Influence of Maternal Diet and Lactation Time on the Exosomal miRNA Cargo in Breast Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Breast Milk Samples
2.2. Exosome and miRNA Isolation
2.3. Reverse Transcription and qPCR
2.4. Mineral Determination by ICP-MS
2.5. Breast Milk Fatty Acids Analysis by GC-FID
2.6. Data Analysis and Statistics
3. Results
3.1. MiRNA Evolution with Lactation Time
3.2. Influence of Maternal Dietary Factors, Fatty Acids and Minerals in miRNA Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization; UNICEF. Global Strategy for Infant and Young Child Feeding; World Health Organization: Geneva, Switzerland, 2003; ISBN 9241562218. [Google Scholar]
- Hennet, T.; Borsig, L. Breastfed at Tiffany’s. Trends Biochem. Sci. 2016, 41, 508–518. [Google Scholar] [CrossRef]
- Sánchez, C.; Franco, L.; Regal, P.; Lamas, A.; Cepeda, A.; Fente, C. Breast Milk: A Source of Functional Compounds with Potential Application in Nutrition and Therapy. Nutrients 2021, 13, 1026. [Google Scholar] [CrossRef]
- Davis, M.K. Breastfeeding and Chronic Disease in Childhood and Adolescence. Pediatr. Clin. N. Am. 2001, 48, 125–141. [Google Scholar] [CrossRef]
- Kosaka, N.; Izumi, H.; Sekine, K.; Ochiya, T. MicroRNA as a New Immune-Regulatory Agent in Breast Milk. Silence 2010, 1, 7. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- van Herwijnen, M.J.C.; Driedonks, T.A.P.; Snoek, B.L.; Kroon, A.M.T.; Kleinjan, M.; Jorritsma, R.; Pieterse, C.M.J.; Hoen, E.N.M.N.; Wauben, M.H.M. Abundantly Present MiRNAs in Milk-Derived Extracellular Vesicles Are Conserved Between Mammals. Front. Nutr. 2018, 5, 81. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhu, Q.; Zhou, X.; Wang, X.; Gao, X.; Li, X. Immune-Related MicroRNAs Are Abundant in Breast Milk Exosomes. Int. J. Biol. Sci. 2011, 8, 118–123. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human Milk MiRNAs Primarily Originate from the Mammary Gland Resulting in Unique MiRNA Profiles of Fractionated Milk. Sci. Rep. 2016, 6, 20680. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Wang, X.; Zhang, H.; Wang, L.; Gao, T. Comparison of MiRNA Profiles in Milk-Derived Extracellular Vesicles and Bovine Mammary Glands. Int. Dairy J. 2022, 134, 105444. [Google Scholar] [CrossRef]
- Xu, Y.R.; Zhao, J.; Huang, H.Y.; Lin, Y.C.D.; Lee, T.Y.; Huang, H.D.; Yang, Y.; Wang, Y.F. Recent Insights into Breast Milk MicroRNA: Their Role as Functional Regulators. Front. Nutr. 2024, 11, 1366435. [Google Scholar] [CrossRef]
- Ahlberg, E.; Al-Kaabawi, A.; Thune, R.; Simpson, M.R.; Pedersen, S.A.; Cione, E.; Jenmalm, M.C.; Tingö, L. Breast Milk MicroRNAs: Potential Players in Oral Tolerance Development. Front. Immunol. 2023, 14, 1154211. [Google Scholar] [CrossRef]
- Galley, J.D.; Besner, G.E. The Therapeutic Potential of Breast Milk-Derived Extracellular Vesicles. Nutrients 2020, 12, 745. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- López de Las Hazas, M.C.; Del Pozo-Acebo, L.; Hansen, M.S.; Gil-Zamorano, J.; Mantilla-Escalante, D.C.; Gómez-Coronado, D.; Marín, F.; Garcia-Ruiz, A.; Rasmussen, J.T.; Dávalos, A. Dietary Bovine Milk MiRNAs Transported in Extracellular Vesicles Are Partially Stable during GI Digestion, Are Bioavailable and Reach Target Tissues but Need a Minimum Dose to Impact on Gene Expression. Eur. J. Nutr. 2022, 61, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Manca, S.; Upadhyaya, B.; Mutai, E.; Desaulniers, A.T.; Cederberg, R.A.; White, B.R.; Zempleni, J. Milk Exosomes Are Bioavailable and Distinct MicroRNA Cargos Have Unique Tissue Distribution Patterns. Sci. Rep. 2018, 8, 11321. [Google Scholar] [CrossRef]
- Frazer, L.C.; Good, M. Intestinal Epithelium in Early Life. Mucosal Immunol. 2022, 15, 1181–1187. [Google Scholar] [CrossRef]
- Torrez Lamberti, M.F.; Parker, L.A.; Gonzalez, C.F.; Lorca, G.L. Pasteurization of Human Milk Affects the MiRNA Cargo of EVs Decreasing Its Immunomodulatory Activity. Sci. Rep. 2023, 13, 10057. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Fernández-Alonso, N.; Tomás-Zapico, C.; Visioli, F.; Iglesias-Gutierrez, E.; Dávalos, A. Breast Milk MicroRNAs Harsh Journey towards Potential Effects in Infant Development and Maturation. Lipid Encapsulation Can Help. Pharmacol. Res. 2018, 132, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Słyk-Gulewska, P.; Kondracka, A.; Kwaśniewska, A. MicroRNA as a New Bioactive Component in Breast Milk. Noncoding RNA Res. 2023, 8, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Leroux, C.; Chervet, M.L.; German, J.B. Perspective: Milk MicroRNAs as Important Players in Infant Physiology and Development. Adv. Nutr. 2021, 12, 1625–1635. [Google Scholar] [CrossRef]
- Chen, W.; Wang, X.; Yan, X.; Yu, Z.; Zhang, J.; Han, S. The Emerging Role of Exosomes in the Pathogenesis, Prognosis and Treatment of Necrotizing Enterocolitis. Am. J. Transl. Res. 2020, 12, 7020. [Google Scholar] [PubMed]
- Guo, M.M.; Zhang, K.; Zhang, J. hui Human Breast Milk–Derived Exosomal MiR-148a-3p Protects Against Necrotizing Enterocolitis by Regulating P53 and Sirtuin 1. Inflammation 2022, 45, 1254–1268. [Google Scholar] [CrossRef] [PubMed]
- Meguid, N.A.; Hemimi, M.; Rashad, M.; Elsaeid, A.; Elpatrik, G.; Zeidan, H.M. Dysregulation of MiR-146a in Human Milk of Mothers Having Children with Autism. Int. J. Dev. Neurosci. 2024, 84, 558–566. [Google Scholar] [CrossRef]
- Van Syoc, E.; Stegman, M.; Sullivan, R.; Confair, A.; Warren, K.; Hicks, S.D. Associations of Maternal Breastmilk MicroRNAs and Infant Obesity Status at 1 Year. Genes 2024, 15, 813. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.D.; Confair, A.; Warren, K.; Chandran, D. Levels of Breast Milk MicroRNAs and Other Non-Coding RNAs Are Impacted by Milk Maturity and Maternal Diet. Front. Immunol. 2022, 12, 785217. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human Milk Cells and Lipids Conserve Numerous Known and Novel MiRNAs, Some of Which Are Differentially Expressed during Lactation. PLoS ONE 2016, 11, e0152610. [Google Scholar] [CrossRef]
- Freiría-Martínez, L.; Iglesias-Martínez-Almeida, M.; Rodríguez-Jamardo, C.; Rivera-Baltanás, T.; Comís-Tuche, M.; Rodrígues-Amorím, D.; Fernández-Palleiro, P.; Blanco-Formoso, M.; Diz-Chaves, Y.; González-Freiria, N.; et al. Human Breast Milk MicroRNAs, Potential Players in the Regulation of Nervous System. Nutrients 2023, 15, 3284. [Google Scholar] [CrossRef]
- Picáns-Leis, R.; Vázquez-Mosquera, M.E.; Pereira-Hernández, M.; Vizoso-Gonzalez, M.; López-Valverde, L.; Barbosa-Gouveia, S.; López-Suárez, O.; López-Sanguos, C.; Bravo, S.B.; Garcia-Gonzalez, M.A. Characterization of the Functional Component in Human Milk and Identification of the Molecular Mechanisms Undergoing Prematurity. Clin. Nutr. 2024, 44, 178–192. [Google Scholar] [CrossRef]
- Gutman-Ido, E.; Reif, S.; Musseri, M.; Schabes, T.; Golan-Gerstl, R. Oxytocin Regulates the Expression of Selected Colostrum-Derived MicroRNAs. J. Pediatr. Gastroenterol. Nutr. 2022, 74, E8–E15. [Google Scholar] [CrossRef]
- Bozack, A.K.; Colicino, E.; Rodosthenous, R.; Bloomquist, T.R.; Baccarelli, A.A.; Wright, R.O.; Wright, R.J.; Lee, A.G. Associations between Maternal Lifetime Stressors and Negative Events in Pregnancy and Breast Milk-Derived Extracellular Vesicle MicroRNAs in the Programming of Intergenerational Stress Mechanisms (PRISM) Pregnancy Cohort. Epigenetics 2021, 16, 389–404. [Google Scholar] [CrossRef]
- Zamanillo, R.; Sánchez, J.; Serra, F.; Palou, A. Breast Milk Supply of Microrna Associated with Leptin and Adiponectin Is Affected by Maternal Overweight/Obesity and Influences Infancy BMI. Nutrients 2019, 11, 2589. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, R.; Díaz-Bao, M.; Cepeda, A.; Regal, P.; Fente, C.A. Fatty Acid Composition of Breast Milk in Galicia (NW Spain): A Cross-Country Comparison. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Fente, C.; Barreiro, R.; López-Racamonde, O.; Cepeda, A.; Regal, P. Association between Breast Milk Mineral Content and Maternal Adherence to Healthy Dietary Patterns in Spain: A Transversal Study. Foods 2020, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, L.; Mulakala, B.K.; Rajasundaram, D.; Gonzalez, S.; Cabrera-Rubio, R.; Martínez-Costa, C.; Collado, M.C. Human Milk MiRNAs Associate to Maternal Dietary Nutrients, Milk Microbiota, Infant Gut Microbiota and Growth. Clin. Nutr. 2023, 42, 2528–2539. [Google Scholar] [CrossRef]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of Maternal Nutrition on Breast-Milk Composition: A Systematic Review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Oliveira, A.; Lopes, C.; Rodríguez-Artalejo, F. Adherence to the Southern European Atlantic Diet and Occurrence of Nonfatal Acute Myocardial Infarction. Am. J. Clin. Nutr. 2010, 92, 211–217. [Google Scholar] [CrossRef]
- Sanjulian, L.; Lamas, A.; Barreiro, R.; Martínez, I.; García-Alonso, L.; Cepeda, A.; Fente, C.; Regal, P. Investigating the Dietary Impact on Trans-Vaccenic Acid (Trans-C18:1 n-7) and Other Beneficial Fatty Acids in Breast Milk and Infant Formulas. Foods 2024, 13, 2164. [Google Scholar] [CrossRef]
- Barreiro, R.; Regal, P.; López-Racamonde, O.; Cepeda, A.; Fente, C. Evolution of Breast Milk Fatty Acids in Spanish Mothers after One Year of Uninterrupted Lactation. Prostaglandins Leukot. Essent. Fat. Acids 2020, 159, 102141. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. MicroRNAs: Milk’s Epigenetic Regulators. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 427–442. [Google Scholar] [CrossRef]
- Melnik, B.C.; Weiskirchen, R.; Schmitz, G. Milk Exosomal MicroRNAs: Friend or Foe?—A Narrative Review. ExRNA 2022, 4, 22. [Google Scholar] [CrossRef]
- Sanjulián, L.; Lamas, A.; Barreiro, R.; Cepeda, A.; Fente, C.A.; Regal, P. Bacterial Diversity of Breast Milk in Healthy Spanish Women: Evolution from Birth to Five Years Postpartum. Nutrients 2021, 13, 2414. [Google Scholar] [CrossRef]
- Tuomi, J.M.; Voorbraak, F.; Jones, D.L.; Ruijter, J.M. Bias in the Cq Value Observed with Hydrolysis Probe Based Quantitative PCR Can Be Corrected with the Estimated PCR Efficiency Value. Methods 2010, 50, 313–322. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; van den hoff, M.J.B.; Moorman, A.F.M. Amplification Efficiency: Linking Baseline and Bias in the Analysis of Quantitative PCR Data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Kooka, A.; Kowatari, K.; Yoshizawa, M.; Chiba, N.; Takaguri, A.; Fukushi, Y.; Hongo, F.; Sato, H.; Wada, S. Expression Profiles of Hsa-MiR-148a-3p and Hsa-MiR-125b-5p in Human Breast Milk and Infant Formulae. Int. Breastfeed. J. 2022, 17, 1. [Google Scholar] [CrossRef]
- Ahlberg, E.; Martí, M.; Govindaraj, D.; Severin, E.; Duchén, K.; Jenmalm, M.C.; Tingö, L. Immune-Related MicroRNAs in Breast Milk and Their Relation to Regulatory T Cells in Breastfed Children. Pediatr. Allergy Immunol. 2023, 34, e13952. [Google Scholar] [CrossRef]
- Melnik, B.C. Milk Disrupts P53 and DNMT1, the Guardians of the Genome: Implications for Acne Vulgaris and Prostate Cancer. Nutr. Metab. 2017, 14, 55. [Google Scholar] [CrossRef]
- Shah, K.B.; Fields, D.A.; Pezant, N.P.; Kharoud, H.K.; Gulati, S.; Jacobs, K.; Gale, C.A.; Kharbanda, E.O.; Nagel, E.M.; Demerath, E.W.; et al. Gestational Diabetes Mellitus Is Associated with Altered Abundance of Exosomal MicroRNAs in Human Milk. Clin. Ther. 2022, 44, 172–185.e1. [Google Scholar] [CrossRef] [PubMed]
- Braconi, C.; Huang, N.; Patel, T. Microrna-Dependent Regulation of DNA Methyltransferase-1 and Tumor Suppressor Gene Expression by Interleukin-6 in Human Malignant Cholangiocytes. Hepatology 2010, 51, 881–890. [Google Scholar] [CrossRef]
- Golan-Gerstl, R.; Elbaum Shiff, Y.; Moshayoff, V.; Schecter, D.; Leshkowitz, D.; Reif, S. Characterization and Biological Function of Milk-Derived MiRNAs. Mol. Nutr. Food Res. 2017, 61, 1700009. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, L.; Li, Y.; Lin, X.; Shen, H.; Cui, K.; Chen, L.; Zhou, F.; Zhao, Q.; Zhang, J.; et al. MIR-148a Inhibits Colitis and Colitis-Associated Tumorigenesis in Mice. Cell Death Differ. 2017, 24, 2199–2209. [Google Scholar] [CrossRef]
- Nair, S.; Guanzon, D.; Jayabalan, N.; Lai, A.; Scholz-Romero, K.; Kalita de Croft, P.; Ormazabal, V.; Palma, C.; Diaz, E.; McCarthy, E.A.; et al. Extracellular Vesicle-Associated MiRNAs Are an Adaptive Response to Gestational Diabetes Mellitus. J. Transl. Med. 2021, 19, 360. [Google Scholar] [CrossRef]
- Martínez, C.; Rodinõ-Janeiro, B.K.; Lobo, B.; Stanifer, M.L.; Klaus, B.; Granzow, M.; González-Castro, A.M.; Salvo-Romero, E.; Alonso-Cotoner, C.; Pigrau, M.; et al. MiR-16 and MiR-125b Are Involved in Barrier Function Dysregulation through the Modulation of Claudin-2 and Cingulin Expression in the Jejunum in IBS with Diarrhoea. Gut 2017, 66, 1537–1538. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.N.; Teh, C.; Shyh-Chang, N.; Xie, H.; Zhou, B.; Korzh, V.; Lodish, H.F.; Lim, B. MicroRNA-125b Is a Novel Negative Regulator of P53. Genes Dev. 2009, 23, 862–876. [Google Scholar] [CrossRef]
- Carrillo-Lozano, E.; Sebastián-Valles, F.; Knott-Torcal, C. Circulating Micrornas in Breast Milk and Their Potential Impact on the Infant. Nutrients 2020, 12, 3066. [Google Scholar] [CrossRef] [PubMed]
- Roshan, R.; Shridhar, S.; Sarangdhar, M.A.; Banik, A.; Chawla, M.; Garg, M.; Singh, V.P.; Pillai, B. Brain-Specific Knockdown of MiR-29 Results in Neuronal Cell Death and Ataxia in Mice. RNA 2014, 20, 1287–1297. [Google Scholar] [CrossRef]
- Durrani-Kolarik, S.; Pool, C.A.; Gray, A.; Heyob, K.M.; Cismowski, M.J.; Pryhuber, G.; Lee, L.J.; Yang, Z.; Tipple, T.E.; Rogers, L.K. MiR-29b Supplementation Decreases Expression of Matrix Proteins and Improves Alveolarization in Mice Exposed to Maternal Inflammation and Neonatal Hyperoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L339–L349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zheng, J.; Han, X.; Zhao, J.; Cheng, S.; Li, A. Bovine Colostrum MiR-30a-5p Targets the NF-ΚB Signaling Pathway to Alleviate Inflammation in Intestinal Epithelial Cells. J. Agric. Food Chem. 2023, 72, 9164–9178. [Google Scholar] [CrossRef]
- Gao, F.; Wu, S.; Zhang, K.; Xu, Z.; Quan, F. Goat Milk Exosomal MicroRNAs Alleviate LPS-Induced Intestinal Inflammation in Mice. Int. J. Biol. Macromol. 2024, 268, 131698. [Google Scholar] [CrossRef]
- Na, R.S.; E, G.X.; Sun, W.; Sun, X.W.; Qiu, X.Y.; Chen, L.P.; Huang, Y.F. Expressional Analysis of Immune-Related MiRNAs in Breast Milk. Genet. Mol. Res. 2015, 14, 11371–11376. [Google Scholar] [CrossRef]
- Schuster, S.; Hechler, C.; Gebauer, C.; Kiess, W.; Kratzsch, J. Leptin in Maternal Serum and Breast Milk: Association with Infants’ Body Weight Gain in a Longitudinal Study over 6 Months of Lactation. Pediatr. Res. 2011, 70, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Wang, J.; Luo, Y.; Hu, J.; Liu, X.; Li, S.; Li, M.; Shi, B.; Hu, L.; Liu, Y.; et al. MicroRNA-200c Affects Milk Fat Synthesis by Targeting PANK3 in Ovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 15601. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human Milk Cells Contain Numerous MiRNAs That May Change with Milk Removal and Regulate Multiple Physiological Processes. Int. J. Mol. Sci. 2016, 17, 956. [Google Scholar] [CrossRef]
- Yun, K.W.; Lee, J.Y.; Yun, S.W.; Lim, I.S.; Choi, E.S. Elevated Serum Level of MicroRNA (MiRNA)-200c and MiRNA-371-5p in Children with Kawasaki Disease. Pediatr. Cardiol. 2014, 35, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Baier, S.R.; Nguyen, C.; Xie, F.; Wood, J.R.; Zempleni, J. MicroRNAs Are Absorbed in Biologically Meaningful Amounts from Nutritionally Relevant Doses of Cow Milk and Affect Gene Expression in Peripheral Blood Mononuclear Cells, HEK-293 Kidney Cell Cultures, and Mouse Livers. J. Nutr. 2014, 144, 1495–1500. [Google Scholar] [CrossRef]
- Witwer, K.; Auerbach, A.; Vyas, G.; Li, A.; Halushka, M. Uptake of Dietary Milk MiRNAs by Adult Humans: A Validation Study. F1000Research 2016, 5, 721. [Google Scholar] [CrossRef]
- Weil, P.P.; Reincke, S.; Hirsch, C.A.; Giachero, F.; Aydin, M.; Scholz, J.; Jönsson, F.; Hagedorn, C.; Nguyen, D.N.; Thymann, T.; et al. Uncovering the Gastrointestinal Passage, Intestinal Epithelial Cellular Uptake, and AGO2 Loading of Milk MiRNAs in Neonates Using XenomiRs as Tracers. Am. J. Clin. Nutr. 2023, 117, 1195–1210. [Google Scholar] [CrossRef] [PubMed]
- Domenichiello, A.F.; Kitson, A.P.; Bazinet, R.P. Is Docosahexaenoic Acid Synthesis from α-Linolenic Acid Sufficient to Supply the Adult Brain? Prog. Lipid Res. 2015, 59, 54–66. [Google Scholar] [CrossRef]
- Petracci, I.; Fedeli, D.; Gabbianelli, R.; Bordoni, L. MiR-21, MiR-148, Fatty Acid Content, and Antioxidant Properties of Raw Cow’s Milk: A Pilot Study. ACS Food Sci. Technol. 2023, 3, 898–908. [Google Scholar] [CrossRef]
- Busch, S.; Auth, E.; Scholl, F.; Huenecke, S.; Koehl, U.; Suess, B.; Steinhilber, D. 5-Lipoxygenase Is a Direct Target of MiR-19a-3p and MiR-125b-5p. J. Immunol. 2015, 194, 1646–1653. [Google Scholar] [CrossRef]
- Hu, F.; Wang, M.; Xiao, T.; Yin, B.; He, L.; Meng, W.; Dong, M.; Liu, F. MiR-30 Promotes Thermogenesis and the Development of Beige Fat by Targeting RIP140. Diabetes 2015, 64, 2056–2068. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Okla, M.; Erickson, A.; Carr, T.; Natarajan, S.K.; Chung, S. Eicosapentaenoic Acid Potentiates Brown Thermogenesis through FFAR4-Dependent up-Regulation of MiR-30b and MiR-378. J. Biol. Chem. 2016, 291, 20551–20562. [Google Scholar] [CrossRef] [PubMed]
- Gharanei, S.; Shabir, K.; Brown, J.E.; Weickert, M.O.; Barber, T.M.; Kyrou, I.; Randeva, H.S. Regulatory MicroRNAs in Brown, Brite and White Adipose Tissue. Cells 2020, 9, 2489. [Google Scholar] [CrossRef]
- Casas-Agustench, P.; Fernandes, F.S.; Tavares Do Carmo, M.G.; Visioli, F.; Herrera, E.; Dávalos, A. Consumption of Distinct Dietary Lipids during Early Pregnancy Differentially Modulates the Expression of MicroRNAs in Mothers and Offspring. PLoS ONE 2015, 10, e0117858. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef]
- Feng, Y.; Xing, Y.; Liu, Z.; Yang, G.; Niu, X.; Gao, D. Integrated Analysis of MicroRNA and MRNA Expression Profiles in Rats with Selenium Deficiency and Identification of Associated MiRNA-MRNA Network. Sci. Rep. 2018, 8, 6601. [Google Scholar] [CrossRef]
- Cui, J.; Liu, H.; Xu, S. Selenium-Deficient Diet Induces Necroptosis in the Pig Brain by Activating TNFR1: Via Mir-29a-3p. Metallomics 2020, 12, 1290–1301. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, D.; Zhou, M.; Shi, H.; Yan, S.; Cai, Y. Regulatory Role of MiR-125a/b in the Suppression by Selenium of Cadmium-Induced Apoptosis via the Mitochondrial Pathway in LLC-PK1 Cells. Chem. Biol. Interact. 2016, 243, 35–44. [Google Scholar] [CrossRef]
- Cao, Y.Z.; Maddox, J.F.; Mastro, A.M.; Scholz, R.W.; Hildenbrandt, G.; Reddy, C.C. Selenium Deficiency Alters the Lipoxygenase Pathway and Mitogenic Response in Bovine Lymphocytes. J. Nutr. 1992, 122, 2121–2127. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
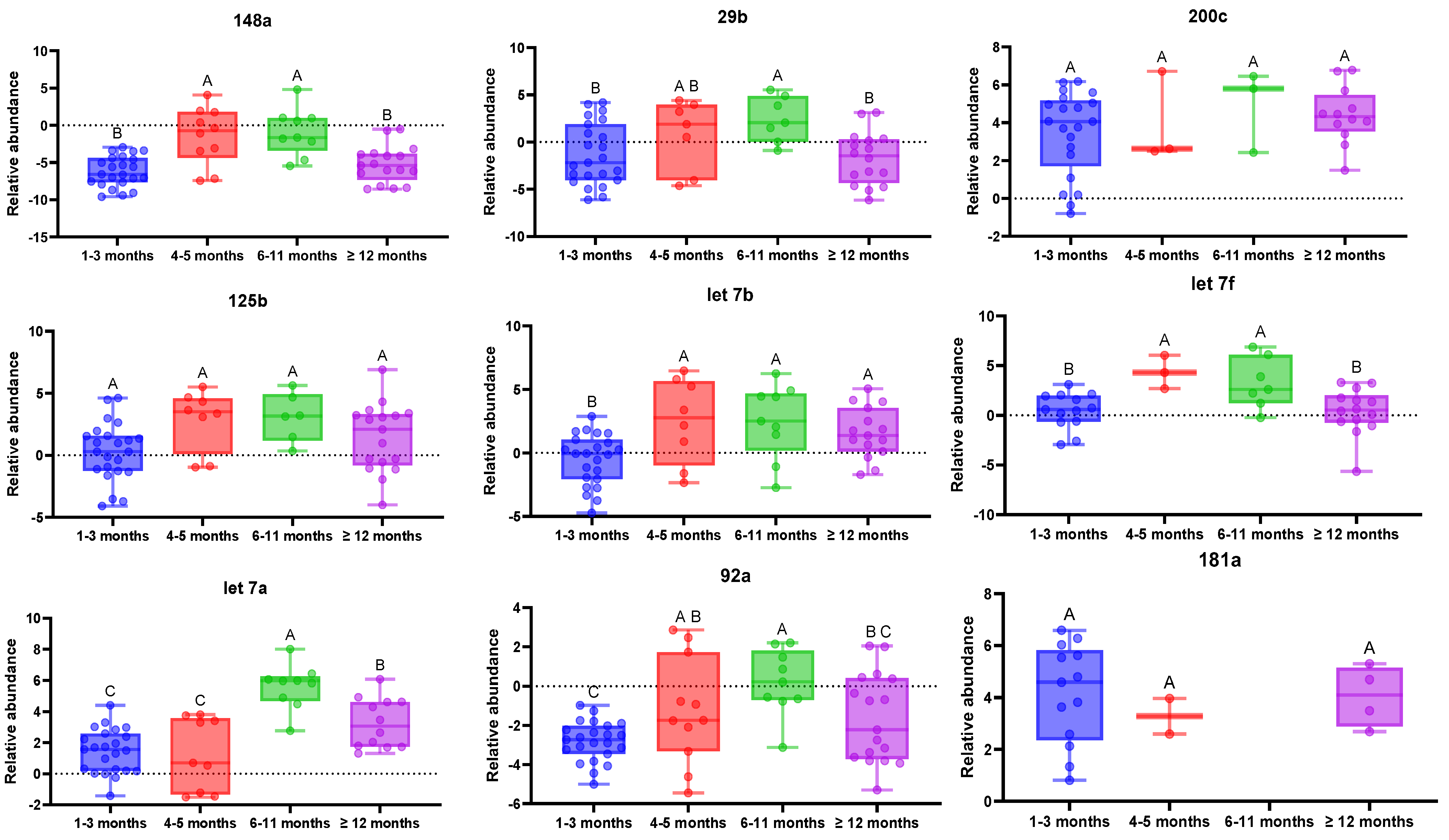
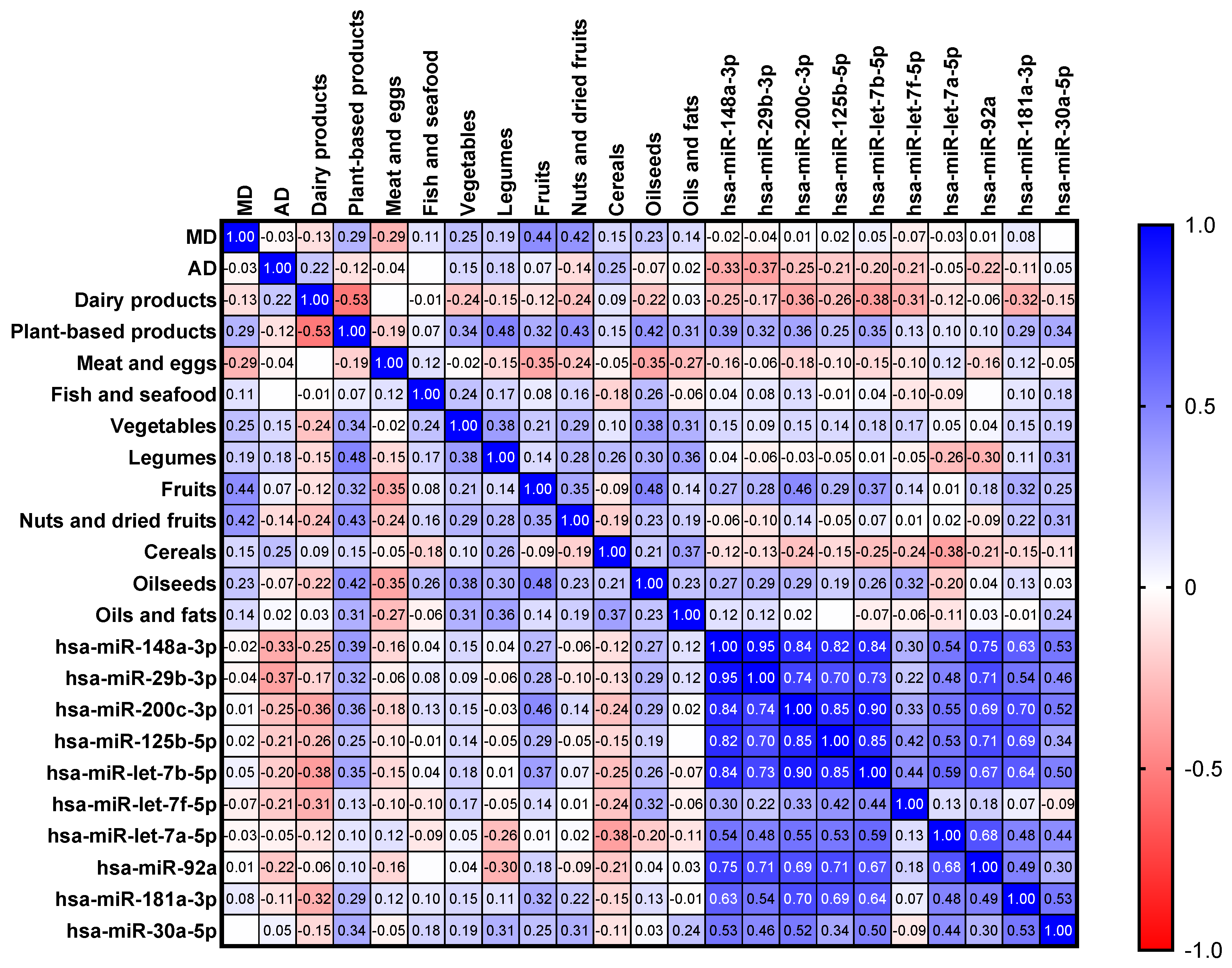
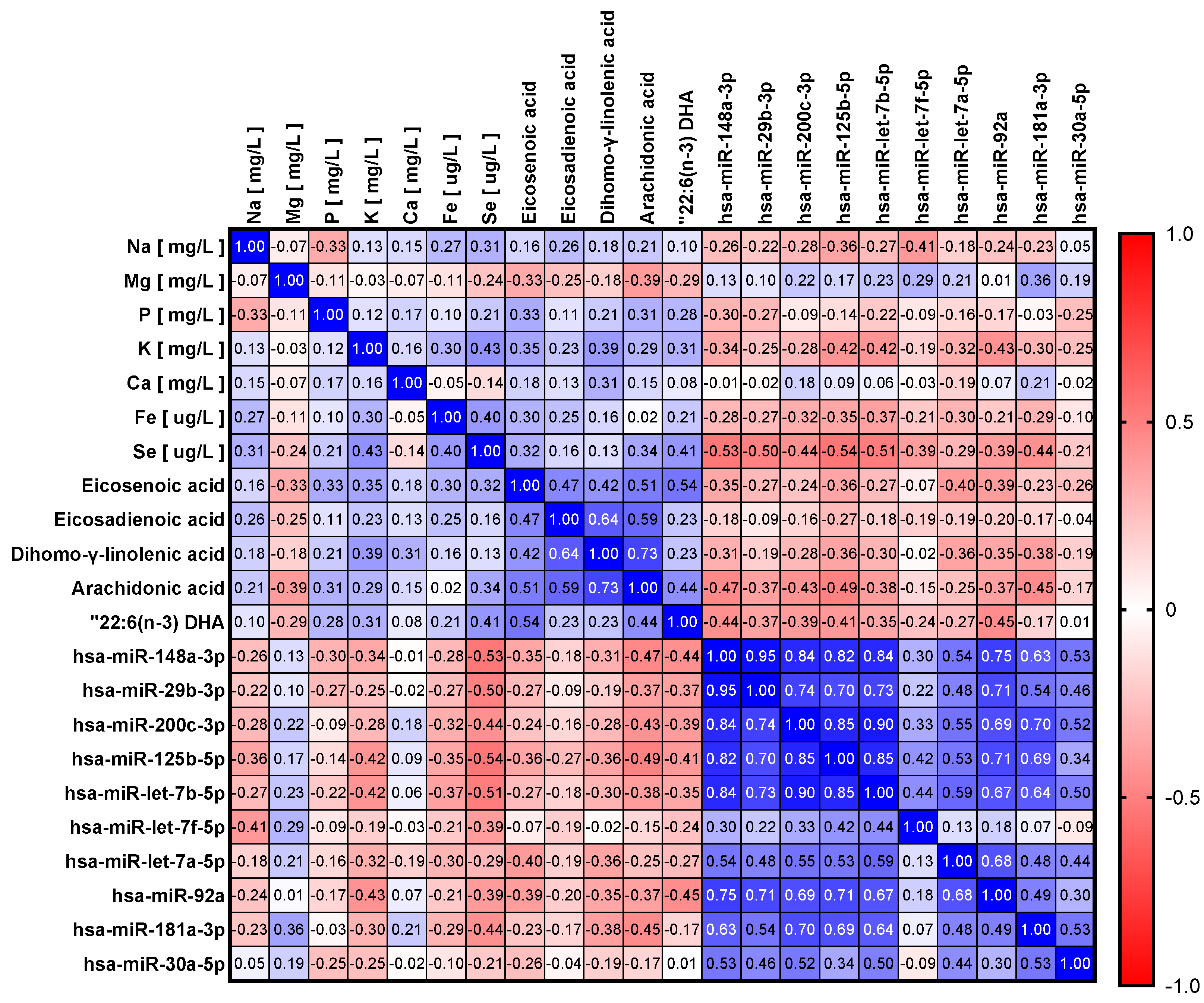
| Mean | Standard Deviation | |
|---|---|---|
| Age (years) | 35.33 | 4.27 |
| Weight (kg) | 64.69 | 10.74 |
| Height (m) | 1.65 | 0.06 |
| BMI (kg/m2) | 23.80 | 3.66 |
| Gestational age at birth (weeks) | 39.72 | 1.29 |
| Weight gain (kg) | 13.32 | 3.71 |
| Infant weight (kg) | 3.35 | 0.45 |
| Lactation time (months) | 10.76 | 13.36 |
| % | ||
| Infant gender: male (%) | 49.12 | |
| Infant gender: female (%) | 50.88 | |
| Caesarean delivery (%) | 13.46 | |
| Parity number: 1st child (%) | 61.40 | |
| Parity number: 2nd child (%) | 36.84 | |
| Parity number: 3rd child (%) | 1.75 | |
| Tandem breastfeeding (%) | 8.47 | |
| Mean | Standard deviation | |
| Adherence to MD (0–14) | 8.30 | 1.87 |
| Adherence to SEAD (0–9) | 3.89 | 1.41 |
| Dairy products (servings/day) | 3.23 | 1.75 |
| Plant-based non-dairy products (servings/day) | 0.27 | 0.75 |
| Meat and eggs (servings/day) | 1.96 | 0.98 |
| Fish and shellfish (servings/day) | 0.85 | 0.44 |
| Vegetables (servings/day) | 4.52 | 3.18 |
| Legumes (servings/day) | 0.2 | 0.22 |
| Fruits (servings/day) | 4.36 | 3.09 |
| Nuts and dried fruit (servings/day) | 0.64 | 1.00 |
| Cereals (servings/day) | 2.81 | 1.39 |
| Oils and fats (servings/day) | 2.35 | 1.31 |
| microRNA | Assay Design | Assay Code * |
|---|---|---|
| hsa-miR-148a-3p | Advanced | 477814_mir |
| hsa-miR-29b-3p | Advanced | 478369_mir |
| hsa-miR-125b-5p | Advanced | 477885_mir |
| hsa-miR-200c-3p | Advanced | 478351_mir |
| hsa-let-7f-5p ¥ | Advanced | 478578_mir |
| hsa-let-7b-5p ¥ | Advanced | 478576_mir |
| hsa-let-7a-5p ¥ | Normal | 000377 |
| hsa-miR-92a | Normal | 000431 |
| hsa-miR-181a-3p | Advanced | 479405_mir |
| hsa-miR-30a-5p | Advanced | 479448_mir |
| hsa-miR-155-5p | Advanced | 483064_mir |
| Amplification (% of Samples) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| microRNA | Cq Mean | Cq SD | Cq Min | Cq Max | All Samples | 1–2 Months | 3–5 Months | 6–11 Months | ≥12 Months |
| hsa-miR-148a-3p | 24.56 | 3.46 | 19.28 | 33.66 | 98.31 | 100.00 | 90.91 | 100.00 | 100.00 |
| hsa-miR-29b-3p | 28.18 | 3.36 | 22.72 | 34.4 | 88.14 | 100.00 | 63.64 | 77.78 | 94.12 |
| hsa-miR-125b-5p | 30.18 | 2.64 | 24.79 | 35.78 | 89.83 | 100.00 | 72.73 | 66.67 | 100.00 |
| hsa-miR-200c-3p | 32.5 | 2.63 | 21.92 | 35.65 | 67.80 | 91.30 | 27.27 | 33.33 | 76.47 |
| hsa-let-7f-5p | 30.1 | 2.54 | 23.23 | 35.74 | 61.02 | 56.52 | 27.27 | 77.78 | 82.35 |
| hsa-let-7b-5p | 29.59 | 2.84 | 22.74 | 35.34 | 94.92 | 100.00 | 72.73 | 100.00 | 100.00 |
| hsa-let-7a-5p | 31.32 | 2.35 | 26.1 | 36.89 | 91.53 | 100.00 | 81.82 | 100.00 | 76.47 |
| hsa-miR-92a | 27.11 | 2.14 | 23.42 | 31.73 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| hsa-miR-181a-3p | 32.89 | 1.68 | 29.68 | 35.46 | 32.20 | 56.52 | 18.18 | 0.00 | 23.53 |
| hsa-miR-30a-5p | 27.3 | 2.03 | 24.53 | 30.69 | 20.34 | 52.17 | 0.00 | 0.00 | 0.00 |
| hsa-miR-155-5p | - | - | - | - | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Group | ||||
|---|---|---|---|---|
| 1–3 Months | 4–5 Months | 6–11 Months | >12 Months | |
| Dairy products (servings/day) | 3.48 ± 1.62 | 2.46 ± 1.59 | 3.35 ± 0.69 | 3.3 ± 2.19 |
| Plant-based non-dairy products (servings/day) | 0.25 ± 0.92 | 0.4 ± 0.52 | 0.04 ± 0.08 | 0.27 ± 0.7 |
| Meat and eggs (servings/day) | 2.18 ± 0.89 | 1.49 ± 0.63 | 1.4 ± 1 | 2.1 ± 1.16 |
| Fish and shellfish (servings/day) | 0.85 ± 0.36 | 0.92 ± 0.7 | 0.6 ± 0.37 | 0.88 ± 0.41 |
| Vegetables (servings/day) | 3.65 ± 1.96 | 6.41 ± 4.68 | 3.85 ± 2.04 | 4.81 ± 3.55 |
| Legumes (servings/day) | 0.15 ± 0.1 | 0.3 ± 0.29 | 0.1 ± 0.04 | 0.25 ± 0.3 |
| Fruits (servings/day) | 3.81 ± 2.81 | 7.24 ± 3.36 | 3.5 ± 2.64 | 3.69 ± 2.74 |
| Nuts and dried fruit (servings/day) | 0.32 ± 0.49 | 0.64 ± 0.81 | 1.18 ± 1.96 | 0.91 ± 1.25 |
| Cereals (servings/day) | 2.8 ± 1.21 | 2.65 ± 1.32 | 1.89 ± 1.32 | 3.16 ± 1.66 |
| Oils and fats (servings/day) | 2.09 ± 1.22 | 2.61 ± 1.28 | 2.24 ± 1.31 | 2.59 ± 1.5 |
| Adherence to MD (normalized to 0-1) | 0.57 ± 0.14 | 0.65 ± 0.09 | 0.59 ± 0.16 | 0.6 ± 0.14 |
| Adherence to SEAD (normalized to 0-1) | 0.42 ± 0.16 | 0.36 ± 0.09 | 0.46 ± 0.12 | 0.48 ± 0.19 |
| Group | ||||
|---|---|---|---|---|
| 1–3 Months | 4–5 Months | 6–11 Months | >12 Months | |
| C6:0 | 0.471 ± 0.315 | 0.571 ± 0.365 | 0.548 ± 0.473 | 0.239 ± 0.113 |
| C8:0 | 0.308 ± 0.083 | 0.256 ± 0.046 | 0.224 ± 0.037 | 0.284 ± 0.055 |
| C10:0 | 1.778 ± 0.454 | 1.595 ± 0.337 | 1.412 ± 0.271 | 1.696 ± 0.274 |
| C11:0 | 0.045 ± 0.018 | 0.053 ± 0.022 | 0.046 ± 0.033 | 0.039 ± 0.016 |
| C12:0 | 9.711 ± 4.215 | 8.657 ± 1.83 | 8.546 ± 1.143 | 11.043 ± 1.829 |
| C13:0 | 0.038 ± 0.011 | 0.032 ± 0.012 | 0.032 ± 0.012 | 0.046 ± 0.013 |
| C14:0 | 6.373 ± 2.436 B | 6.484 ± 1.99 B | 6.999 ± 1.27 B | 10.281 ± 1.986 A |
| C14:1 (n-5) | 0.222 ± 0.11 | 0.189 ± 0.064 | 0.168 ± 0.07 | 0.207 ± 0.119 |
| C15:0 | 0.255 ± 0.078 | 0.213 ± 0.085 | 0.208 ± 0.093 | 0.216 ± 0.076 |
| C16:0 | 19.578 ± 2.217 | 18.07 ± 3.102 | 17.788 ± 4.34 | 17.518 ± 2.227 |
| C16:1 (n-9) | 0.575 ± 0.133 | 0.513 ± 0.081 | 0.476 ± 0.108 | 0.575 ± 0.156 |
| C16:1 (n-7) | 2.406 ± 0.891 | 2.196 ± 0.81 | 1.969 ± 0.682 | 2.238 ± 0.839 |
| C16:1 (n-5) | 0.071 ± 0.029 | 0.057 ± 0.012 | 0.054 ± 0.018 | 0.061 ± 0.027 |
| C16:1 (n-13)t | 0.08 ± 0.037 | 0.072 ± 0.049 | 0.069 ± 0.032 | 0.054 ± 0.019 |
| C17:0 | 0.304 ± 0.055 | 0.274 ± 0.084 | 0.267 ± 0.09 | 0.236 ± 0.049 |
| C17:1 (n-9) | 0.224 ± 0.078 | 0.185 ± 0.056 | 0.167 ± 0.041 | 0.201 ± 0.063 |
| C18:0 | 7.034 ± 1.881 | 6.624 ± 1.305 | 6.229 ± 1.75 | 4.918 ± 0.925 |
| C18:1 (n-9) | 27.93 ± 6.531 | 31.562 ± 6.291 | 35.889 ± 8.35 | 27.602 ± 8.369 |
| C18:1 (n-7) | 0.731 ± 0.137 | 0.693 ± 0.124 | 0.619 ± 0.11 | 0.695 ± 0.135 |
| C18:2 (n-6) | 15.95 ± 4.379 | 15.919 ± 4.922 | 13.376 ± 3.718 | 16.04 ± 4.337 |
| C18:2 (n-6)9,12t | 0.159 ± 0.045 | 0.157 ± 0.045 | 0.13 ± 0.039 | 0.163 ± 0.06 |
| C18:2 (n-6)9t,12 | 0.129 ± 0.036 | 0.128 ± 0.031 | 0.11 ± 0.031 | 0.138 ± 0.049 |
| C18:3 (n-6) | 0.127 ± 0.085 | 0.14 ± 0.053 | 0.075 ± 0.046 | 0.086 ± 0.051 |
| C18:3 (n-3) | 0.586 ± 0.256 | 0.676 ± 0.269 | 0.594 ± 0.175 | 1.008 ± 0.905 |
| C18:2 (n-7)9,11t | 0.609 ± 0.175 | 0.487 ± 0.14 | 0.477 ± 0.164 | 0.539 ± 0.212 |
| C18:4 (n-3) | 0.122 ± 0.044 | 0.13 ± 0.046 | 0.117 ± 0.064 | 0.108 ± 0.039 |
| C18:2 (n-6)10t,12 | 0.341 ± 0.129 | 0.34 ± 0.105 | 0.318 ± 0.153 | 0.315 ± 0.113 |
| C20:0 | 0.164 ± 0.04 A | 0.174 ± 0.041 A | 0.146 ± 0.034 A,B | 0.122 ± 0.021 B |
| C20:1 (n-11) | 0.068 ± 0.019 | 0.081 ± 0.04 | 0.075 ± 0.048 | 0.077 ± 0.044 |
| C20:1 (n-9) | 0.504 ± 0.113 A | 0.413 ± 0.091 A,B | 0.361 ± 0.059 B | 0.41 ± 0.122 A,B |
| C20:2 (n-6) | 0.366 ± 0.084 A | 0.275 ± 0.078 B | 0.246 ± 0.032 B | 0.328 ± 0.091 A,B |
| C20:3 (n-6) | 0.612 ± 0.174 A | 0.417 ± 0.084 B | 0.32 ± 0.07 B | 0.447 ± 0.165 B |
| C20:4 (n-6) | 0.663 ± 0.143 A | 0.509 ± 0.103 A,B | 0.409 ± 0.089 B | 0.639 ± 0.214 A |
| C20:3 (n-3) | 0.066 ± 0.038 | 0.08 ± 0.055 | 0.073 ± 0.07 | 0.055 ± 0.022 |
| C20:4 (n-3) | 0.101 ± 0.033 | 0.1 ± 0.05 | 0.109 ± 0.095 | 0.059 ± 0.028 |
| C20:5 (n-3) | 0.134 ± 0.095 | 0.155 ± 0.089 | 0.133 ± 0.107 | 0.097 ± 0.063 |
| C22:0 | 0.071 ± 0.023 | 0.076 ± 0.03 | 0.063 ± 0.016 | 0.059 ± 0.014 |
| C22:1 (n-11) | 0.084 ± 0.047 | 0.093 ± 0.086 | 0.054 ± 0.025 | 0.035 ± 0.016 |
| C22:1 (n-9) | 0.104 ± 0.026 | 0.095 ± 0.025 | 0.088 ± 0.03 | 0.079 ± 0.02 |
| C22:5 (n-3) | 0.137 ± 0.053 | 0.127 ± 0.033 | 0.112 ± 0.024 | 0.148 ± 0.067 |
| C24:0 | 0.052 ± 0.048 | 0.075 ± 0.071 | 0.046 ± 0.031 | 0.028 ± 0.013 |
| C22:6 (n-3) | 0.497 ± 0.354 | 0.351 ± 0.19 | 0.328 ± 0.26 | 0.494 ± 0.298 |
| Total SFA | 45.931 ± 6.985 | 43.078 ± 6.119 | 42.431 ± 7.569 | 46.508 ± 3.958 |
| Total MUFA | 33.515 ± 6.991 | 36.927 ± 5.996 | 40.671 ± 8.099 | 32.801 ± 7.672 |
| Total PUFA | 20.483 ± 4.675 | 19.92 ± 5.065 | 16.834 ± 3.788 | 20.635 ± 5.685 |
| Total PUFA n-3 | 1.545 ± 0.612 | 1.562 ± 0.381 | 1.383 ± 0.565 | 1.94 ± 1.127 |
| Total PUFA n-6 | 17.988 ± 4.619 | 17.532 ± 5.067 | 14.656 ± 3.67 | 17.865 ± 4.843 |
| CLAs | 0.95 ± 0.251 | 0.826 ± 0.24 | 0.796 ± 0.303 | 0.854 ± 0.315 |
| Group | ||||
|---|---|---|---|---|
| 1–3 Months | 4–5 Months | 6–11 Months | >12 Months | |
| Na (mg/L) | 144.4 ± 69.57 | 101.15 ± 49.65 | 109.16 ± 46.91 | 232.46 ± 188.79 |
| Mg (mg/L) | 31.64 ± 6.31 | 32.74 ± 5.73 | 34.34 ± 2.4 | 35.63 ± 8.89 |
| P (mg/L) | 133.02 ± 25.91 | 119.75 ± 10.27 | 119.32 ± 26.5 | 132.05 ± 39.49 |
| K (mg/L) | 502.54 ± 65.4 A | 415.57 ± 32.32 B | 451.94 ± 68.39 A,B | 420.95 ± 53.92 A,B |
| Ca (mg/L) | 301.61 ± 71.15 | 267.48 ± 36.73 | 255.53 ± 17.64 | 246.85 ± 49.1 |
| Fe (mg/L) | 226.72 ± 93.41 | 196.48 ± 90.22 | 170.93 ± 96.83 | 219.98 ± 94.48 |
| Se (µg/L) | 12.47 ± 4.22 | 7.62 ± 1.35 | 10.36 ± 7.44 | 12.12 ± 5.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanjulián, L.; Lamas, A.; Barreiro, R.; Cepeda, A.; Fente, C.; Regal, P. Influence of Maternal Diet and Lactation Time on the Exosomal miRNA Cargo in Breast Milk. Foods 2025, 14, 1003. https://doi.org/10.3390/foods14061003
Sanjulián L, Lamas A, Barreiro R, Cepeda A, Fente C, Regal P. Influence of Maternal Diet and Lactation Time on the Exosomal miRNA Cargo in Breast Milk. Foods. 2025; 14(6):1003. https://doi.org/10.3390/foods14061003
Chicago/Turabian StyleSanjulián, Laura, Alexandre Lamas, Rocío Barreiro, Alberto Cepeda, Cristina Fente, and Patricia Regal. 2025. "Influence of Maternal Diet and Lactation Time on the Exosomal miRNA Cargo in Breast Milk" Foods 14, no. 6: 1003. https://doi.org/10.3390/foods14061003
APA StyleSanjulián, L., Lamas, A., Barreiro, R., Cepeda, A., Fente, C., & Regal, P. (2025). Influence of Maternal Diet and Lactation Time on the Exosomal miRNA Cargo in Breast Milk. Foods, 14(6), 1003. https://doi.org/10.3390/foods14061003










