Effectiveness of Cinnamon Oil Embedded Chitosan–Gelatin Film in Inhibiting Rhizopus oryzae, R. microsporus, and Syncephalastrum racemosum and Controlling Rice Weevil Infestation on Paddy Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Cinnamon-Oil-Embedded Chitosan–Gelatin Film
2.1.1. Extraction of Cinnamon Oil
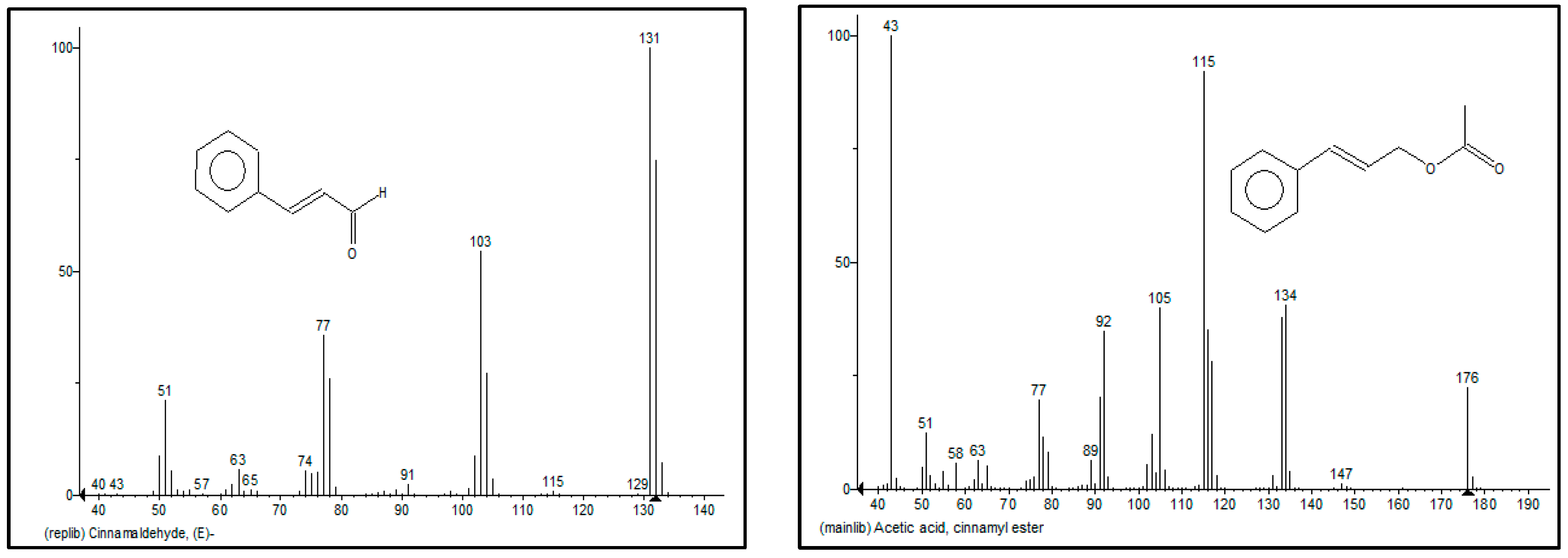
2.1.2. Gas Chromatography/Mass Spectrometry Analysis of Cinnamon Oil
2.1.3. Cinnamon-Oil-Embedded Chitosan–Gelatin Film (CO–C:G Film)
2.2. Fungal Strains and Preparation of Spore Solution
2.2.1. Fungal Strains
2.2.2. Preparation of Fungal Spore Solution and Rice Weevils
Fungal Spore Solution
Rice Weevils
2.3. Infection of Fungi and Rice Weevil on Paddy Rice
2.4. Evaluation of Fungal Growth and Rice Weevil Mortality
2.5. Statistical Analysis
3. Results and Discussions
3.1. Chemical Composition of Cinnamon Oil
3.2. Influence of the Cinnamon-Oil-Embeded Chitosan–Gelatin Films on R. oryzae 01, R. microsporus 01, and S. racemorium 01 Growth on Paddy Rice
3.3. Influence of the CO–C:G Film on Rice Weevil on Paddy Rice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Zoreky, N.S.; Saleh, F.A. Limited survey on aflatoxin contamination in rice. Saudi J. Biol. Sci. 2019, 26, 225–231. [Google Scholar] [CrossRef]
- Devi, K.S.; Ponnarasi, T. An Economic Analysis of Modern Rice Production Technology and its Adoption Behaviour in Tamil Nadu. Agric. Econ. Res. Rev. 2009, 22, 341–348. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J.W. Limiting mycotoxins in stored wheat. Food Addit. Contam. 2010, 27, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.N.; Reddy, C.S.; Muralidharan, K. Detection of Aspergillus spp. and aflatoxin B1 in rice in India. Food Microbiol. 2009, 26, 27–31. [Google Scholar] [CrossRef]
- Phan, L.T.K.; Tran, T.M.; Audenaert, K.; Jacxsens, L.; Eeckhout, M. Contamination of Fusarium proliferatum and Aspergillus flavus in the Rice Chain Linked to Crop Seasons, Cultivation Regions, and Traditional Agricultural Practices in Mekong Delta, Vietnam. Foods 2021, 10, 2064. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kalita, P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Dillahunty, A.L.; Siebenmorgen, T.J.; Mauromoustakos, A. Effect of temperature, exposure duration, and moisture content on color and viscosity of rice. Cereal Chem. 2001, 78, 559–563. [Google Scholar] [CrossRef]
- Phillips, S.; Mitfa, R.; Wallbridge, A. Rice yellowing during drying delays. J. Stored Prod. Res. 1989, 25, 155–164. [Google Scholar] [CrossRef]
- Makun, A.; Gbodi, A.; Akanya, H.; Salako, A.; Ogbadu, H. Fungi and some mycotoxins contaminating rice (Oryza Sativa) in Niger State, Nigeria. Afr. J. Biotechnol. 2007, 6, 99–108. [Google Scholar]
- Phillips, S.; Widjaja, S.; Wallbridge, A.; Cooke, R. Rice yellowing during post-harvest drying by aeration and during storage. J. Stored Prod. Res. 1988, 24, 173–181. [Google Scholar] [CrossRef]
- Schroeder, H.W. Relation between storage fungis and damage in high moisture rice in aerated storage. Phytopathology 1963, 53, 804–808. [Google Scholar]
- Yadav, A.; Kumar, N.; Upadhyay, A.; Singh, A.; Anurag, R.K.; Pandiselvam, R. Effect of mango kernel seed starch-based active edible coating functionalized with lemongrass essential oil on the shelf-life of guava fruit. Qual. Assur. Saf. Crops Foods 2022, 14, 103–115. [Google Scholar] [CrossRef]
- Phan, L.T.K.; Le, A.T.H.; Hoang, N.T.N.; Debonne, E.; De Saeger, S.; Eeckhout, M.; Jacxsens, L. Evaluation of the efficacy of cinnamon oil on Aspergillus flavus and Fusarium proliferatum growth and mycotoxin production on paddy and polished rice: Towards a mitigation strategy. Int. J. Food Microbiol. 2024, 415, 110636. [Google Scholar] [CrossRef]
- Marei, G.I.K.; Abdel Rasoul, M.A.; Abdelgaleil, S.A.M. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic. Biochem. Physiol. 2012, 103, 56–61. [Google Scholar] [CrossRef]
- Soylu, E.M.; Kurt, Ş.; Soylu, S. In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 2010, 143, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of essential oils and their components to improve safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 311–319. [Google Scholar] [CrossRef]
- Tserennadmid, R.; Takó, M.; Galgóczy, L.; Papp, T.; Pesti, M.; Vágvölgyi, C.; Almássy, K.; Krisch, J. Anti yeast activities of some essential oils in growth medium, fruit juices and milk. Int. J. Food Microbiol. 2011, 144, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Moon, S.S.; Doyle, M.P.; McWatters, K.H. Inactivation of Escherichia coli O157:H7, Salmonella enterica serotype enteritidis, and Listeria monocytogenes on lettuce by hydrogen peroxide and lactic acid and by hydrogen peroxide with mild heat. J. Food Prot. 2002, 65, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Samadi, N.; Abadian, N.; Bakhtiari, D.; Fazeli, M.R.; Jamalifar, H. Efficacy of detergents and fresh produce disinfectants against microorganisms associated with mixed raw vegetables. J. Food Prot. 2009, 72, 1486–1490. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, L.; Phelan, V.H.; Doyle, M.P. Efficacy of antimicrobial agents in lettuce leaf processing water for control of Escherichia coli O157:H7. J. Food Prot. 2009, 72, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Alonso, P.; Fernández-Pastor, S.; Guerrero, A. Application of Cinnamon Essential Oil in Active Food Packaging: A Review. Appl. Sci. 2024, 14, 6554. [Google Scholar] [CrossRef]
- Faleiro, M.L.; Miguel, M.G.; Ladeiro, F.; Venâncio, F.; Tavares, R.; Brito, J.C.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antimicrobial activity of essential oils isolated from Portuguese endemic species of Thymus. Lett. Appl. Microbiol. 2003, 36, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT-Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Grossmann, M.V.E.; Yamashita, F.; Pined, E.A.G. Antimicrobial, mechanical, and barrier properties of cassava starch-chitosan films incorporated with oregano essential oil. J. Agric. Food Chem. 2009, 57, 7499–7504. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of Essential Oils in Bioactive Edible Coatings: A Review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Rodríguez-Lázaro, D.; Domínguez, R.; Zhong, J.; Lorenzo, J.M. The Role of Essential Oils against Pathogenic Escherichia coli in Food Products. Microorganisms 2020, 8, 924. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.I.S.; Marques, C.; Mota, J.; Pedroso, L.; Lima, A. Applications of Essential Oils as Antibacterial Agents in Minimally Processed Fruits and Vegetables—A Review. Microorganisms 2022, 10, 760. [Google Scholar] [CrossRef]
- Phan, L.T.K.; Nguyen, H.X.; De Saeger, S.; Jacxsens, L.; Eeckhout, M.; Devlieghere, F. Predictive modelling of the radial growth of Aspergillus flavus and Fusarium proliferatum on paddy and white rice (Oryza sativa). Int. J. Food Microbiol. 2022, 375, 109743. [Google Scholar] [CrossRef]
- Yogendrarajah, P.; Vermeulen, A.; Jacxsens, L.; Mavromichali, E.; De Saeger, S.; De Meulenaer, B.; Devlieghere, F. Mycotoxin production and predictive modelling kinetics on the growth of Aspergillus flavus and Aspergillus parasiticus isolates in whole black peppercorns (Piper nigrum L.). Int. J. Food Microbiol. 2016, 228, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Chutia, M.; Deka Bhuyan, P.; Pathak, M.G.; Sarma, T.C.; Boruah, P. Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from North East India. LWT-Food Sci. Technol. 2009, 42, 777–780. [Google Scholar] [CrossRef]
- Xing, F.; Hua, H.; Selvaraj, J.N.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Growth inhibition and morphological alterations of Fusarium verticillioides by cinnamon oil and cinnamaldehyde. Food Control. 2014, 46, 343–350. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control. 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Farzaneh, M.; Kiani, H.; Sharifi, R.; Reisi, M.; Hadian, J. Chemical composition and antifungal effects of three species of Satureja (S. hortensis, S. spicigera, and S. khuzistanica) essential oils on the main pathogens of strawberry fruit. Postharvest Biol. Technol. 2015, 109, 145–151. [Google Scholar] [CrossRef]
- Ni, Z.J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based Complement Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Shang, B.; Wang, L.; Lu, Z.; Liu, Y. Cinnamaldehyde inhibits fungal growth and aflatoxin B1 biosynthesis by modulating the oxidative stress response of Aspergillus flavus. Appl. Microbiol. Biotechnol. 2016, 100, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Z.; Liu, L.; Qu, S.; Mao, Y.; Peng, X.; Li, Y.; Tian, J. Cinnamaldehyde inhibits Candida albicans growth by causing apoptosis and its treatment on vulvovaginal candidiasis and oropharyngeal candidiasis. Appl. Microbiol. Biotechnol. 2019, 103, 9037–9055. [Google Scholar] [CrossRef]
- OuYang, Q.; Duan, X.; Li, L.; Tao, N. Cinnamaldehyde exerts its antifungal activity by disrupting the cell wall integrity of Geotrichum citri-aurantii. Front. Microbiol. 2019, 10, 414800. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Xing, F.; Selvaraj, J.N.; Wang, Y.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Inhibitory effect of essential oils on Aspergillus ochraceus growth and ochratoxin A production. PLoS ONE 2014, 9, e108285. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential Oils and Their Application in Food Safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Cardador, M.J.; Gallego, M. Effect of the chlorinated washing of minimally processed vegetables on the generation of haloacetic acids. J. Agric. Food Chem. 2012, 60, 7326–7332. [Google Scholar] [CrossRef]
- Klingelhöfer, D.; Zhu, Y.; Braun, M.; Bendels, M.H.K.; Brüggmann, D.; Groneberg, D.A. Aflatoxin—Publication analysis of a global health threat. Food Control. 2018, 89, 280–290. [Google Scholar] [CrossRef]
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. A review. Food Control. 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in Food and Feed: Present Status and Future Concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef]
- Matan, N.; Nisoa, M.; Matan, N. Antibacterial activity of essential oils and their main components enhanced by atmospheric RF plasma. Food Control. 2014, 39, 97–99. [Google Scholar] [CrossRef]
- Tzortzakis, N.G.; Economakis, C.D. Antifungal activity of lemongrass (Cympopogon citratus L.) essential oil against key postharvest pathogens. Innov. Food Sci. Emerg. Technol. 2007, 8, 253–258. [Google Scholar] [CrossRef]
- Groopman, J.D.; Kensler, T.W.; Wild, C.P. Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Annu. Rev. Public Health 2008, 29, 187–203. [Google Scholar] [CrossRef]
- Nasulhah Kasim, N.; Nursyimi Azlina Syed Ismail, S.; Masdar, N.; Ab Hamid, F.; Nawawi, W. Extraction and Potential of Cinnamon Essential Oil towards Repellency and Insecticidal Activity. Int. J. Sci. Res. Publ. 2014, 4, 1–6. [Google Scholar]
- Brari, J.; Thakur, D.R. Insecticidal efficacy of essential oil from Cinnamomum zeylanicum Blume and its two major constituents against Callosobruchus maculatus (F.) and Sitophilus oryzae (L.). J. Agric. Technol. 2015, 11, 1323–1336. [Google Scholar]
- Binseena, S.R.; Anitha, N.; Paul, A.; Amritha, V.S.; Anith, K.N. Management of rice weevil, Sitophilus oryzae using essential volatile oils. Entomon 2018, 43, 277–280. [Google Scholar] [CrossRef]
- Stefanazzi, N.; Stadler, T.; Ferrero, A. Composition and toxic, repellent and feeding deterrent activity of essential oils against the stored-grain pests Tribolium castaneum (Coleoptera: Tenebrionidae) and Sitophilus oryzae (Coleoptera: Curculionidae). Pest Manag. Sci. 2011, 67, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.; Adak, T.; Pandi, G.; Gowda, G. Ecofriendly approach for rice weevil (Sitophilus oryzae) (Coleoptera: Curculionidae) management using fumigant oils. In Proceedings of the 10th International Conference on Controlled Atmosphere and Fumigation in Stored Products, New Delhi, India, 6–11 November 2016; pp. 16–21. [Google Scholar]
- Houghton, P.J.; Ren, Y.; Howes, M.J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef] [PubMed]




| Compounds | Retention Time (min) | Amount (%) | MS Compatibility |
|---|---|---|---|
| α-Pinene | 10.545 | 0.34 | 91.5 |
| o-Cymene | 13.233 | 2.14 | 94.4 |
| D-Limonene | 13.363 | 0.41 | 90.5 |
| Eucalyptol | 13.466 | 0.08 | 91.5 |
| Linalool | 15.411 | 1.09 | 81.7 |
| α-Terpineol | 18.412 | 0.05 | 90.7 |
| Cinnamaldehyde | 20.441 | 89.29 | 90.6 |
| Eugenol | 22.628 | 0.37 | 93.5 |
| Caryophyllene | 24.417 | 1.86 | 92.8 |
| Cinnamyl acetate | 24.788 | 4.38 | 91.9 |
| Water Activity (aw) | Fungi | C:G | Thickness (mm) | Cinnamon Oil—CO (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.75 | 1.0 | 1.25 | Control 1 (cm3/Day) | 2.5 | 5.0 | Control 2 (cm3/Day) | |||||||||
| Mean ± SD (cm3/Day) | I (%) | Mean ± SD (cm3/Day) | I (%) | Mean ± SD (cm3/Day) | I (%) | Mean ± SD (cm3/Day) | I (%) | Mean ± SD (cm3/Day) | I (%) | ||||||
| 0.71 | RO01; RM01 and SR01 | 1:1 and 1:2 | 0.165; 0.183 and 0.287 | – | – | – | – | – | – | – | – | – | – | – | – |
| 0.95 | R. oryzae 01 | 1:1 | 0.165 | 8.94 aA ± 0.04 | 15.67 | 8.05 bA ± 0.04 | 24.06 | 6.57 cA ± 0.02 | 37.83 | 10.00 ± 0.00 | 6.40 bB ± 0.33 | 44.51 | 8.12 aA ± 0.54 | 29.62 | 11.54 ± 0.00 |
| 0.183 | 7.78 aB ± 0.04 | 26.56 | 7.03 bB ± 0.03 | 33.56 | 5.63 cB ± 0.03 | 46.67 | 5.33 aC ± 0.32 | 53.78 | 4.08 bB ± 0.27 | 64.62 | |||||
| 0.287 | 7.48 aC ± 0.02 | 29.33 | 6.91 bC ± 0.05 | 34.67 | 3.04 cC ± 0.03 | 70.83 | 8.02 aA ± 0.10 | 30.51 | 8.18 aA ± 0.17 | 29.12 | |||||
| 1:2 | 0.165 | 9.67 aA ± 0.03 | 8.89 | 8.30 bA ± 0.02 | 21.67 | 7.46 cA ± 0.04 | 29.50 | 8.25 bB ± 0.27 | 28.51 | 10.30 aB ± 0.30 | 10.73 | ||||
| 0.183 | 9.02 aB ± 0.02 | 15.00 | 7.77 bB ± 0.04 | 26.78 | 6.06 cB ± 0.03 | 42.61 | 7.65 bB ± 0.10 | 33.73 | 8.69 aC ± 0.16 | 24.73 | |||||
| 0.287 | 8.23 aC ± 0.03 | 22.33 | 7.18 bC ± 0.02 | 32.28 | 4.57 cC ± 0.02 | 56.50 | 11.29 aA ± 0.35 | 2.18 | 10.97 aA ± 0.13 | 4.90 | |||||
| R. microsporus 01 | 1:1 | 0.165 | 16.10 aA ± 0.72 | 14.11 | 15.51 aA ± 0.08 | 17.28 | 15.21 aA ± 0.48 | 18.89 | 24.02 ± 0.02 | 10.83 aA ± 0.01 | 54.90 | 10.87 bA ± 0.02 | 55.14 | 24.02 ± 0.02 | |
| 0.183 | 14.23 aB ± 0.50 | 24.11 | 13.74 aA ± 0.69 | 26.72 | 13.46 aAB ± 0.43 | 28.22 | 9.29 aB ± 0.03 | 61.33 | 8.76 bB ± 0.02 | 63.55 | |||||
| 0.287 | 13.02 aB ± 0.08 | 30.58 | 10.98 aB ± 0.45 | 41.42 | 8.38 aB ± 0.03 | 55.33 | 9.11 aC ± 0.06 | 62.08 | 8.17 bC ± 0.05 | 65.97 | |||||
| 1:2 | 0.165 | 18.75 aA ± 0.00 | 0.00 | 18.75 aA ± 0.00 | 0.00 | 16.14 bA ± 0.68 | 13.94 | 11.54 aA ± 0.00 | 51.96 | 11.15 bA ± 0.03 | 53.56 | ||||
| 0.183 | 16.98 aB ± 0.87 | 9.44 | 16.75 aB ± 0.53 | 10.67 | 15.49 aA ± 0.16 | 17.39 | 10.94 aB ± 0.06 | 54.45 | 9.48 bB ± 0.03 | 60.53 | |||||
| 0.287 | 14.56 aC ± 0.08 | 2.36 | 14.18 aC ± 0.54 | 24.39 | 11.58 bB ± 0.78 | 38.22 | 10.47 aC ± 0.06 | 56.39 | 9.29 bC ± 0.05 | 61.33 | |||||
| S. racemosum 01 | 1:1 | 0.165 | 8.23 aA ± 0.13 | 45.11 | 6.8 bA ± 0.12 | 54.67 | 6.38 bA ± 0.2 | 57.44 | 15.00 ± 0.00 | 7.04 aA ± 0.21 | 29.56 | 6.31 bA ± 0.23 | 36.94 | 10.00 ± 0.00 | |
| 0.183 | 7.09 aB ± 0.06 | 52.72 | 6.23 bB ± 0.09 | 58.44 | 5.04 cB ± 0.43 | 66.39 | 6.7 aAB ± 0.07 | 33.00 | 5.39 bAB ± 0.46 | 46.11 | |||||
| 0.287 | 6.41 aC ± 0.13 | 57.28 | 5.74 bC ± 0.18 | 61.72 | 4.11 cC ± 0.17 | 72.61 | 6.32 aB ± 0.08 | 36.83 | 5.11 bB ± 0.03 | 48.89 | |||||
| 1:2 | 0.165 | 14.25 aA ± 0.19 | 5.00 | 10.85 bA ± 0.41 | 27.67 | 9.05 cA ± 0.16 | 39.67 | 9.56 aA ± 0.05 | 4.39 | 7.82 bA ± 0.23 | 21.78 | ||||
| 0.183 | 13.38 aB ± 0.21 | 10.83 | 9.51 bB ± 0.21 | 36.61 | 8.38 cB ± 0.26 | 44.17 | 9.32 aB ± 0.02 | 6.72 | 7.63 bA ± 0.06 | 23.72 | |||||
| 0.287 | 12.64 aC ± 0.08 | 15.72 | 8.46 bC ± 0.06 | 43.61 | 7.63 cC ± 0.09 | 49.17 | 9.05 aC ± 0.01 | 9.44 | 7.14 bB ± 0.13 | 28.56 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, L.T.K.; Huynh, V.T.M.; Bui, N.M.; Le, A.T.H. Effectiveness of Cinnamon Oil Embedded Chitosan–Gelatin Film in Inhibiting Rhizopus oryzae, R. microsporus, and Syncephalastrum racemosum and Controlling Rice Weevil Infestation on Paddy Rice. Foods 2025, 14, 807. https://doi.org/10.3390/foods14050807
Phan LTK, Huynh VTM, Bui NM, Le ATH. Effectiveness of Cinnamon Oil Embedded Chitosan–Gelatin Film in Inhibiting Rhizopus oryzae, R. microsporus, and Syncephalastrum racemosum and Controlling Rice Weevil Infestation on Paddy Rice. Foods. 2025; 14(5):807. https://doi.org/10.3390/foods14050807
Chicago/Turabian StylePhan, Lien Thi Kim, Vi Thi Mi Huynh, Nhat Minh Bui, and Anh Thi Hong Le. 2025. "Effectiveness of Cinnamon Oil Embedded Chitosan–Gelatin Film in Inhibiting Rhizopus oryzae, R. microsporus, and Syncephalastrum racemosum and Controlling Rice Weevil Infestation on Paddy Rice" Foods 14, no. 5: 807. https://doi.org/10.3390/foods14050807
APA StylePhan, L. T. K., Huynh, V. T. M., Bui, N. M., & Le, A. T. H. (2025). Effectiveness of Cinnamon Oil Embedded Chitosan–Gelatin Film in Inhibiting Rhizopus oryzae, R. microsporus, and Syncephalastrum racemosum and Controlling Rice Weevil Infestation on Paddy Rice. Foods, 14(5), 807. https://doi.org/10.3390/foods14050807






