Pan, F.; Stöppelmann, F.; Liang, J.; Qin, D.; Xiang, C.; Rigling, M.; Hannemann, L.; Wagner, T.; Zhang, Y.; et al. Unlocking the potential of odor-induced sugar reduction: An updated review of the underlying mechanisms, substance selections, and technical methodologies. Trends Food Sci. Technol. 2024, 147, 104423. [Google Scholar] [CrossRef]
- Chen, Y.P.; Ding, Z.; Yu, Y.; He, P.; Zhou, Y.; Liu, Y.; Feng, X. Recent advances in investigating odor-taste interactions: Psychophysics, neuroscience, and microfluidic techniques. Trends Food Sci. Technol. 2023, 138, 500–510. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, W.; Li, Z.; Zhao, W.; Liu, C.; Zhu, B.; He, P.; Tang, S.; Wu, Y.; Yang, J.; et al. Rethinking Sweetener Discovering: Multiparameter Modeling of Molecular Docking Results between the T1R2–T1R3 Receptor and Compounds with Different Tastes. J. Agric. Food. Chem. 2024, 72, 7336–7343. [Google Scholar] [CrossRef]
- Akishino, M.; Aoki, Y.; Baba, H.; Asakawa, M.; Hama, Y.; Mitsutake, S. Red algae-derived isofloridoside activates the sweet taste receptor T1R2/T1R3. Food Biosci. 2022, 50, 102186. [Google Scholar] [CrossRef]
| No. | Compounds | Odor Description | Threshold in Water (μg/L) | Threshold in 2.5% Fructose Solution (μg/L) | Ratio (Fructose Solution: Water) | Concentration in Watermelon Juice (μg/L) | OAV |
|---|---|---|---|---|---|---|---|
| 1 | Ethyl acetate | Fruity, green, sweety | 5 | 11.57 | 2.31 | 112.35 | 10 |
| 2 | Ethyl propionate | Fruity, sweety | 10 | 12.36 | 1.24 | 14.57 | 1 |
| 3 | Octanal | Citrus, green | 0.8 | 4.27 | 5.34 | 32.91 | 8 |
| 4 | (E,E)-2,4-hexadienal | Muskmelon, floral, green | 1.8 | 5.08 | 2.82 | 124.35 | 24 |
| 5 | (E)-2-octenal | Citrus, green | 2.7 | 4.11 | 1.52 | 55.46 | 14 |
| 6 | Methylheptenone | Citrus, apple, green | 68 | 63.41 | 0.93 | 111.67 | 2 |
| 7 | Geranyl acetone | Fruity, floral, green | 60 | 63.42 | 1.06 | 65.85 | 1 |
| Combination | Binding Energy (kcal/mol) | Hydrogen Bonding Residue Amino Acid | Hydrophobic Residue Amino Acid |
|---|---|---|---|
| T1R2/T1R3–fructose | −5.7 | Asp278, Ser303, Ser165, Asp142, Lys65 | Pro277, Glu302, Asn143, Tyr103 |
| T1R2/T1R3–ethyl acetate–fructose | −5.77 | Asp278, Ser303, Ser165, Asp142, Lys65, Tyr103, Glu302 | Pro277, Asn143 |
| T1R2/T1R3–ethyl propionate–fructose | −5.563 | Ser303, Glu302, Ser165, Asp142 | Val384, Asn143 |
| T1R2/T1R3–octanal–fructose | −5.714 | Tyr103, Lys65 | Pro277, Ser303, Asn143, Asp278, Ser165, Asp142, Glu302 |
| T1R2/T1R3-(E,E)–2,4-hexadienal–fructose | −5.711 | Lsy65, Ser303, Glu302, Ser165 | Val384, Leu71, Asp142, Ile67 |
| T1R2/T1R3–(E)-2-octenal–fructose | −5.625 | Asp142, Ser165, Glu302, Ser303 | Asn143, Lys65, Ile67 |
| T1R2/T1R3–methyl heptenone–fructose | −5.282 | Asp439, Arg172, Ser186, Asp443, His189 | Val444, Ala445, Leu471, Pro470, Pro185 |
| T1R2/T1R3–geranyl acetone–fructose | −5.38 | Asn70, Val384, Val385, Gly381, Ser380, Il306 | Arg383, Ile67, Phe462, Leu377 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Sun, S.; Yang, F.; Zhen, S.; Xiong, X.; Liu, Y.; Bi, S. Revealing Volatile Odor Compounds in Watermelon Juice to Enhance Fructose Sweetness Perception: Sensory Evaluation and Molecular Docking Techniques. Foods 2025, 14, 1034. https://doi.org/10.3390/foods14061034
Dai Y, Sun S, Yang F, Zhen S, Xiong X, Liu Y, Bi S. Revealing Volatile Odor Compounds in Watermelon Juice to Enhance Fructose Sweetness Perception: Sensory Evaluation and Molecular Docking Techniques. Foods. 2025; 14(6):1034. https://doi.org/10.3390/foods14061034
Chicago/Turabian StyleDai, Yixin, Shuang Sun, Fan Yang, Shaobo Zhen, Xiaoying Xiong, Ye Liu, and Shuang Bi. 2025. "Revealing Volatile Odor Compounds in Watermelon Juice to Enhance Fructose Sweetness Perception: Sensory Evaluation and Molecular Docking Techniques" Foods 14, no. 6: 1034. https://doi.org/10.3390/foods14061034
APA StyleDai, Y., Sun, S., Yang, F., Zhen, S., Xiong, X., Liu, Y., & Bi, S. (2025). Revealing Volatile Odor Compounds in Watermelon Juice to Enhance Fructose Sweetness Perception: Sensory Evaluation and Molecular Docking Techniques. Foods, 14(6), 1034. https://doi.org/10.3390/foods14061034






