Preparation, Characterization, and Safety Evaluation of a Submicron Emulsion Processed Using High-Pressure Homogenization to Protect Bitter Melon Seed Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Preparation of the BMSOSE
2.4. Characterization of BMSOSE
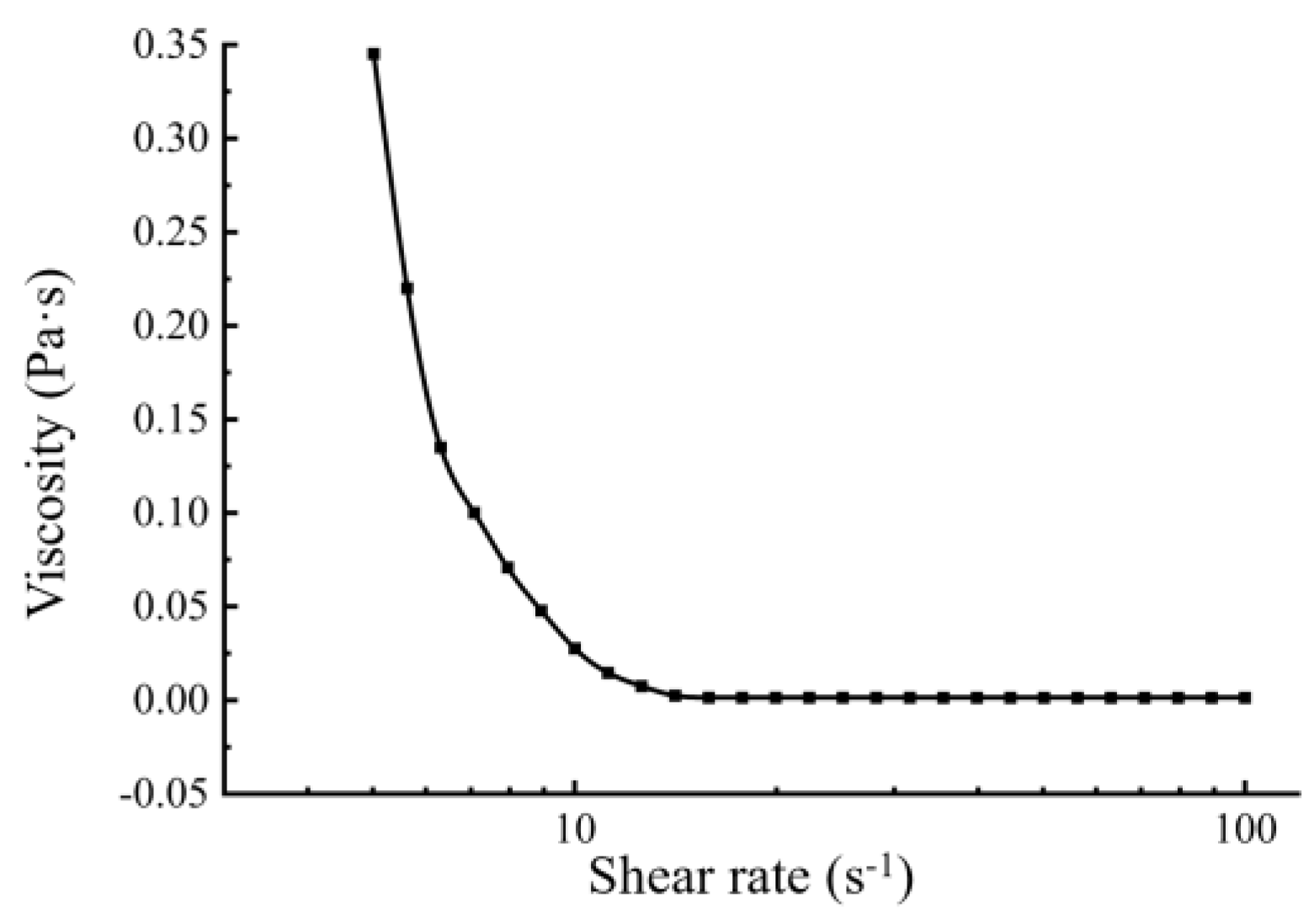
2.5. Apparent Viscosity
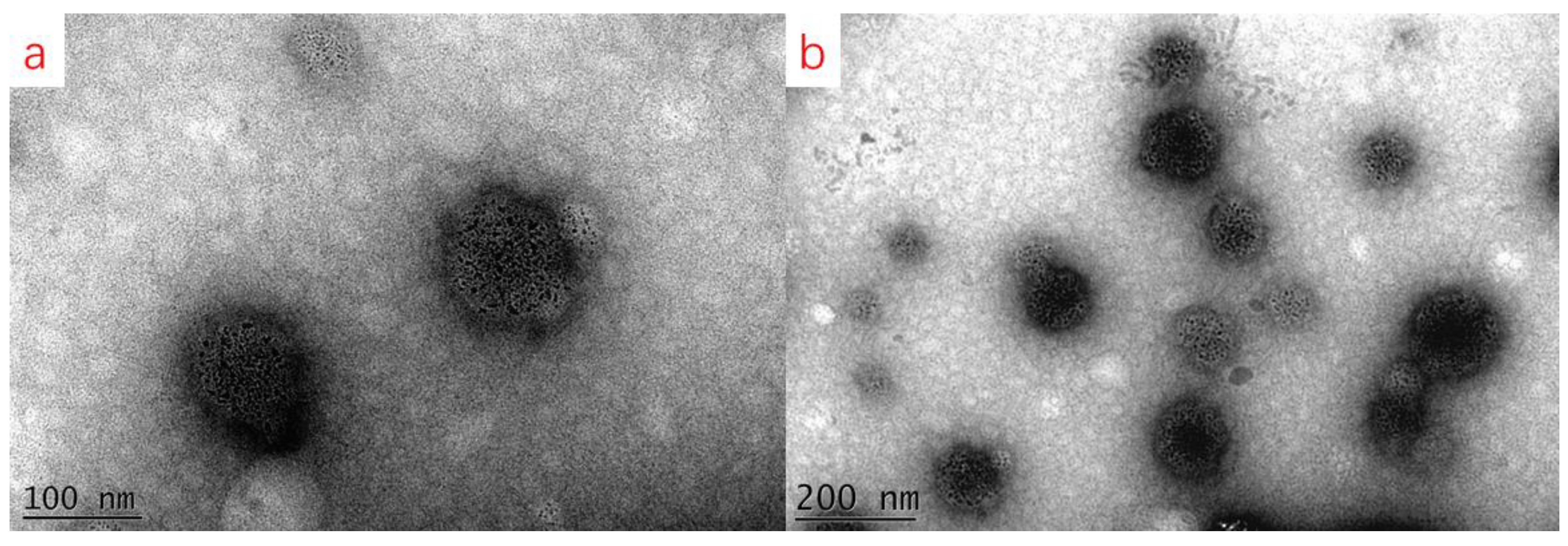
2.6. Morphology Observation
2.7. Fatty Acid Composition Determination
2.8. Safety Verification of the BMSOSE
2.8.1. Hemolysis Test In Vitro
2.8.2. Acute Toxicity Test
2.9. Statistical Analysis
3. Results and Discussion
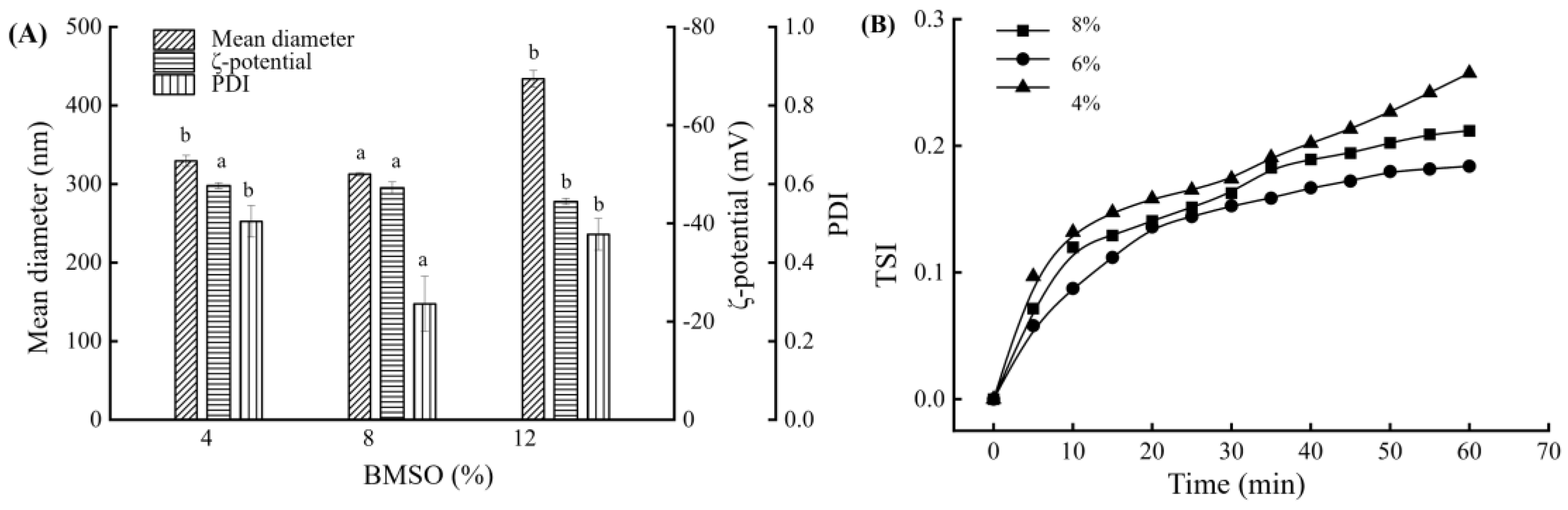
3.1. Influence of BMSO Concentration on the BMSOSE
3.2. Influence of Emulsifier Concentration on the BMSOSE
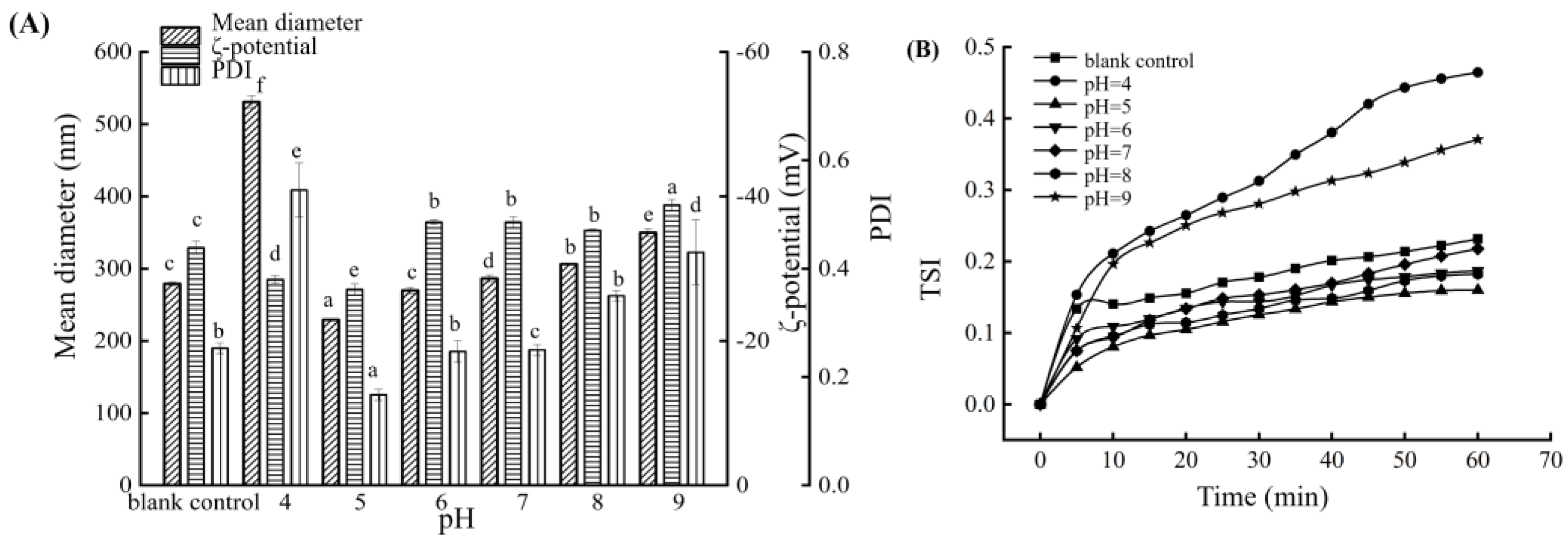
3.3. Influence of pH on the BMSOSE
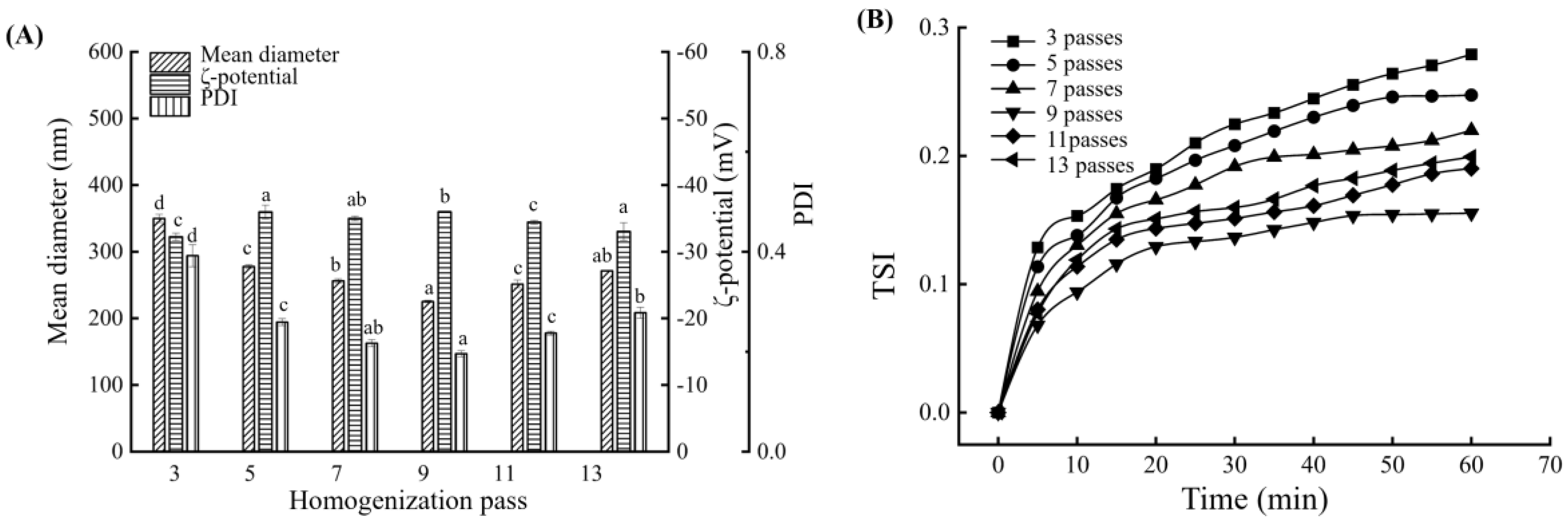
3.4. Influence of Homogenization Time on the BMSOSE
3.5. Physical Properties of the BMSOSE
3.6. Fatty Acid Composition of the BMSOSE
3.7. Hemolysis Testing of the BMSOSE
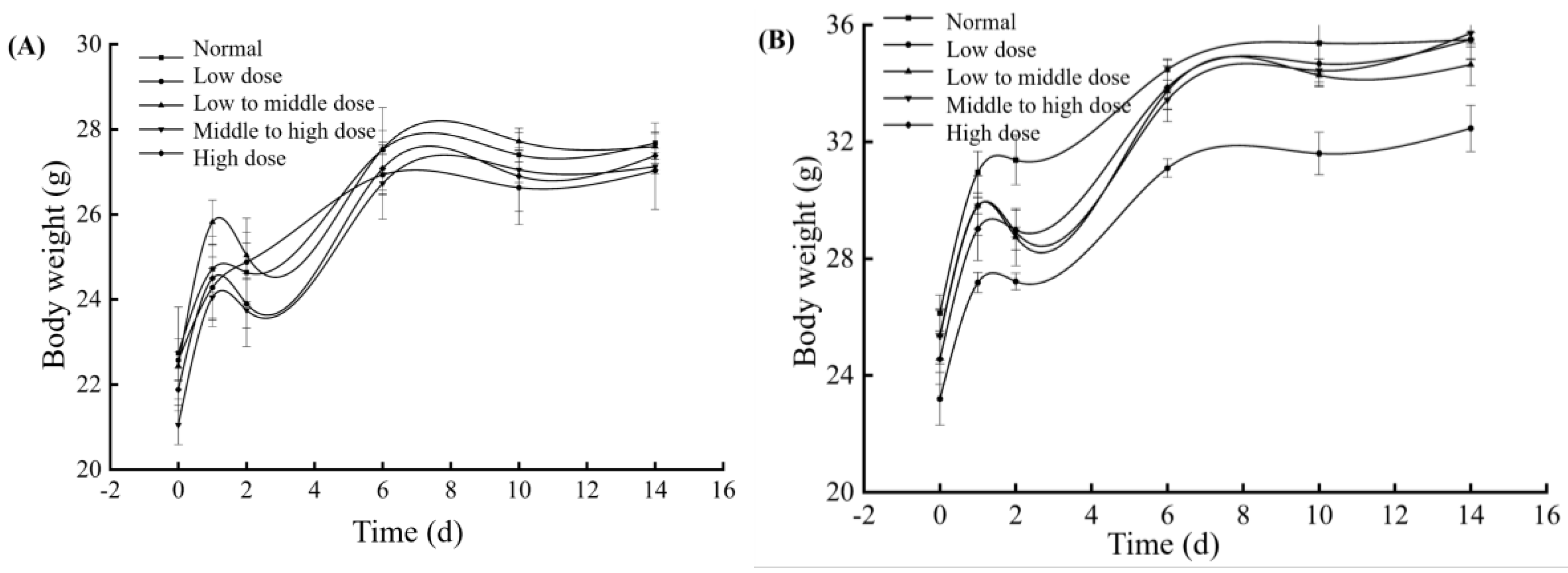
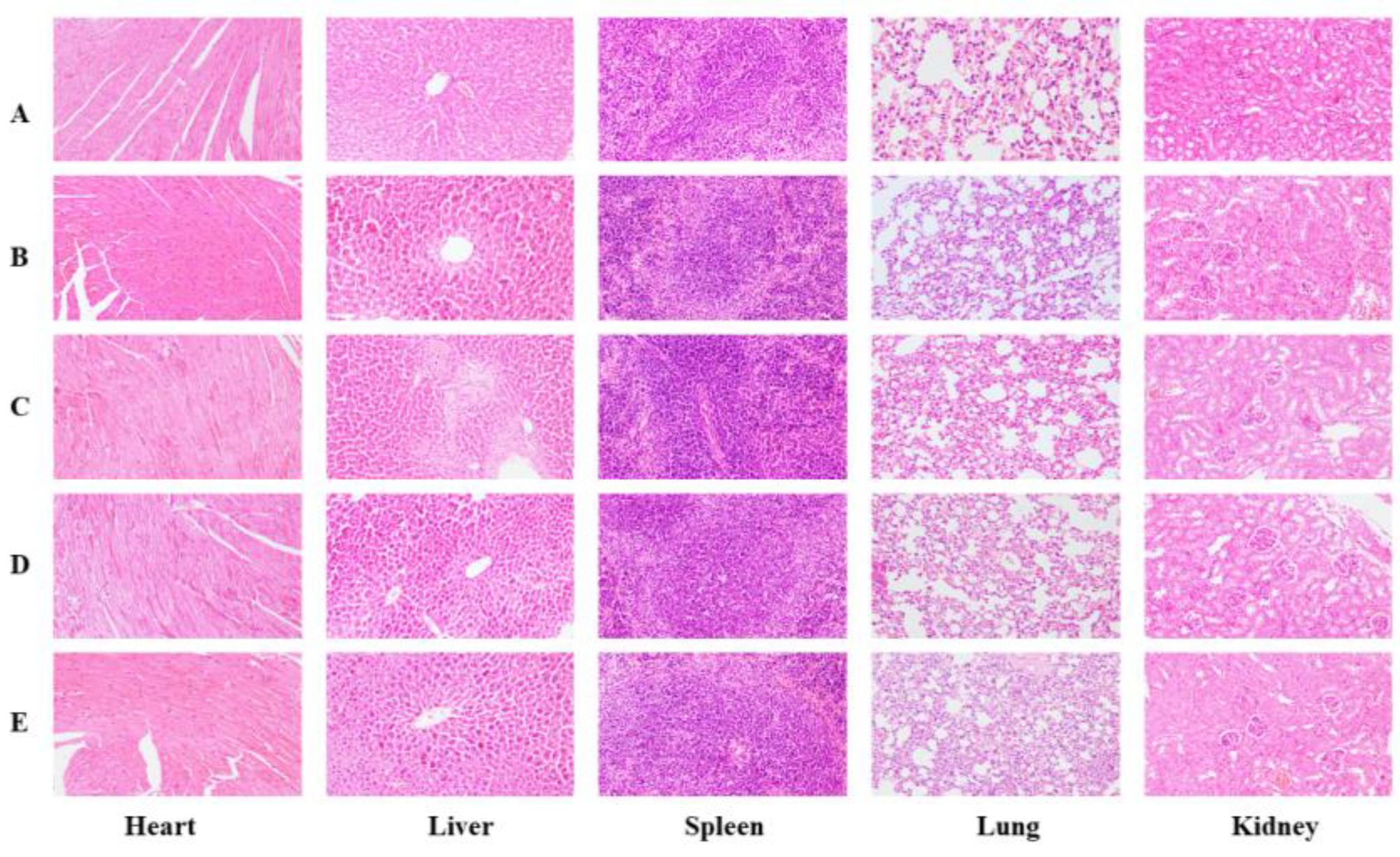
3.8. Acute Toxicity of the BMSOSE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anjum, F.; Shahid, M.; Bukhari, S.A.; Anwar, S.; Latif, S. Study of Quality Characteristics and Efficacy of Extraction Solvent/Technique on the Antioxidant Activity of Bitter Gourd Seed. J. Food Process. Technol. 2012, 4, 1000205. [Google Scholar] [CrossRef]
- Braca, A.; Siciliano, T.; D’Arrigo, M.; Germanò, M.P. Chemical composition and antimicrobial activity of Momordica charantia seed essential oil. Fitoterapia 2008, 79, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Białek, A.; Jelińska, M.; Białek, M.; Lepionka, T.; Czerwonka, M.; Czauderna, M. The Effect of Diet Supplementation with Pomegranate and Bitter Melon on Lipidomic Profile of Serum and Cancerous Tissues of Rats with Mammary Tumours. Antioxidants 2020, 9, 243. [Google Scholar] [CrossRef]
- Kilcar, A.Y.; Yildiz, O.; Dogan, T.; Sulu, E.; Takan, G.; Muftuler, F.Z.B. The Effect of Bitter Melon (Momordica charantia) Extract on the Uptake of (99m)Tc Labeled Paclitaxel: In Vitro Monitoring in Breast Cancer Cells. Anticancer Agents Med. Chem. 2020, 20, 1497–1503. [Google Scholar] [CrossRef]
- Ahmad, Z.; Zamhuri, K.F.; Yaacob, A.; Siong, C.H.; Selvarajah, M.; Ismail, A.; Hakim, M.N. In vitro anti-diabetic activities and chemical analysis of polypeptide-k and oil isolated from seeds of Momordica charantia (bitter gourd). Molecules 2012, 17, 9631–9640. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Su, H.M.; Chen, S.H.; Hsieh, W.T.; Chyuan, J.H.; Chao, P.M. Roles of Peroxisome Proliferator-Activated Receptor α in Bitter Melon Seed Oil-Corrected Lipid Disorders and Conversion of α-Eleostearic Acid into Rumenic Acid in C57BL/6J Mice. Nutrients 2016, 8, 805. [Google Scholar] [CrossRef]
- Chan, F.K.; Hsu, C.; Li, T.C.; Chen, W.H.; Tseng, K.T.; Chao, P.M. Bitter melon seed oil increases mitochondrial content in gastrocnemius muscle and improves running endurance in sedentary C57BL/6J mice. J. Nutr. Biochem. 2018, 58, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Yoshime, L.T.; De Melo, I.L.P.; Sattler, J.A.G.; de Carvalho, E.B.T.; Mancini-Filho, J. Bitter gourd (Momordica charantia L.) seed oil as a naturally rich source of bioactive compounds for nutraceutical purposes. Nutrire 2016, 41, 12. [Google Scholar] [CrossRef]
- Chen, G.C.; Chen, W.H.; Tseng, K.T.; Chao, P.M. The anti-adiposity effect of bitter melon seed oil is solely attributed to its fatty acid components. Lipids Health Dis. 2017, 16, 186. [Google Scholar] [CrossRef]
- Dhar, P.; Chattopadhyay, K.; Bhattacharyya, D.; Roychoudhury, A.; Biswas, A.; Ghosh, S. Antioxidative effect of conjugated linolenic acid in diabetic and non-diabetic blood: An in vitro study. J. Oleo Sci. 2006, 56, 19–24. [Google Scholar] [CrossRef]
- Zhuo, R.J.; Wang, F.; Zhang, X.H.; Zhang, J.J.; Xu, J.; Dong, W.; Zou, Z.Q. A-eleostearic acid inhibits growth and induces apoptosis in breast cancer cells via HER2/HER3 signaling. Mol. Med. Rep. 2014, 9, 993–998. [Google Scholar] [CrossRef][Green Version]
- Cao, S.Q.; Zhang, K.Y.; Yan, X.; Ma, Y. Preparation and evaluation of paclitaxel and Brucea javanica oil core-matched nanoemulsions to treat cancer in vitro and in vivo. Chin. Herb. Med. SciVerse Sci. 2018, 10, 310–317. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, W.; Liu, H.; Deng, F.; Wang, W.; Qin, L. Preparation of injectable clopidogrel loaded submicron emulsion for enhancing physicochemical stability and anti-thrombotic efficacy. Int. J. Pharm. 2022, 611, 121323. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Q.; Li, G.; Xu, J.H.; Zhang, J.; Zhang, Z.Z.; Xiao, H.Y.; Li, X.F. Combination of submicroemulsion and phospholipid complex for novel delivery of ursodeoxycholic acid. Pharm. Dev. Technol. 2014, 19, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Sung, K.C.; Hu, O.Y.; Yeh, C.H.; Fang, J.Y. Submicron lipid emulsion as a drug delivery system for nalbuphine and its prodrugs. J. Control. Release 2006, 115, 140–149. [Google Scholar] [CrossRef]
- Wen, L. Distribution of 10-Hydroxy Camptothecin in Arterial and Intravenous Plasma of Rats with 2 Kinds of Routes of Administration. China Pharm. 2013, 24, 1176–1179. [Google Scholar]
- Zhang, R.; Wang, B.; Zhao, H.; Wei, C.; Guo, R. Tissue distribution of Curcumol in rats after intravenous injection of zedoary turmeric oil fat emulsion. Asian J. Pharmacodyn. Pharmacokinet. 2009, 9, 51–57. [Google Scholar]
- Krista, H.; Braylee, G.; Laura, G.; Paul, E.W. Lipid emulsions in parenteral nutrition: Does it matter? Curr. Opin. Crit. Care 2023, 29, 1070–5295. [Google Scholar]
- Plasencia, J.; Inkson, N.; Nydal, O.J. Research on the viscosity of stabilized emulsions in different pipe diameters using pressure drop and phase inversion. Exp. Comput. Multiph. Flow 2021, 4, 241–263. [Google Scholar] [CrossRef]
- Johnson, A.; Woernley, D.; Pressman, D. Sedimentation properties of human and sheep erythrocyte hemolysins. J. Immunol. 1957, 79, 234–237. [Google Scholar] [CrossRef]
- Cesário, F.R.; de Albuquerque, T.R.; de Lacerda, G.M.; de Oliveira, M.R.; da Silva, B.A.; Rodrigues, L.B.; Martins, A.O.; da Silva Almeida, J.R.; Vale, M.L.; Coutinho, H.D.; et al. Chemical fingerprint, acute oral toxicity and anti-inflammatory activity of the hydroalcoholic extract of leaves from Tocoyena formosa (Cham. & Schlecht.) K. Schum. Saudi J. Biol. Sci. 2019, 26, 873–880. [Google Scholar]
- Dhaval, M.; Vaghela, P.; Patel, K.; Sojitra, K.; Patel, M.; Patel, S.; Dudhat, K.; Shah, S.; Manek, R.; Parmar, R. Lipid-based emulsion drug delivery systems—A comprehensive review. Drug Deliv. Transl. Res. 2022, 12, 1616–1639. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, Y.; Zhai, J.; Su, Y.; Gu, L.; Chang, C.; Yang, Y. Enhancing the oxidative stability of algal oil powders stabilized by egg yolk granules/lecithin composites. Food Chem. 2021, 345, 128782. [Google Scholar] [CrossRef]
- Wan, Z.; Xie, F.; Wang, L.; Zhang, G.; Zhang, H. Preparation and Evaluation of Cabazitaxel-Loaded Bovine Serum Albumin Nanoparticles for Prostate Cancer. Int. J. Nanomed. 2020, 15, 5333–5344. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Tolerability of hypertonic injectables. Int. J. Pharm. 2015, 490, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Udomrati, S.; Khalid, N.; Gohtani, S.; Nakajima, M.; Uemura, K.; Kobayashi, I. Formulation and characterization of esterified xylo-oligosaccharides-stabilized oil-in-water emulsions using microchannel emulsification. Colloids Surf. B Biointerfaces 2016, 148, 333–342. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Zhu, D.; Hu, S.; Kang, Z.; Ma, H. Effect of dynamic ultra-high pressure homogenization on the structure and functional properties of whey protein. J. Food Sci. Technol. 2020, 57, 1301–1309. [Google Scholar] [CrossRef]
- Arinina, M.P.; Zuev, K.V.; Kulichikhin, V.G.; Malkin, A.Y. Effect of Composition and Interfacial Tension on the Rheology and Morphology of Heavy Oil-In-Water Emulsions. ACS Omega 2020, 5, 16460–16469. [Google Scholar] [CrossRef]
- Chung, W.Y.; Jadhav, S.; Hsu, P.K.; Kuan, C.M. Evaluation of acute and sub-chronic toxicity of bitter melon seed extract in Wistar rats. Toxicol. Rep. 2022, 29, 1024–1034. [Google Scholar] [CrossRef]




| Tube Number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 2% red cell suspension (RCS) | 2.5 | 2.5 | 2.5 | 2.5 | 0 |
| 0.9% sodium chloride solution/mL | 2.2 | 2.2 | 2.5 | 0 | 4.7 |
| Water/mL | 0 | 0 | 0 | 2.5 | 0 |
| Bitter melon oil submicron emulsion/mL | 0.3 | 0.3 | 0 | 0 | 0.3 |
| Determination Criteria for Hemolysis Result | Phenomenon |
|---|---|
| Hemolysis | The solution was clear red, and there was no cell residue at the bottom of the tube. |
| Part of the hemolysis | The solution was clear red or brown with a small number of cells at the bottom of the tube. |
| No hemolysis | The red blood cells were sinking, and the upper fluid was clear and colorless. |
| Erythrocyte aggregation | The red blood cells aggregated and did not disperse after vibration. |
| Fatty Acid Composition | Relative Amount (%) | |
|---|---|---|
| BMSO | BMSO from BMSOSE | |
| C16:0 (Palmitic acid) | 1.68 ± 0.02 | 1.92 ± 0.04 |
| C18:0 (Stearic acid) | 16.8 ± 0.36 | 17.37 ± 0.57 |
| C18:1 (Oleic acid) | 4.34 ± 0.04 | 4.26 ± 0.03 |
| C18:2 (Linoleic acid) | 0.44 ± 0.01 | 0.42 ± 0.03 |
| C20:0 (Arachidic acid) | 6.13 ± 0.01 | 6.12 ± 0.07 |
| C18:3 (α-eleostearic acid) | 54.9 ± 0.60 | 51.83 ± 0.04 |
| C18:3 (CLN-B) | 3.63 ± 0.04 | 4.83 ± 0.13 |
| C18:3 (CLN-C) | 12.08 ± 0.94 | 13.76 ± 0.44 |
| Gender | Group | Cardiac Index/% | Renal Index/% | Liver Index/% | Spleen Index/% |
|---|---|---|---|---|---|
| Female | Normal | 0.53 ± 0.07 | 1.33 ± 0.06 | 4.18 ± 0.06 | 0.38 ± 0.04 |
| Low dose | 0.60 ± 0.06 | 1.25 ± 0.06 | 4.18 ± 0.36 | 0.41 ± 0.11 | |
| Low to middle dose | 0.53 ± 0.12 | 1.33 ± 0.04 | 4.75 ± 0.66 | 0.40 ± 0.04 | |
| Middle to high dose | 0.58 ± 0.00 | 01.22 ± 0.15 | 4.78 ± 0.26 | 0.50 ± 0.02 | |
| High dose | 0.62 ± 0.04 | 1.35 ± 0.09 | 4.34 ± 0.29 | 0.38 ± 0.04 | |
| Male | Normal | 0.51 ± 0.05 | 0.80 ± 0.23 b | 4.56 ± 0.19 | 0.38 ± 0.08 |
| Low dose | 0.52 ± 0.06 | 1.60 ± 0.16 a | 4.90 ± 0.29 | 0.45 ± 0.06 | |
| Low to middle dose | 0.54 ± 0.11 | 0.84 ± 0.12 b | 4.90 ± 0.47 | 0.44 ± 0.07 | |
| Middle to high dose | 0.54 ± 0.11 | 0.90 ± 0.08 b | 4.61 ± 0.17 | 0.38 ± 0.05 | |
| High dose | 0.54 ± 0.07 | 0.83 ± 0.08 b | 4.63 ± 0.41 | 0.36 ± 0.009 |
| Gender | Group | GPT/(U/L) | GOT/(U/L) | CR/(μmol/L) |
|---|---|---|---|---|
| Female | Normal | 16.51 ± 4.07 | 22.04 ± 7.00 | 72.99 ± 8.18 |
| Low dose | 17.76 ± 2.17 | 25.04 ± 4.60 | 80.18 ± 5.20 | |
| Low to middle dose | 14.69 ± 3.04 | 28.67 ± 1.20 | 80.08 ± 12.47 | |
| Middle to high dose | 16.95 ± 3.50 | 22.49 ± 8.39 | 88.75 ± 9.10 | |
| High dose | 13.35 ± 3.55 | 17.57 ± 3.04 | 77.26 ± 7.65 | |
| Male | Normal | 21.36 ± 5.89 | 21.03 ± 4.06 | 74.20 ± 1.84 |
| Low dose | 20.86 ± 7.08 | 24.99 ± 5.09 | 75.75 ± 4.13 | |
| Low to middle dose | 16.08 ± 1.67 | 31.27 ± 7.74 | 90.92 ± 2.29 | |
| Middle to high dose | 17.82 ± 8.11 | 24.59 ± 3.49 | 80.94 ± 4.99 | |
| High dose | 20.41 ± 1.99 | 20.86 ± 5.13 | 74.71 ± 7.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Guo, H.; Shuai, X.; Ma, Y.; Zhang, R.; Wu, M.; He, J.; Ling, J. Preparation, Characterization, and Safety Evaluation of a Submicron Emulsion Processed Using High-Pressure Homogenization to Protect Bitter Melon Seed Oil. Foods 2025, 14, 850. https://doi.org/10.3390/foods14050850
Wang H, Guo H, Shuai X, Ma Y, Zhang R, Wu M, He J, Ling J. Preparation, Characterization, and Safety Evaluation of a Submicron Emulsion Processed Using High-Pressure Homogenization to Protect Bitter Melon Seed Oil. Foods. 2025; 14(5):850. https://doi.org/10.3390/foods14050850
Chicago/Turabian StyleWang, Huiling, Heng Guo, Xiaoyan Shuai, Yan Ma, Rui Zhang, Muci Wu, Jingren He, and Jiayan Ling. 2025. "Preparation, Characterization, and Safety Evaluation of a Submicron Emulsion Processed Using High-Pressure Homogenization to Protect Bitter Melon Seed Oil" Foods 14, no. 5: 850. https://doi.org/10.3390/foods14050850
APA StyleWang, H., Guo, H., Shuai, X., Ma, Y., Zhang, R., Wu, M., He, J., & Ling, J. (2025). Preparation, Characterization, and Safety Evaluation of a Submicron Emulsion Processed Using High-Pressure Homogenization to Protect Bitter Melon Seed Oil. Foods, 14(5), 850. https://doi.org/10.3390/foods14050850











