Integrated Metagenomic and LC–MS/MS Analysis Reveals the Biogenic Amine-Producing Strains of Two Typical Chinese Traditional Fish Products: Fermented Mandarin Fish (Siniperca chuatsi) and Semi-Dried Yellow Croaker (Larimichthys crocea)
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. Biogenic Amine Analysis
2.3. Total Visible Count and Microbial Community Analysis
2.4. Bacterial Diversity Analysis by the Culture-Dependent Method
2.4.1. Screening of BA-Producing Bacteria
2.4.2. Identification of BA-Producing Bacteria
2.4.3. Determination of BA-Producing Capacity of Bacteria
2.5. Statistical Analysis
3. Results and Discussion
3.1. BA Levels and Microbial Counts in Fish Products
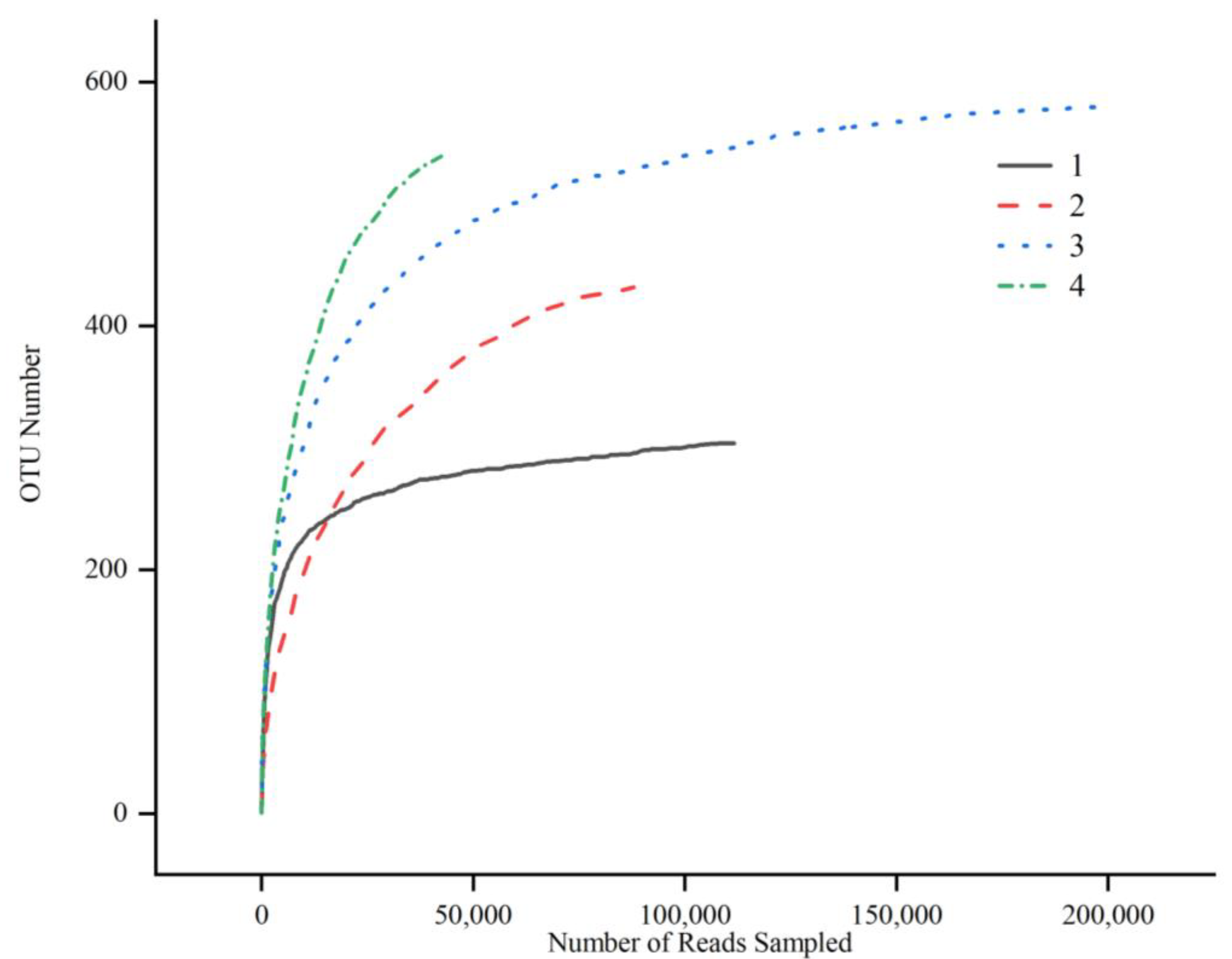
3.2. Alpha Diversity in Fish Products
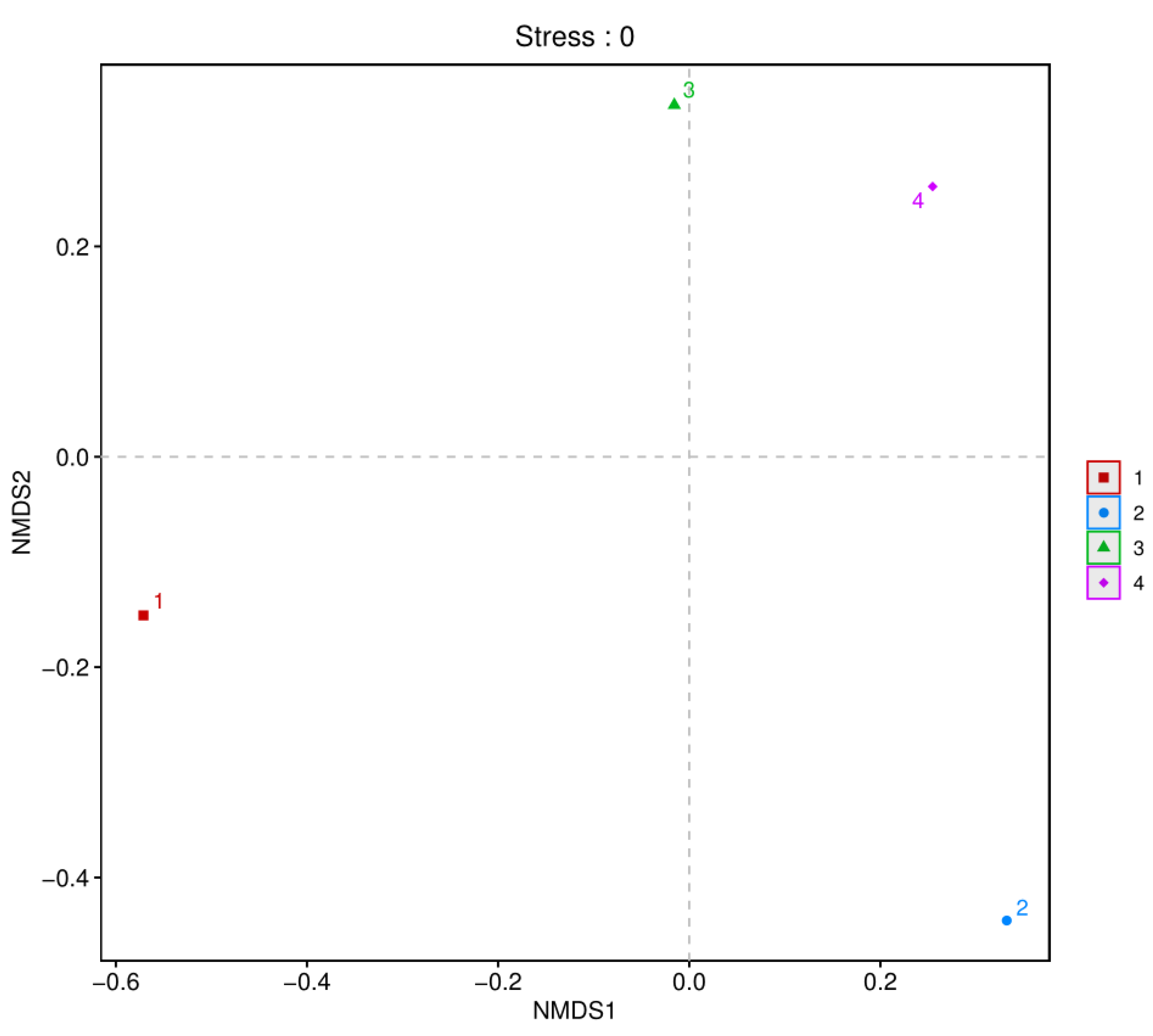
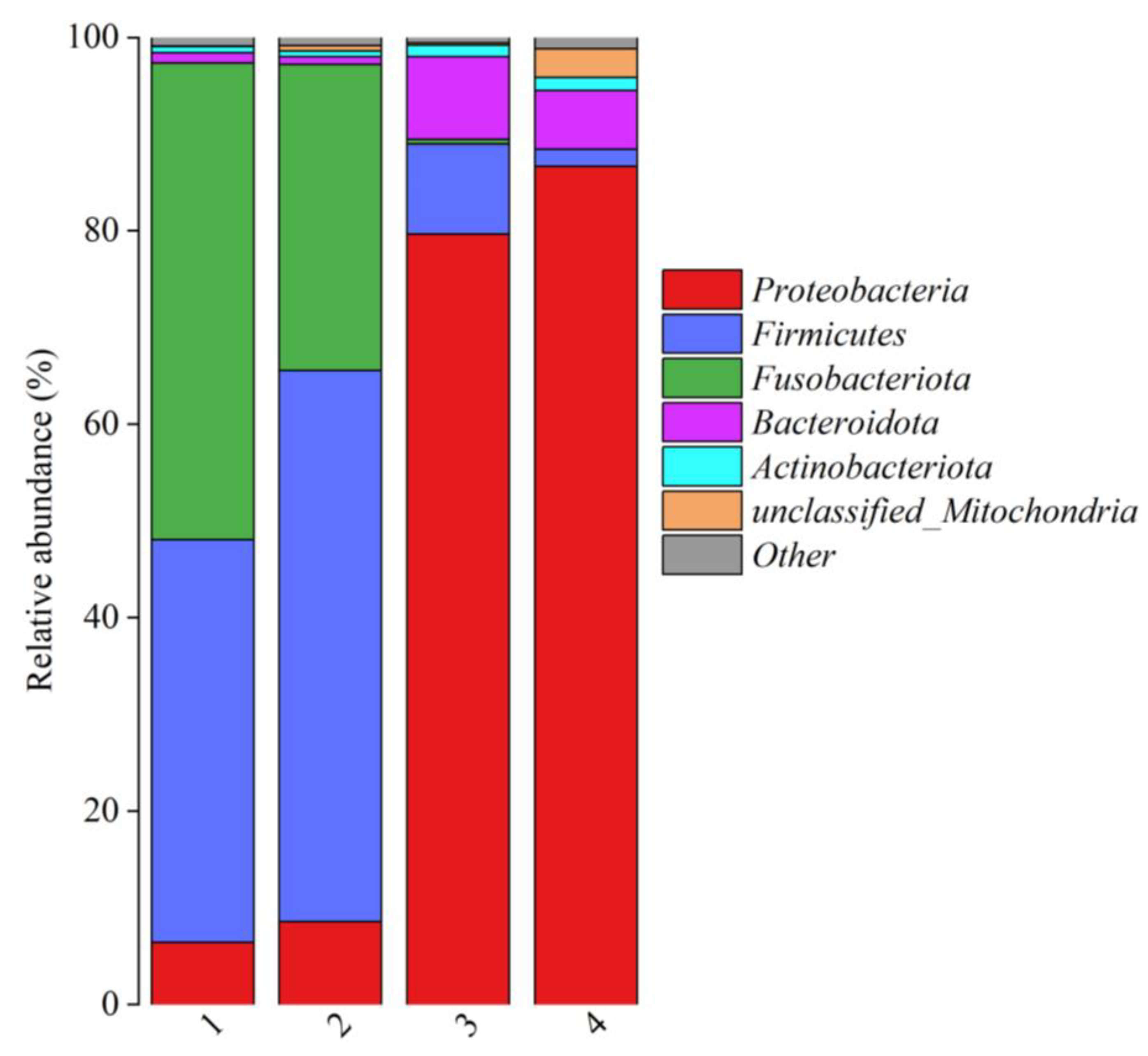
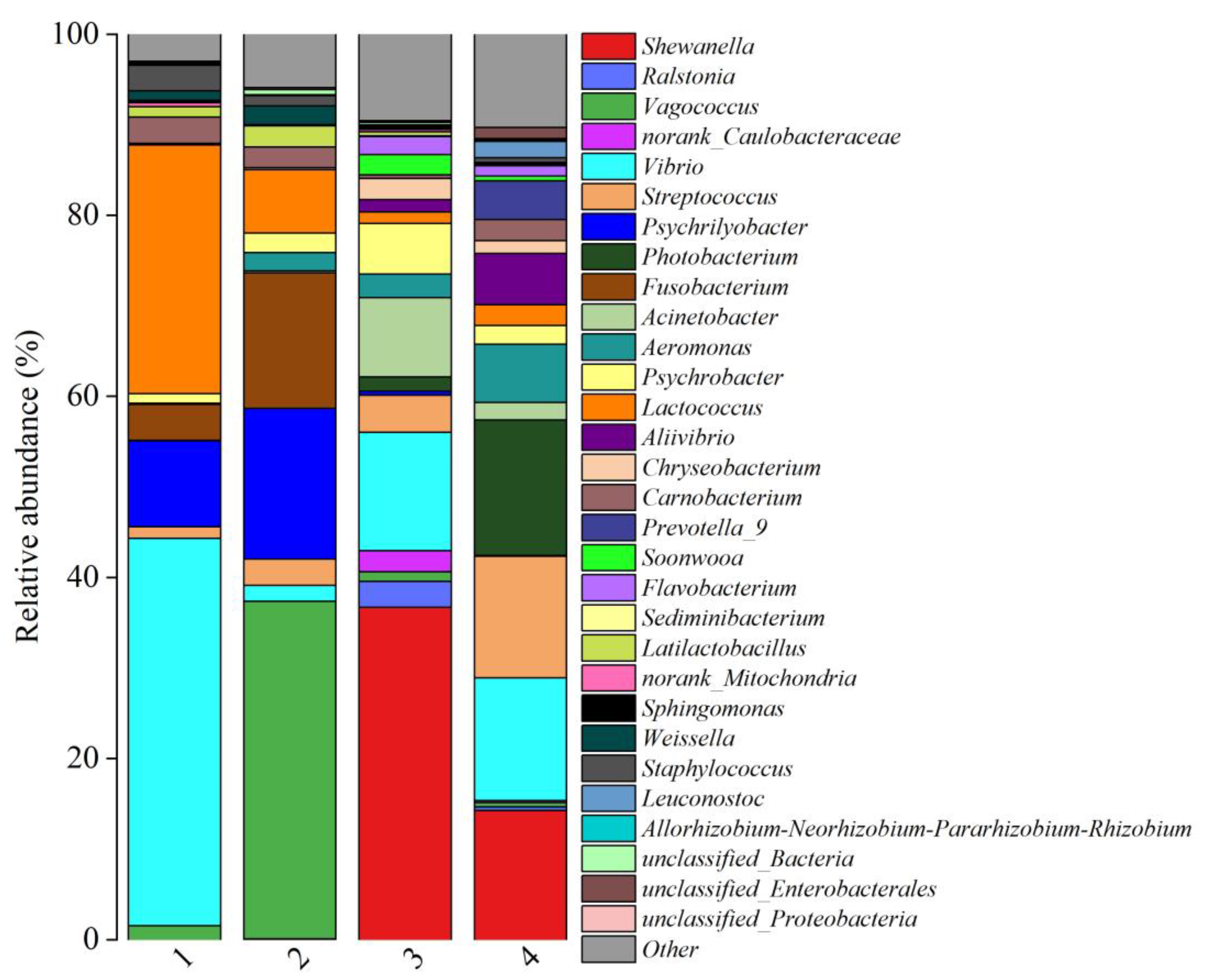
3.3. Composition of Bacterial Communities in Fish Products
3.4. Screening and Identification of BA-Producing Bacteria
3.5. Aminogenic Capacity of BA-Producing Bacteria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, J.; Fu, J.; Zhu, Z.; Cao, Z.; Zhang, M.; Yuan, Y.; Chai, T.; Chen, Y. Flavor characteristics of semi-dried yellow croaker (Pseudosciaena crocea) with KCl and ultrasound under sodium-reduced conditions before and after low temperature vacuum heating. Food Chem. 2023, 426, 136574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, Y.; Wu, Y.; Li, C.; Li, L.; Zhao, Y.; Hu, X.; Wei, Y.; Huang, H. Comparison of the microbial community and flavor compounds in fermented mandarin fish (Siniperca chuatsi): Three typical types of Chinese fermented mandarin fish products. Food Res. Int. 2021, 144, 110365. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, C.; He, R.; Zhang, Y.; Wang, B.; Zhang, Z.; Ho, C. Research advances on biogenic amines in traditional fermented foods: Emphasis on formation mechanism, detection and control methods. Food Chem. 2023, 405, 134911. [Google Scholar] [CrossRef]
- Jaguey-Hernández, Y.; Aguilar-Arteaga, K.; Ojeda-Ramirez, D.; Añorve-Morga, J.; González-Olivares, L.G.; Castañeda-Ovando, A. Biogenic amines levels in food processing: Efforts for their control in foodstuffs. Food Res. Int. 2021, 144, 110341. [Google Scholar] [CrossRef]
- FDA. FDA and EPA safety levels in regulations and guidance. In Department of Health and Human Services, 4th ed.; Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Seafood, Fish and Fishery Products Hazards and Controls, Appendix 5: Maitland, FL, USA, 2020; pp. 5–7. [Google Scholar]
- Tian, X.; Gao, P.; Xu, Y.; Xia, W.; Jiang, Q. Reduction of biogenic amines accumulation with improved flavor of low-salt fermented bream (Parabramis pekinensis) by two-stage fermentation with different temperature. Food Biosci. 2021, 44, 101438. [Google Scholar] [CrossRef]
- Dabadé, D.S.; Jacxsens, L.; Miclotte, L.; Abatih, E.; Devlieghere, F.; De Meulenaer, B. Survey of multiple biogenic amines and correlation to microbiological quality and free amino acids in foods. Food Control 2021, 120, 107497. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, S.; Lv, J.; Sun, Z.; Xu, W.; Ji, C.; Liang, H.; Li, S.; Yu, C.; Lin, X. Microbial succession and the changes of flavor and aroma in Chouguiyu, a traditional Chinese fermented fish. Food Biosci. 2020, 37, 100725. [Google Scholar] [CrossRef]
- Halász, A.; Baráth, Á.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994, 5, 42–49. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, C.; Huang, D.; Yang, G.; Tang, Y.; Shi, Y.; Kong, K.; Cao, P.; Cai, Y. Determination of 8 biogenic amines in aquatic products and their derived products by high-performance liquid chromatography-tandem mass spectrometry without derivatization. Food Chem. 2021, 361, 130044. [Google Scholar] [CrossRef]
- Deng, H.; Wu, G.; Zhou, L.; Chen, X.; Guo, L.; Luo, S.; Yin, Q. Microbial contribution to 14 biogenic amines accumulation in refrigerated raw and deep-fried hairtails (Trichiurus lepturus). Food Chem. 2024, 443, 138509. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Y.; Huang, H.; Xu, Q. Microbial succession of grass carp (Ctenopharyngodon idellus) filets during storage at 4°C and its contribution to biogenic amines’ formation. Int. J. Food Microbiol. 2014, 190, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Li, C.; Li, L.; Yang, L.; Wu, Y.; Chen, S.; Zhao, Y. Novel insight into physicochemical and flavor formation in naturally fermented tilapia sausage based on microbial metabolic network. Food Res. Int. 2021, 141, 110122. [Google Scholar] [CrossRef] [PubMed]
- Aregbe, A.Y.; Mu, T.; Sun, H. Effect of different pretreatment on the microbial diversity of fermented potato revealed by high-throughput sequencing. Food Chem. 2019, 290, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Eun, J. Shotgun metagenomics approach reveals the bacterial community and metabolic pathways in commercial hongeo product, a traditional Korean fermented skate product. Food Res. Int. 2020, 131, 109030. [Google Scholar] [CrossRef]
- Lin, Y.T.; Whitman, W.B.; Coleman, D.C.; Jin, S.H.; Wang, H.C.; Chiu, C.Y. Soil bacterial communities at the treeline in subtropical alpine areas. Catena 2021, 201, 105205. [Google Scholar] [CrossRef]
- Bhutia, M.O.; Thapa, N.; Shangpliang, H.N.J.; Tamang, J.P. Metataxonomic profiling of bacterial communities and their predictive functional profiles in traditionally preserved meat products of Sikkim state in India. Food Res. Int. 2021, 140, 110002. [Google Scholar] [CrossRef]
- Bhutia, M.O.; Thapa, N.; Shangpliang, H.N.J.; Tamang, J.P. High-throughput sequence analysis of bacterial communities and their predictive functionalities in traditionally preserved fish products of Sikkim, India. Food Res. Int. 2020, 143, 109885. [Google Scholar] [CrossRef]
- Ding, T.; Li, Y. Biogenic amines are important indices for characterizing the freshness and hygienic quality of aquatic products: A review. LWT–Food Sci. Technol. 2024, 194, 115793. [Google Scholar] [CrossRef]
- Lou, X.; Zhai, D.; Yang, H. Changes of metabolite profiles of fish models inoculated with Shewanella baltica during spoilage. Food Control 2021, 123, 107697. [Google Scholar] [CrossRef]
- Dalgaard, P.; Madsen, H.L.; Samieian, N.; Emborg, J. Biogenic amine formation and microbial spoilage in chilled garfish (Belone belone belone)–effect of modified atmosphere packaging and previous frozen storage. J. Appl. Microbiol. 2006, 101, 80–95. [Google Scholar] [CrossRef]
- Belleggia, L.; Osimani, A. Fermented fish and fermented fish-based products, an ever-growing source of microbial diversity: A literature review. Food Res. Int. 2023, 172, 113112. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Han, X.; Han, B.; Deng, H.; Wu, T.; Zhao, X.; Huang, W.; Zhan, J.; You, L. Survey of the biogenic amines in craft beer from the Chinese market and the analysis of the formation regularity during beer fermentation. Food Chem. 2023, 405A, 134861. [Google Scholar] [CrossRef]
- Saticioglu, I.B.; Yardimci, B.; Altun, S.; Duman, M. A comprehensive perspective on a Vagococcus salmoninarum outbreak in rainbow trout broodstock. Aquaculture 2021, 545, 737224. [Google Scholar] [CrossRef]
- Henríquez-Aedo, K.; Durán, D.; Garcia, A.; Hengst, M.B.; Aranda, M. Identification of biogenic amines-producing lactic acid bacteria isolated from spontaneous malolactic fermentation of chilean red wines. LWT–Food Sci. Technol. 2016, 68, 183–189. [Google Scholar] [CrossRef]
- Ding, A.; Zhu, M.; Qian, X.; Li, J.; Xiong, G.; Shi, L.; Wu, W.; Wang, L. Identification of amine-producing bacterium (APB) in Chinese fermented mandarin fish (Chouguiyu) by high-throughput sequencing and culture-dependent methods. J. Food Compos. Anal. 2024, 133, 106368. [Google Scholar] [CrossRef]
- Li, B.; Lu, S. The importance of amine-degrading enzymes on the biogenic amine degradation in fermented foods: A review. Process Biochem. 2020, 99, 331–339. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Meng, J.; Yang, Q.; Wan, W.; Zhu, Q.; Zeng, X. Physicochemical properties and adaptability of amine-producing Enterobacteriaceae isolated from traditional Chinese fermented fish (Suan yu). Food Chem. 2022, 369, 130885. [Google Scholar] [CrossRef]
- Lavizzari, T.; Breccia, M.; Bover-Cid, S.; Vidal-Carou, M.C.; Veciana-Nogués, M.T. Histamine, cadaverine, and putrescine produced in vitro by Enterobacteriaceae and Pseudomonadaceae isolated from spinach. J. Food Prot. 2010, 73, 385–389. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhang, G.; Bi, J.; Hao, H.; Hou, H. The role and the determination of the LuxI protein binding targets in the formation of biogenic amines in Hafnia alvei H4. Int. J. Food Microbiol. 2025, 426, 110928. [Google Scholar] [CrossRef]
- Benkerroum, N. Biogenic amines in dairy products: Origin, incidence, and control means. Compr. Rev. Food Sci. Food Saf. 2016, 15, 801–826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Lin, X.; Liang, H.; Zhang, S.; Ji, C. Lactobacillus strains inhibit biogenic amine formation in salted mackerel (Scomberomorus niphonius). LWT–Food Sci. Technol. 2022, 155, 112851. [Google Scholar] [CrossRef]
- Wunderlichová, L.; Buňková, L.; Koutný, M.; Jančová, P.; Buňka, B. Formation, Degradation, and Detoxification of Putrescine by Foodborne Bacteria: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1012–1030. [Google Scholar] [CrossRef]
- Lee, S.G.; Chang, H.C. Assessment of Bacillus subtilis SN7 as a starter culture for Cheonggukjang, a Korean traditional fermented soybean food, and its capability to control Bacillus cereus in Cheonggukjang. Food Control 2017, 73, 946–953. [Google Scholar] [CrossRef]
- Shan, K.; Yao, Y.; Wang, J.; Zhou, T.; Zeng, X.; Zhang, M.; Ke, W.; He, H.; Li, C. Effect of probiotic Bacillus cereus DM423 on the flavor formation of fermented sausage. Food Res. Int. 2023, 172, 113210. [Google Scholar] [CrossRef]
- Lerfall, J.; Shumilina, E.; Jakobsen, A.N. The significance of Shewanella sp. strain HSO12, Photobacterium phosphoreum strain HS254 and packaging gas composition in quality deterioration of fresh saithe fillets. LWT–Food Sci. Technol. 2022, 154, 112636. [Google Scholar] [CrossRef]
- Lou, X.; Hai, Y.; Le, Y.; Ran, X.; Yang, H. Metabolic and enzymatic changes of Shewanella baltica in golden pomfret broths during spoilage. Food Control 2023, 144, 109341. [Google Scholar] [CrossRef]
- Bao, X.; Wang, F.; Yang, R.; Zhang, Y.; Fu, L.; Wang, Y. Ornithine Decarboxylation System of Shewanella baltica Regulates Putrescine Production and Acid Resistance. J. Food Prot. 2021, 84, 303–309. [Google Scholar] [CrossRef]
- Bjornsdottir-Butler, K.; May, S.; Hayes, M.; Abraham, A.; Benner, R.A., Jr. Characterization of a novel enzyme from Photobacterium phosphoreum with histidine decarboxylase activity. Int. J. Food Microbiol. 2020, 334, 108815. [Google Scholar] [CrossRef]
- Bjornsdottir-Butler, K.; Abraham, A.; Harper, A.; Dunlap, P.V.; Benner, R.A. Biogenic amine production by and phylogenetic analysis of 23 Photobacterium species. J. Food Prot. 2018, 81, 1264–1274. [Google Scholar] [CrossRef]
- Kämpfer, P. Acinetobacter. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 11–17. [Google Scholar]
- Guan, T.; Fu, S.; Wu, X.; Yu, H.; Liu, Y. Bioturbation effect of artificial inoculation on the flavor metabolites and bacterial communities in the Chinese Mao-tofu fermentation. Food Chem. X 2024, 21, 101133. [Google Scholar] [CrossRef]

| Fish Product | Sample No. | Region | mg/kg | Log CFU/g | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAD | TYR | TRY | HIS | PUT | 2-PHE | Total BAs | PCA | |||
| Fermented mandarin fish | 1 | Shanghai | 36.90 ± 2.55 a | ND | ND | 8.28 ± 0.18 a | 56.84 ± 5.35 a | 0.36 ± 0.02 a | 102.38 ± 8.10 a | 4.9 ± 0.1 a |
| 2 | Huangshan | 29.66 ± 1.87 b | 15.89 ± 1.00 a | ND | 6.94 ± 0.36 b | 49.97 ± 3.22 a | 0.21 ± 0.02 b | 102.67 ± 6.47 a | 5.3 ± 0.1 b | |
| Semi-dried yellow croaker | 3 | Ningde | 3.94 ± 0.15 c | ND | ND | 1.82 ± 0.06 c | 4.17 ± 0.11 b | ND | 9.93 ± 0.32 b | 3.2 ± 0.1 c |
| 4 | Zhoushan | 2.23 ± 0.14 d | 1.67 ± 0.08 b | 0.91 ± 0.05 | 1.68 ± 0.07 c | ND | 0.66 ± 0.02 c | 7.15 ± 0.36 c | 3.6 ± 0.1 d | |
| Samples from Different Regions | Sample No. | Region | Number | OTUs | Shannon | Chao | Ace | Simpson | Shannoneven | Coverage |
|---|---|---|---|---|---|---|---|---|---|---|
| Fermented mandarin fish | 1 | Shanghai | 111,634 a | 304 a | 2.43 a | 313.63 a | 317.93 a | 0.26 a | 0.42 a | 0.9998 a |
| 2 | Huangshan | 87,888 b | 432 ac | 2.51 a | 440.25 ac | 455.11 ac | 0.19 a | 0.41 a | 0.9995 a | |
| Semi-dried yellow croaker | 3 | Ningde | 201,288 c | 581 bc | 3.53 a | 587.12 bc | 596.30 bc | 0.08 b | 0.55 a | 0.9998 a |
| 4 | Zhoushan | 44,199 d | 542 bc | 3.52 a | 560.33 bc | 575.38 bc | 0.07 b | 0.56 a | 0.9985 a |
| Fish Product | Sample No. | Region | Isolates No. (n = 33) | Species | Identity (%) |
|---|---|---|---|---|---|
| Fermented mandarin fish | 1 | Shanghai | S1, S2 | Lactobacillus sakei | 99.76 |
| S3, S4 | Bacillus cereus | 98.85 | |||
| S5 | Hafnia alvei | 100.00 | |||
| S6 | Psychrobacter faecalis | 97.89 | |||
| S7 | Vagococcus fluvialis | 99.90 | |||
| 2 | Huangshan | H1, H2 | Lactobacillus sakei | 99.97 | |
| H3, H4 | Hafnia alvei | 100.00 | |||
| H5 | Bacillus cereus | 99.65 | |||
| H6 | Psychrobacter faecalis | 99.51 | |||
| H7 | Aeromonas veronii | 99.97 | |||
| H8 | Staphylococcus caprae | 99.73 | |||
| H9 | Lactococcus garvieae | 98.98 | |||
| Semi-dried yellow croaker | 3 | Ningde | N1, N2, N3 | Shewanella baltica | 98.66 |
| N4 | Ralstonia pickettii | 100.00 | |||
| N5 | Aeromonas veronii | 99.68 | |||
| N6 | Acinetobacter johnsonii | 99.55 | |||
| N7 | Psychrobacter faecalis | 100.00 | |||
| 4 | Zhoushan | Z1, Z2, Z3 | Shewanella baltica | 99.97 | |
| Z4, Z5 | Aeromonas veronii | 99.98 | |||
| Z6 | Streptococcus parauberis | 97.16 | |||
| Z7 | Vibrio aphrogenes | 98.94 | |||
| Z8 | Photobacterium phosphoreum | 98.97 | |||
| Z9 | Prevotella 9 sp. | 99.69 | |||
| Z10 | Aliivibrio salmonicida | 99.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Chi, H.; Peng, D.; Jiang, M.; Wang, C.; Zhang, H.; Kang, W.; Li, L. Integrated Metagenomic and LC–MS/MS Analysis Reveals the Biogenic Amine-Producing Strains of Two Typical Chinese Traditional Fish Products: Fermented Mandarin Fish (Siniperca chuatsi) and Semi-Dried Yellow Croaker (Larimichthys crocea). Foods 2025, 14, 1016. https://doi.org/10.3390/foods14061016
Zhang X, Chi H, Peng D, Jiang M, Wang C, Zhang H, Kang W, Li L. Integrated Metagenomic and LC–MS/MS Analysis Reveals the Biogenic Amine-Producing Strains of Two Typical Chinese Traditional Fish Products: Fermented Mandarin Fish (Siniperca chuatsi) and Semi-Dried Yellow Croaker (Larimichthys crocea). Foods. 2025; 14(6):1016. https://doi.org/10.3390/foods14061016
Chicago/Turabian StyleZhang, Xuan, Hai Chi, Di Peng, Mei Jiang, Cuihua Wang, Haiyan Zhang, Wei Kang, and Lei Li. 2025. "Integrated Metagenomic and LC–MS/MS Analysis Reveals the Biogenic Amine-Producing Strains of Two Typical Chinese Traditional Fish Products: Fermented Mandarin Fish (Siniperca chuatsi) and Semi-Dried Yellow Croaker (Larimichthys crocea)" Foods 14, no. 6: 1016. https://doi.org/10.3390/foods14061016
APA StyleZhang, X., Chi, H., Peng, D., Jiang, M., Wang, C., Zhang, H., Kang, W., & Li, L. (2025). Integrated Metagenomic and LC–MS/MS Analysis Reveals the Biogenic Amine-Producing Strains of Two Typical Chinese Traditional Fish Products: Fermented Mandarin Fish (Siniperca chuatsi) and Semi-Dried Yellow Croaker (Larimichthys crocea). Foods, 14(6), 1016. https://doi.org/10.3390/foods14061016






