Effect of Non-Meat Protein Addition on the 3D Printing Performance of Chicken Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rheology Measurement
2.3. Printing Process
2.4. Texture Profile Analysis (TPA)
2.5. Cooking Loss
2.6. Low-Field Nuclear Magnetic Resonance (LF-NMR)
2.7. Scanning Electron Microscopy (SEM)
2.8. Statistical Analysis
3. Results
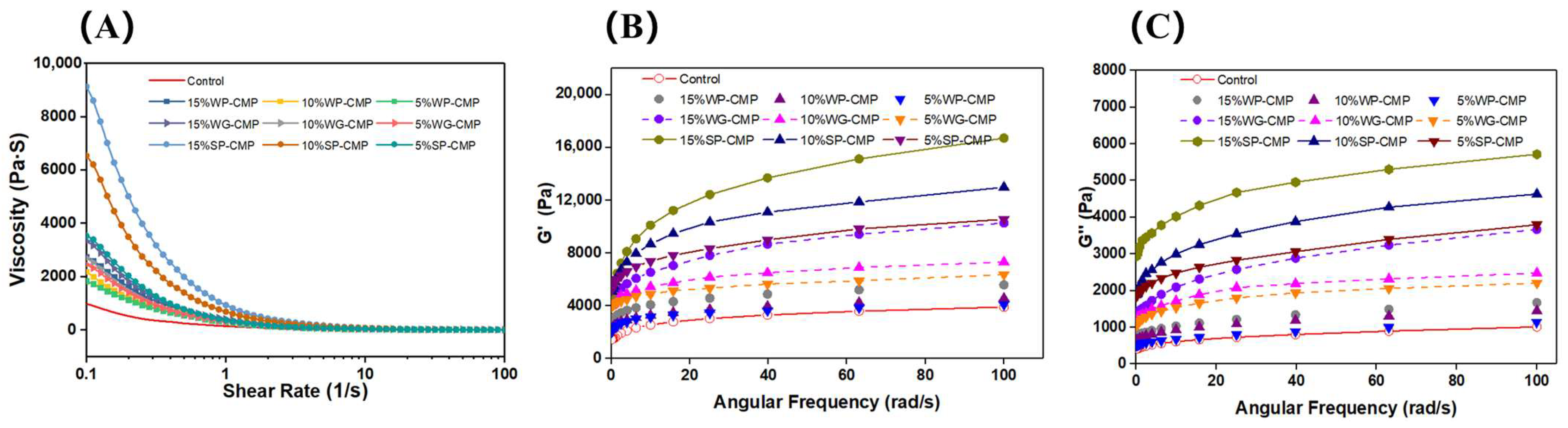
3.1. Rheological Properties
3.2. Texture Profile Analysis
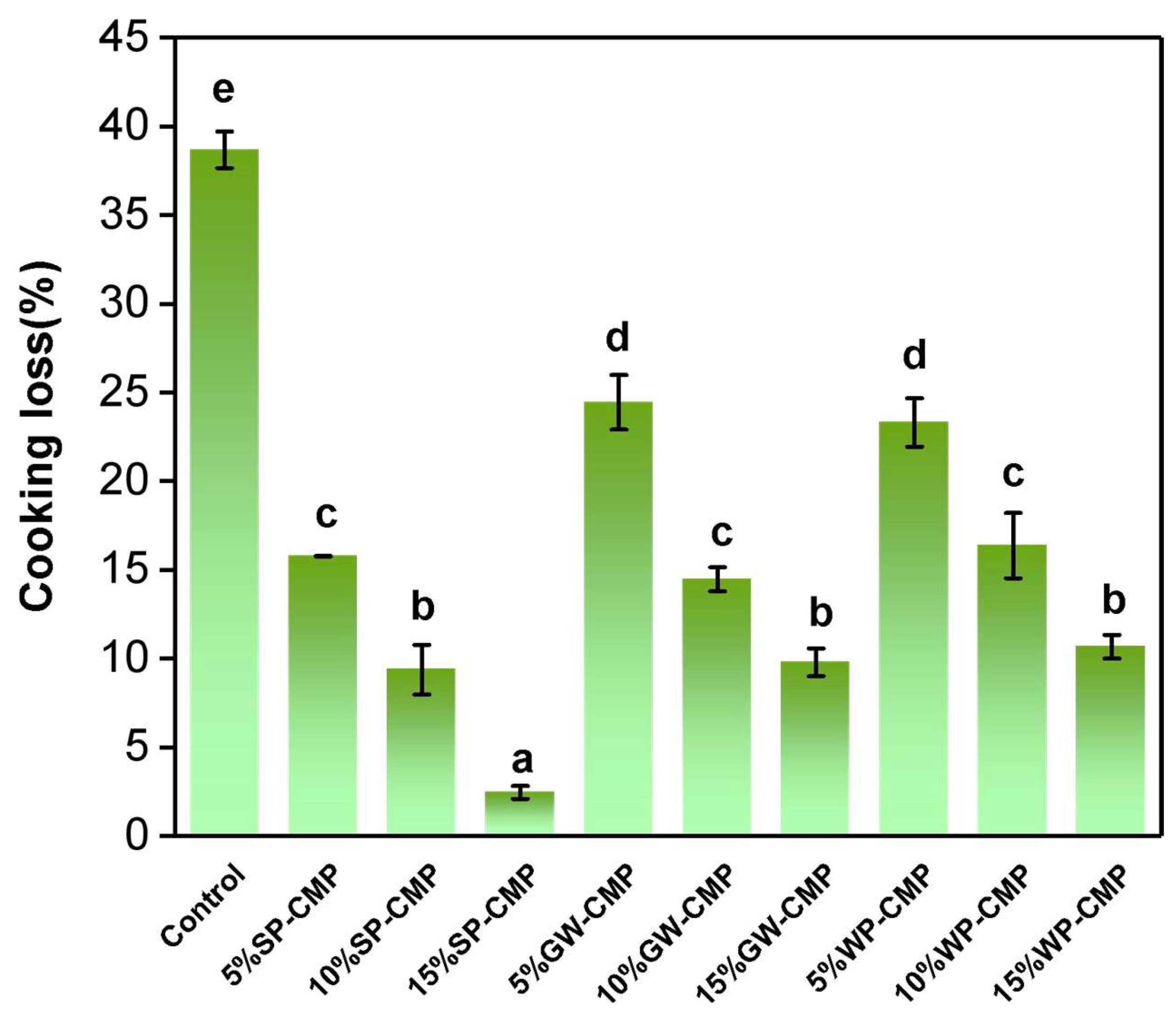
3.3. Cooking Loss of Gels
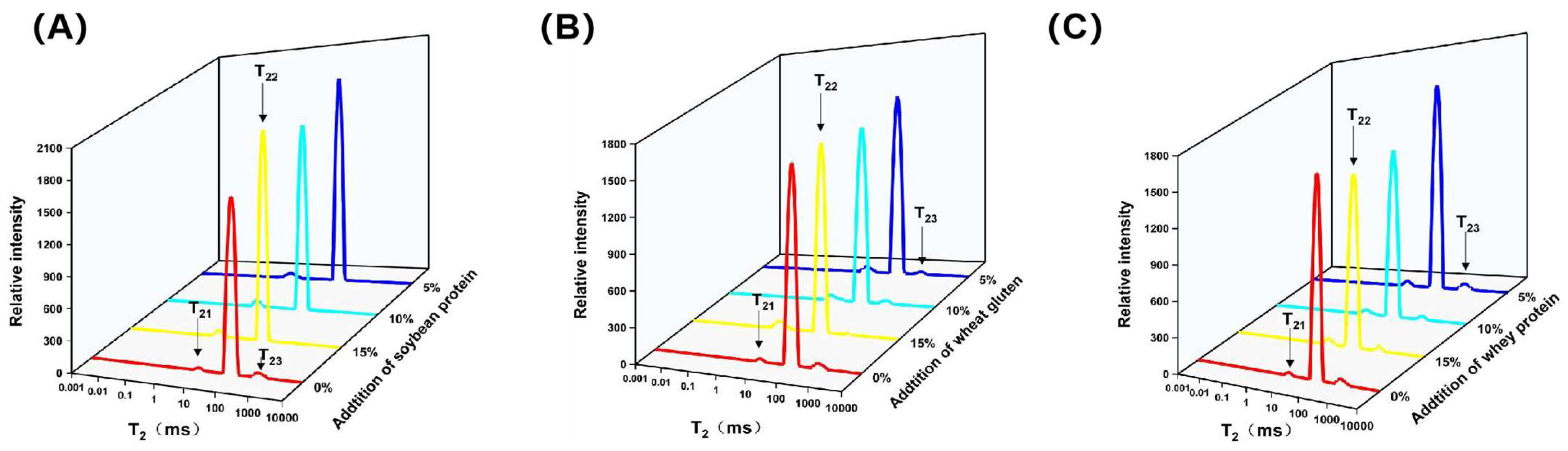
3.4. LF-NMR Analysis
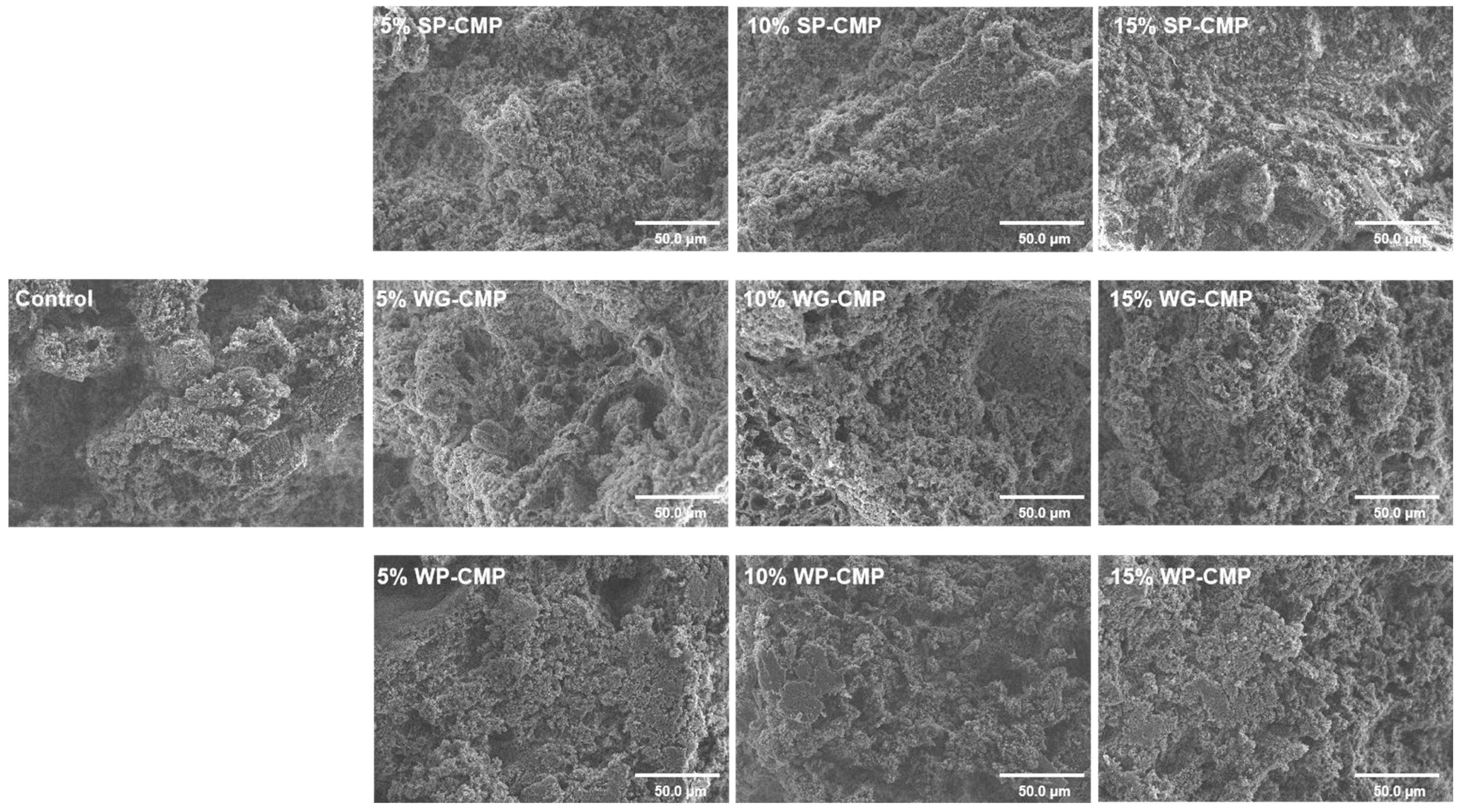
3.5. Microstructure of Gels
3.6. Three-Dimensional Printing Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nawa, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W.; Zhao, Y.; Nawab, Y.; Li, K.; Xiao, M.; et al. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139. [Google Scholar] [CrossRef]
- Belova, A.V.; Smutka, L.; Rosochatecká, E. World chicken meat market–its development and current status. Acta Univ. Agric. Silvic. Mendel. Brun. 2012, 60, 15–30. [Google Scholar] [CrossRef]
- Cui, B.; Wang, L.D.L.; Chen, X.; Xu, M.Y.; Ke, J.; Tian, Y. Chicken meat taste preferences, perceived risk of human infection with avian influenza virus, and self-reported chicken meat consumption in China. Prev. Vet. Med. 2022, 203, 105658. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S. Digital phenoty: A game changer for the broiler industry. Animals 2023, 13, 2585. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, W.; Yan, L.; Huang, D.; Lin, L. Extrusion-based food printing for digitalized food design and nutrition control. J. Food Eng. 2018, 220, 1–11. [Google Scholar] [CrossRef]
- Ramachandraiah, K. Potential development of sustainable 3D-printed meat analogues: A review. Sustainability 2021, 13, 938. [Google Scholar] [CrossRef]
- Nachal, N.; Moses, J.A.; Karthik, P.; Anandharamakrishnan, C. Applications of 3D printing in food processing. Food Eng. Rev. 2019, 11, 123–141. [Google Scholar] [CrossRef]
- Lipton, J.; Arnold, D.; Nigl, F.; Lopez, N.; Cohen, D.; Norén, N.; Lipson, H. Multi-material food printing with complex internal structure suitable for conventional post-processing. In Proceedings of the 2010 International Solid Freeform Fabrication Symposium, Austin, TX, USA, 23 September 2010. [Google Scholar] [CrossRef]
- Dick, A.; Bhandari, B.; Prakash, S. Effect of reheating method on the post-processing characterisation of 3D printed meat products for dysphagia patients. LWT 2021, 150, 111915. [Google Scholar] [CrossRef]
- Dong, X.; Pan, Y.; Zhao, W.; Huang, Y.; Qu, W.; Pan, J.; Qi, H.; Prakash, S. Impact of microbial transglutaminase on 3D printing quality of Scomberomorus niphonius surimi. LWT 2020, 124, 109123. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Wei, S.; Xia, Q.; Pan, Y.; Ji, H.; Deng, C.; Hao, J.; Liu, S. Insight into the correlations among rheological behaviour, protein molecular structure and 3D printability during the processing of surimi from golden pompano (Trachinotus ovatus). Food Chem. 2022, 371, 131046. [Google Scholar] [CrossRef]
- Dong, H.; Wang, P.; Yang, Z.; Xu, X. 3D printing based on meat materials: Challenges and opportunities. Curr. Res. Food Sci. 2023, 6, 100423. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.; Bhandari, B.; Prakash, S. Printability and textural assessment of modified-texture cooked beef pastes for dysphagia patients. Future Foods 2021, 3, 100006. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Bhandari, B.; Yang, C. Investigation on fish surimi gel as promising food material for 3D printing. J. Food Eng. 2018, 220, 101–108. [Google Scholar] [CrossRef]
- Dong, X.; Huang, Y.; Pan, Y.; Wang, K.; Prakash, S.; Zhu, B. Investigation of sweet potato starch as a structural enhancer for three-dimensional printing of Scomberomorus niphonius surimi. J. Texture Stud. 2019, 50, 316–324. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Z.; Pan, Y.; Jiang, P.; Pan, J.; Yu, C.; Dong, X. Effect of κ-carrageenan on quality improvement of 3D printed Hypophthalmichthys molitrix-sea cucumber compound surimi product. LWT 2022, 154, 112279. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Q.; Yan, B.; Gao, W.; Jiao, X.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. Synergistic effect of microwave 3D print and transglutaminase on the self-gelation of surimi during printing. Innov. Food Sci. Emerg. Technol. 2021, 67, 102546. [Google Scholar] [CrossRef]
- Bulut, E.G.; Candoğan, K. Development and characterization of a 3D printed functional chicken meat based snack: Optimization of process parameters and gelatin level. LWT 2022, 154, 112768. [Google Scholar] [CrossRef]
- D’Almeida, A.P.; de Albuquerque, T.L. Is It Possible to Produce Meat Without Animals? The Potential of Microorganisms as Protein Sources. Fermentation 2025, 11, 24. [Google Scholar] [CrossRef]
- Muniz, E.N.; Montenegro, R.T.Q.; da Silva, D.N.; D’Almeida, A.P.; Gonçalves, L.R.B.; de Albuquerque, T.L. Advances in Biotechnological Strategies for Sustainable Production of Non-Animal Proteins: Challenges, Innovations, and Applications. Fermentation 2024, 10, 638. [Google Scholar] [CrossRef]
- Bohrer, B.M. Nutrient density and nutritional value of meat products and non-meat foods high in protein. Trends Food Sci. Technol. 2017, 65, 103–112. [Google Scholar] [CrossRef]
- Liu, L.; Meng, Y.; Dai, X.; Chen, K.; Zhu, Y. 3D printing complex egg white protein objects: Properties and optimization. Food Bioprocess Technol. 2019, 12, 267–279. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Wei, G.; Ma, Y.; Bhandari, B.; Zhou, P. 3D printed milk protein food simulant: Improving the printing performance of milk protein concentration by incorporating whey protein isolate. Innov. Food Sci. Emerg. Technol. 2018, 49, 116–126. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Bhandari, B. 3D printing of steak-like foods based on textured soybean protein. Foods 2011, 10, 2011. [Google Scholar] [CrossRef]
- Herrero, A.M.; Carmona, P.; Cofrades, S.; Jiménez-Colmenero, F. Raman spectroscopic determination of structural changes in meat batters upon soy protein addition and heat treatment. Food Res. Int. 2008, 41, 765–772. [Google Scholar] [CrossRef]
- Li, Y.; Sukmanov, V.; Kang, Z.L.; Ma, H. Effect of soy protein isolate on the techno—Functional properties and protein conformation of low-sodium pork meat batters treated by high pressure. J. Food Process Eng. 2020, 43, e13343. [Google Scholar] [CrossRef]
- Gao, X.; Xiong, G.; Fu, L.; Liu, S. Water distribution of raw and heat-induced gelation of minced pork paste prepared by soy protein isolates and carrageenan: Ingredients modify the gelation of minced pork. J. Food Process. Preserv. 2019, 43, e14221. [Google Scholar] [CrossRef]
- Chen, J.; Mu, T.; Goffin, D.; Blecker, C.; Richard, G.; Richel, A.; Haubruge, E. Application of soy protein isolate and hydrocolloids based mixtures as promising food material in 3D food printing. J. Food Eng. 2019, 261, 76–86. [Google Scholar] [CrossRef]
- Qiu, Y.; McClements, D.J.; Chen, J.; Li, C.; Liu, C.; Dai, T. Construction of 3D printed meat analogs from plant-based proteins: Improving the printing performance of soy protein-and gluten-based pastes facilitated by rice protein. Food Res. Int. 2023, 167, 112635. [Google Scholar] [CrossRef]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat analogues: Health promising sustainable meat substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef]
- Jiang, Q.; Wei, X.; Liu, Q.; Zhang, T.; Chen, Q.; Yu, X.; Jiang, H. Rheo-fermentation properties of bread dough with different gluten contents processed by 3D printing. Food Chem. 2024, 433, 137318. [Google Scholar] [CrossRef]
- Cheng, Z.; Qiu, Y.; Bian, M.; He, Y.; Xu, S.; Li, Y.; Ahmad, I.; Ding, Y.; Lyu, F. Effect of insoluble dietary fiber on printing properties and molecular interactions of 3D-printed soy protein isolate-wheat gluten plant-based meats. Int. J. Biol. Macromol. 2024, 258, 128803. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimons, S.M.; Mulvihill, D.M.; Morris, E.R. Co-gels of whey protein isolate with crosslinked waxy maize starch: Analysis of solvent partition and phase structure by polymer blending laws. Food Hydrocoll. 2008, 22, 468–484. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, M.; Chen, H. Effect of whey protein on the 3D printing performance of konjac hybrid gel. LWT 2021, 140, 110716. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Reetu; Punia, S.; Dhakane-Lad, J.; Singh, S.; Dhumal, S.; Pradhan, C.P.; Bhushan, B.; et al. Functional characterization of plant-based protein to determine its quality for food applications. Food Hydrocoll. 2022, 123, 106986. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, B.; Fu, H.; Li, J.; Ji, L.; Gong, H.; Zhang, R.; Liu, J.; Yu, H. The development process of plant-based meat alternatives: Raw material formulations and processing strategies. Food Res. Int. 2023, 167, 112689. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.; Bhandari, B.; Prakash, S. 3D printing of meat. Meat Sci. 2019, 153, 35–44. [Google Scholar] [CrossRef]
- Huang, M.; Wang, H.; Xu, X.; Lu, X.; Song, X.; Zhou, G. Effects of nanoemulsion-based edible coatings with composite mixture of rosemary extract and ε-poly-L-lysine on the shelf life of ready-to-eat carbonado chicken. Food Hydrocoll. 2020, 102, 105576. [Google Scholar] [CrossRef]
- Yang, G.; Tao, Y.; Wang, P.; Xu, X.; Zhu, X. Optimizing 3D printing of chicken meat by response surface methodology and genetic algorithm: Feasibility study of 3D printed chicken product. LWT 2022, 154, 112693. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, L.; Zou, Y.; Tong, Z.; Han, S.; Wang, S. 3D food printing: Main components selection by considering rheological properties. Crit. Rev. Food Sci. Nutr. 2019, 59, 2335–2347. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, M.; Bhandari, B.; Liu, Y. Investigation on lemon juice gel as food material for 3D printing and optimization of printing parameters. LWT 2018, 87, 67–76. [Google Scholar] [CrossRef]
- Binsi, P.K.; Shamasundar, B.A.; Dileep, A.O.; Badii, F.; Howell, N.K. Rheological and functional properties of gelatin from the skin of Bigeye snapper (Priacanthus hamrur) fish: Influence of gelatin on the gel-forming ability of fish mince. Food Hydrocoll. 2009, 23, 132–145. [Google Scholar] [CrossRef]
- Wagner, J.; Biliaderis, C.G.; Moschakis, T. Whey proteins: Musings on denaturation, aggregate formation and gelation. Crit. Rev. Food Sci. Nutr. 2020, 60, 3793–3806. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Chen, C.; Zheng, L.; Zhou, C.; Cai, K. Effect of high pressure processing on the gel properties of salt-soluble meat protein containing CaCl2 and κ-carrageenan. Meat Sci. 2013, 95, 22–26. [Google Scholar] [CrossRef]
- Cheng, Q.; Sun, D.W. Factors affecting the water holding capacity of red meat products: A review of recent research advances. Crit. Rev. Food Sci. Nutr. 2008, 48, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Z.; Wang, Y.; Xue, Y.; Xue, C. Effects of konjac glucomannan on heat-induced changes of physicochemical and structural properties of surimi gels. Food Res. Int. 2016, 83, 152–161. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Benjakul, S.; Visessanguan, W.; Lanier, T.C. Chicken plasma protein affects gelation of surimi from bigeye snapper (Priacanthus tayenus). Food Hydrocoll. 2004, 18, 259–270. [Google Scholar] [CrossRef]

| Ingredients (g) | Control | 5%SP-CMP | 10%SP-CMP | 15%SP-CMP | 5%WG-CMP | 10%WG-CMP | 15%WG-CMP | 5%WP-CMP | 10%WP-CMP | 15%WP-CMP |
|---|---|---|---|---|---|---|---|---|---|---|
| CMP | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| SP | 0 | 5 | 10 | 15 | 0 | 0 | 0 | 0 | 0 | 0 |
| WG | 0 | 0 | 0 | 0 | 5 | 10 | 15 | 0 | 0 | 0 |
| WP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 10 | 15 |
| Hardness (g) | Springiness | Cohesiveness | Gumminess | Resilience | |
|---|---|---|---|---|---|
| control | 826.02 ± 61.91 a | 0.811 ± 0.002 a | 0.69 ± 0.01 b | 568.95 ± 43.85 a | 0.27 ± 0.01 a |
| 5% SP-CMP | 1097.69 ± 62.58 c | 0.87 ± 0.01 d | 0.72 ± 0.01 d | 715.46 ± 64.97 d | 0.30 ± 0.01 cd |
| 10% SP-CMP | 1238.22 ± 94.22 de | 0.90 ± 0.01 e | 0.71 ± 0.02 cd | 826.80 ± 35.47 e | 0.342 ± 0.001 e |
| 15% SP-CMP | 1991.40 ± 88.22 g | 0.922 ± 0.002 f | 0.72 ± 0.01 d | 1299.14 ± 21.21 g | 0.34 ± 0.01 e |
| 5% WG-CMP | 1050.68 ± 58.11 c | 0.84 ± 0.01 c | 0.692 ± 0.004 bc | 672.06 ± 60.00 cd | 0.28 ± 0.01 ab |
| 10% WG-CMP | 1177.88 ± 72.30 cd | 0.87 ± 0.01 d | 0.70 ± 0.01 bcd | 836.08 ± 56.59 e | 0.29 ± 0.01 bc |
| 15% WG-CMP | 1418.08 ± 55.19 f | 0.892 ± 0.001 e | 0.72 ± 0.01 d | 1019.80 ± 48.84 f | 0.34 ± 0.01 e |
| 5% WP-CMP | 837.78 ± 13.58 a | 0.82 ± 0.01 ab | 0.62 ± 0.01 a | 523.59 ± 10.34 a | 0.272 ± 0.001 a |
| 10% WP-CMP | 944.10 ± 32.98 b | 0.83 ± 0.01 bc | 0.63 ± 0.01 a | 610.12 ± 57.85 bc | 0.27 ± 0.01 a |
| 15% WP-CMP | 1062.48 ± 38.35 c | 0.84 ± 0.01 c | 0.63 ± 0.01 a | 738.11 ± 62.40 d | 0.28 ± 0.01 ab |
| T21 (ms) | T22 (ms) | T23 (ms) | P21 (%) | P22 (%) | P23 (%) | |
|---|---|---|---|---|---|---|
| Control | 7.75 ± 0.32 f | 71.49 ± 0.00 g | 489.49 ± 17.24 f | 1.09 ± 0.06 a | 96.23 ± 0.22 de | 2.68 ± 0.24 g |
| 5%SP-CMP | 1.82 ± 0.08 a | 56.07 ± 0.00 e | 460.59 ± 0.00 e | 3.69 ± 0.06 f | 95.86 ± 0.01 cd | 0.45 ± 0.05 b |
| 10%SP-CMP | 1.63 ± 0.08 a | 45.21 ± 2.14 c | 381.27 ± 18.06 c | 3.30 ± 0.04 de | 96.53 ± 0.97 e | 0.12 ± 0.02 a |
| 15%SP-CMP | 1.59 ± 0.00 a | 37.40 ± 0.00 a | 329.27 ± 7.00 b | 3.33 ± 0.05 de | 96.35 ± 0.43 e | 0.051 ± 0.003 a |
| 5%WG-CMP | 5.81 ± 0.12 e | 62.51 ± 2.96 f | 364.95 ± 6.44 c | 3.08 ± 0.11 bc | 95.47 ± 0.33 c | 1.26 ± 0.06 c |
| 10%WG-CMP | 4.95 ± 0.40 bc | 53.16 ± 2.52 d | 335.75 ± 4.53 b | 3.24 ± 0.05 cd | 94.78 ± 0.08 b | 1.98 ± 0.10 e |
| 15%WG-CMP | 4.68 ± 0.22 bc | 40.56 ± 0.00 b | 282.64 ± 1.15 a | 5.33 ± 0.08 g | 93.81 ± 0.45 a | 1.06 ± 0.24 c |
| 5%WP-CMP | 5.82 ± 0.47 e | 60.80 ± 0.00 f | 521.59 ± 20.52 g | 2.95 ± 0.07 b | 94.79 ± 0.06 b | 2.25 ± 0.13 f |
| 10%WP-CMP | 5.22 ± 0.24 cd | 51.71 ± 0.00 d | 405.04 ± 11.55 d | 4.20 ± 0.24 h | 93.92 ± 0.25 a | 1.88 ± 0.07 de |
| 15%WP-CMP | 4.32 ± 0.20 b | 43.98 ± 0.00 c | 361.23 ± 0.00 c | 3.46 ± 0.19 e | 94.86 ± 0.17 b | 1.68 ± 0.09 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Huang, M.; Chen, D.; Xiao, E.; Li, Y. Effect of Non-Meat Protein Addition on the 3D Printing Performance of Chicken Meat. Foods 2025, 14, 1015. https://doi.org/10.3390/foods14061015
Li X, Huang M, Chen D, Xiao E, Li Y. Effect of Non-Meat Protein Addition on the 3D Printing Performance of Chicken Meat. Foods. 2025; 14(6):1015. https://doi.org/10.3390/foods14061015
Chicago/Turabian StyleLi, Xin, Mingyuan Huang, Dan Chen, Enquan Xiao, and Yuqing Li. 2025. "Effect of Non-Meat Protein Addition on the 3D Printing Performance of Chicken Meat" Foods 14, no. 6: 1015. https://doi.org/10.3390/foods14061015
APA StyleLi, X., Huang, M., Chen, D., Xiao, E., & Li, Y. (2025). Effect of Non-Meat Protein Addition on the 3D Printing Performance of Chicken Meat. Foods, 14(6), 1015. https://doi.org/10.3390/foods14061015







